Introduction
The management of rectal cancer has evolved over the
past decades, with improvements in surgical techniques and adjuvant
therapy. Studies involving advanced rectal cancer have demonstrated
that preoperative or postoperative radiotherapy significantly
reduces the risk of local recurrence and cancer-specific mortality,
compared to surgery alone (1,2).
Furthermore, the addition of 5-fluorouracil (5-FU) to preoperative
radiotherapy has been shown to improve the pathological complete
response rate and locoregional control, compared to radiotherapy
alone (3,4). Thus, preoperative radiotherapy and
concurrent 5-FU administration has become a standard treatment in
locally advanced rectal cancer. However, disease recurrence remains
the major cause of mortality in these patients and recent efforts,
involving the incorporation of newer cytotoxic or
molecular-targeted agents into chemoradiotherapy regimens, have
been reported to improve oncological outcome. The addition of
oxaliplatin or irinotecan to 5-FU administered concurrently with
radiotherapy, resulted in a more favorable pathological response in
certain phase I/II trials (5,6),
while the addition of other chemotherapeutic drugs significantly
increased toxicity or exhibited no oncological improvement
(7,8). Similarly, incorporating anti-vascular
endothelial growth factor (VEGF) or anti-epidermal growth factor
receptor therapies into preoperative chemoradiotherapy in patients
with locally advanced rectal cancer did not significantly enhance
pathological downstaging, although few randomized trials have been
undertaken (9).
Our previous study demonstrated that preoperative
chemoradiotherapy enhanced tumor-specific VEGF expression,
particularly in individual cancer cells in vitro and in
vivo(10). We also
demonstrated that VEGF gene expression in pre-irradiation tumor
biopsies may be a useful marker in predicting tumor responsiveness
to preoperative chemoradiotherapy, as well as disease recurrence
(11). Subsequently, we
hypothesized that dose-intensified treatment strategies, including
the anti-VEGF antibody, in a preoperative setting, may be an ideal
adjuvant treatment. This is attributed to the fact that
angiogenesis is required for tumor growth and malignant progression
and VEGF is a crucial regulator of this process. However, there
have been no studies on chemoradiotherapy with bevacizumab as
preoperative treatment in Japanese patients with locally advanced
rectal cancer, although more active preoperative chemoradiotherapy,
using bevacizumab, oxaliplatin and fluoropyrimidine for locally
advanced rectal cancer, has already been reported in Western
countries (12,13). The present clinical phase I study
was conducted to determine the maximum tolerated dose (MTD) and
recommended dose (RD) of standard 5-FU/l-leucovorin (5-FU/LV) with
bevacizumab and radiation in Japanese patients with locally
advanced rectal cancer. We also evaluated adverse effects caused by
the treatment and resectability of locally advanced rectal cancer,
including pathological response rates.
Patients and methods
Ethics and patient consent
The present study was reviewed and approved by the
Mie University Institutional Review Board and the study was
performed in accordance with the Helsinki Declaration of 1975, as
revised in 2000. Patients were required to provide written informed
consent prior to enrollment.
Eligibility criteria
Eligible patients had previously untreated stage T3
or T4 locally advanced rectal cancer, as determined by computed
tomography (CT), magnetic resonance imaging (MRI) or endoscopic
ultrasound criteria and had histologically confirmed adenocarcinoma
prior to surgery. Eligible patients also had an Eastern Cooperative
Oncology Group (ECOG) performance status of 0–1 and a survival
expectation of >3 months. Additional eligibility criteria
included: age 20–80 years at enrollment; no severe compromise of
main organ functions (including bone marrow, lung, liver and
kidney) or blood biochemistry (leukocyte count
4,000–12,000/mm3; platelet count
≥100×103/μl; hemoglobin concentration ≥9.0 g/dl;
total bilirubin concentration ≤1.5 × upper limit of normal; serum
aspartate aminotransferase and alanine aminotransferase levels
<2.5 × upper limit of normal; and serum creatinine concentration
<1.5 × upper limit of normal).
The exclusion criteria were patients with potential
risk factors for bevacizumab, 5-FU and LV-related adverse events.
The risk factors included: patients with child-bearing potential or
lactation; clinical evidence of thrombosis, requiring medication;
uncontrolled hypertension; clinically significant cardiovascular or
pulmonary disease; clinical evidence of gastrointestinal bleeding;
bowel obstruction and perforation; active inflammatory bowel
disease; active infection; brain metastasis; synchronous or
metachronous malignancy; major surgical procedure within 28 days of
study enrollment; and any other cases regarded as inadequate for
enrollment by the investigator.
Pretreatment evaluation
Complete clinical and radiographic staging was
performed prior to enrollment in the study. Patients underwent the
following evaluations: history and physical examination; CT scan of
the chest, abdomen and pelvis; chest X-ray; colonoscopy; barium
enema; endoscopic ultrasound; and pelvic MRI. A complete blood
count with differential analysis, serum chemistry tests,
urinalysis, electrocardiogram, carcinoembryonic antigen and
carbohydrate antigen 19-9 levels were obtained. During treatment,
patients were evaluated at least weekly via a history and physical
examination and they underwent a complete blood count with
differential analysis, electrolyte analysis, liver function tests,
chemistry panel assays, coagulation panel assays and
urinalysis.
Treatment
Radiation therapy
The patients underwent the 4-field
(anterior-posterior, posterior-anterior and right and left
laterals) approach and radiation therapy was delivered using a
10-MV linear accelerator. Patients were treated in the prone
position, using a dedicated device to minimize exposure of the
small bowel. A CT-based treatment planning system was mandatory for
defining the planning target volume, including the primary tumor,
internal iliac lymph nodes and the surrounding mesorectum.
Radiotherapy was administered in fractions of 1.8 Gy/day, 5
days/week, for 5 weeks. The total dose of radiation delivered was
45 Gy.
Chemotherapy
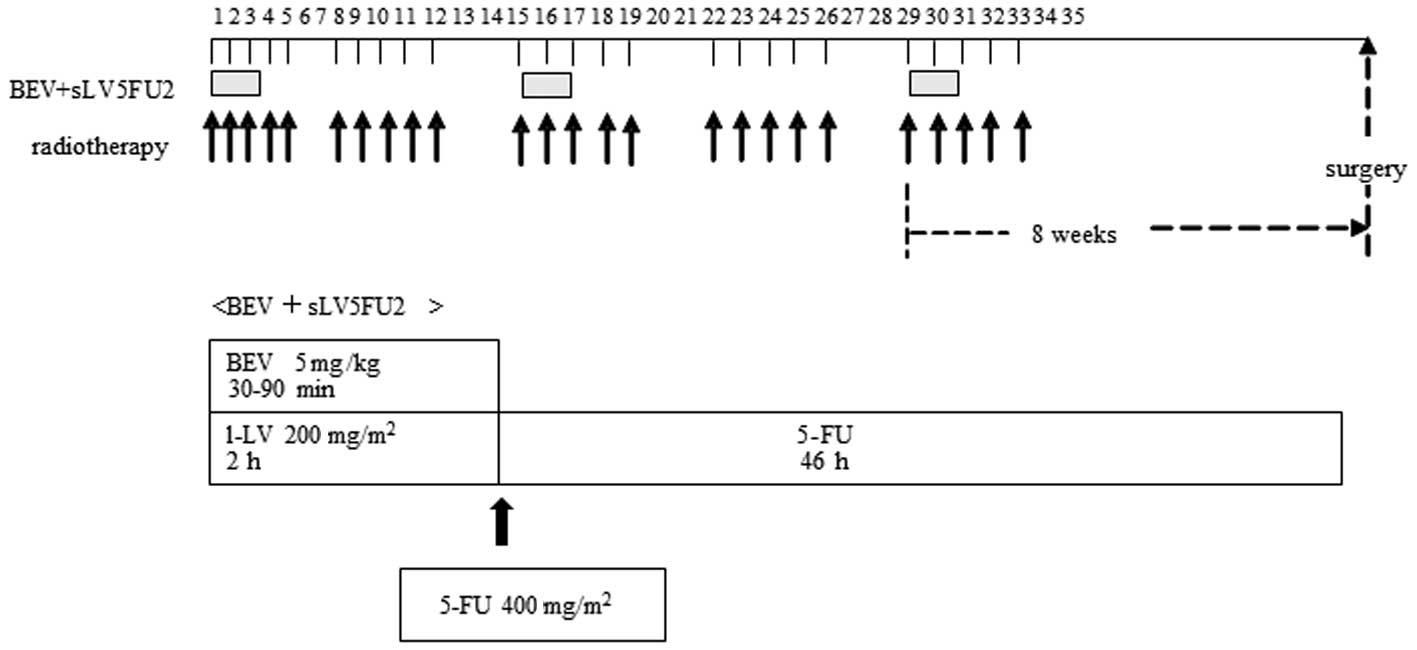
During radiation therapy, patients received three
courses of the simplified LV and 5-FU regimen (sLV5FU2) with
bevacizumab. The sLV5FU2 regimen consisted of 200 mg/m2
of LV, administered continuously by intravenous (i.v.) infusion
over 2 h, followed by 400 mg/m2 of 5-FU, administered by
i.v. bolus injection, at an initial loading dose of 2,000
mg/m2 over 46 h. The sLV5FU2 was administered every 2
weeks during radiotherapy and bevacizumab was infused at a fixed
dose of 5 mg/kg over 30–90 min on Days 1, 15 and 29. Table I provides a summary of 5-FU dose
levels delivered by continuous i.v. infusion. 5-FU administration
was initiated at 2,000 mg/m2, with a planned dose
escalation to dose levels 2 and 3. A treatment schema is shown in
Fig. 1. The patients were
premedicated prior to chemotherapy treatment to minimize nausea and
vomiting, usually with 5-HT3 receptor antagonists.
 | Table ISummary of 5-fluorouracil
(5-FU)/l-leucovorin/bevacizumab dose levels used in combination
with radiation therapy. |
Table I
Summary of 5-fluorouracil
(5-FU)/l-leucovorin/bevacizumab dose levels used in combination
with radiation therapy.
| Level | Bevacizumab
(kg/m2) | 5-FU civ
(mg/m2) | 5-FU bolus
(mg/m2) | L-leucovorin
(mg/m2) |
|---|
| −1 | 5 | 1,600 | 400 | 200 |
| 1 | 5 | 2,000 | 400 | 200 |
| 2 | 5 | 2,400 | 400 | 200 |
| 3 | 5 | 3,000 | 400 | 200 |
Surgery
Six to 8 weeks following the completion of
chemoradiotherapy, patients were preoperatively assessed by their
respective surgeons for tumor resectability. The assessment
included a history and physical examination and
proctosigmoidoscopy. Following thorough exploration of the
peritoneal cavity for metastases, a total mesorectal excision was
performed in conjunction with a colonic J-pouch-anal anastomosis
(CAA), low anterior resection (LAR) or abdominoperineal resection
(APR). A diverting ileostomy was used in the patients who had
undergone a sphincter-saving procedure.
Assessment
Adverse events were assessed at least weekly during
radiation therapy, using the National Cancer Institute common
toxicity criteria (version 3.0). Side effects were managed
aggressively with standard supportive measures. Resectability was
also evaluated and a complete pathological response was defined as
no evidence of malignancy in the specimen.
Study design, definitions and
endpoints
The primary endpoint of this study was the
determination of the MTD and the RD. The secondary endpoint was
evaluation of the extent and frequency of the adverse events and
the resectability of locally advanced rectal cancer. The
dose-limiting toxicity (DLT) was defined as Grade 4 hematological
toxicity; fever with Grade 3 neutropenia; Grade 3 or higher
thrombocytopenia; Grade 3 or higher non-hematological toxicity,
despite adequate supportive care; any single interruption of
radiation therapy of ≥7 days or >2 interruptions per radiation
course; any delay in the completion of radiation therapy of >14
days; and any treatment-related hospitalization or death. The MTD
was defined as the dose level that produced DLT in at least two out
of three patients, or two out of six patients. If DLT occurred in
one of the first three patients, three additional patients were
assigned to receive the same dose level. If none of the three
patients initially receiving a given dose level exhibited DLT, or
if one out of six patients exhibited DLT, the dose was increased to
the next level. Dose escalation was not allowed in the same
patient. The dose level immediately below the MTD was considered
the RD for phase II studies. In this study, we also compared the
adverse events of enrolled patients with those from our
conventional chemoradiotherapy (sLV5FU2) treatment group.
Results
Patient characteristics
From December, 2009 through May, 2012, six patients
treated at our hospital were enrolled in the study. Patient
characteristics are summarized in Table II. Five patients had an ECOG
performance status (PS) of 0 and one patient had a PS of 1.
 | Table IIPatient characteristics. |
Table II
Patient characteristics.
| Characteristics | |
|---|
| Age in years
(range) | 65 (57–75) |
| Gender | |
| Male | 4 |
| Female | 2 |
| Performance
status | |
| 0 | 5 |
| 1 | 1 |
| Tumor site | |
| Rb | 4 |
| Rb-P | 2 |
| Clinical stage
(pretreatment) | |
| T3N0M0 | 2 |
| T3N1M0 | 1 |
| T4N1M0 | 1 |
| T4N2M0 | 2 |
DLT and RD levels
The acute toxicities that were observed are listed
in Table III. Three patients were
initially enrolled at dose level 1. Two patients had perianal
dermatitis (one had Grade 1 and the other Grade 2). In addition,
two patients experienced diarrhea (one had Grade 2 and the other
Grade 3) during week 3 of radiotherapy, prior to the third course
of sLV5FU2 with bevacizumab. One patient had Grade 1 neutropenia,
but there were no severe hematological toxicities. In the first
three enrolled patients, chemoradiotherapy was completed according
to the protocol, with the exception of one patient with refractory
Grade 2 diarrhea who was able to complete radiation therapy without
receiving a third course of sLV5FU2/bevacizumab. Of the first three
patients enrolled in the study, one patient exhibited DLT due to
diarrhea. Subsequently, three additional patients were enrolled at
the same dose level 1. Of these additional three patients, the
first patient had Grade 2 neutropenia, Grade 2 stomatitis and Grade
2 perianal dermatitis; however, chemoradiotherapy was completed
according to the protocol. The second patient had Grade 2 vomiting,
Grade 3 diarrhea and Grade 2 perianal dermatitis during week 4 and
required hospitalization and infusion therapy, which constituted a
protocol-defined DLT. Following a 1-week treatment interruption,
the patient was able to complete radiotherapy, without receiving a
final course of sLV5FU2/bevacizumab. The third patient developed
Grade 3 neutropenia and Grade 2 diarrhea during Week 2 of
radiotherapy. Although the second course of sLV5FU2/bevacizumab was
postponed for 1 week, the patient was able to complete
chemoradiotherapy without receiving a final course of chemotherapy.
None of the six patients experienced Grade 4 toxicities. Two out of
the six patients exhibited DLT, therefore, level 1 was designated
as the MTD. To determine the LD, toxicities were also compared with
those of six patients who had received standard preoperative
chemoradiotherapy with sLV5FU2 during the same period. The sLV5FU2
regimen of the control group consisted of 200 mg/m2 of
LV, administered by continuous i.v. infusion over 2 h, followed by
400 mg/m2 of 5-FU, administered by i.v. bolus injection
at a total dose of 2,400 mg/m2 over 46 h; the total dose
of radiation delivered was 45 Gy. Patient characteristics of the
control group were matched for the study. In the control group, two
patients had Grade 3 neutropenia, but the others had no toxicities
≥Grade 3. Frequently occurring toxicities were diarrhea in four
patients (one of Grade 1 and three of Grade 2) and there were three
patients with Grade 3 perianal dermatitis. There were no
significant differences in toxicity between the study group and the
control group. Therefore, dose level 1 was determined to have
acceptable toxicity as the LD and was declared the recommended
phase II dose.
 | Table IIIToxicity parameters evaluated in the
study and matched control group. |
Table III
Toxicity parameters evaluated in the
study and matched control group.
| Dose level 1 | Control |
|---|
|
|
|---|
| Toxicity | G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 |
|---|
| Hematological | | | | | | | | |
| Neutropenia | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 0 |
| Anemia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Non-hematological | | | | | | | | |
| Nausea | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Vomiting | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stomatitis | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Fatigue | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Diarrhea | 0 | 2 | 2 | 0 | 1 | 3 | 0 | 0 |
| Skin (perianal
dermatitis) | 2 | 3 | 0 | 0 | 0 | 3 | 0 | 0 |
Efficacy measures
The patients were evaluable for tumor response. Four
out of the six patients exhibited a radiographic partial response
as their best response and two exhibited radiographically stable
disease. Details of treatment response for the patients are
presented in Table IV. Laparotomy
was performed in the patients. One patient underwent LAR with
colonic J-pouch reconstruction, four underwent CAA and one
underwent APR. The postoperative complications were perineal wound
infection in one patient (16.7%), pelvic abscess in one patient
(16.7%) and pouch-vaginal fistula in one patient (16.7%). With
regard to surgical curability, two patients had a
microscopically-positive resection margin (R1) and four patients
had complete resection (R0). Two patients exhibited a pathological
complete response (pCR rate, 33.3%) and one exhibited a high
pathological response.
 | Table IVIndividual patient characteristics
and treatment response. |
Table IV
Individual patient characteristics
and treatment response.
| Patient | Dose level | Pretreatment
stage | Dose-limiting
toxicity | Radiographic
response | Pathological
effectsa Grade | Surgical
outcome |
|---|
| 1 | 1 | T3N0M0 | No | PR | 2 | R0 |
| 2 | 1 | T4N1M0 | Yes | PR | 3b | R0 |
| 3 | 1 | T4N2M0 | No | SD | 1a | R1 |
| 4 | 1 | T4N2M0 | No | PR | 1b | R1 |
| 5 | 1 | T3N0M0 | Yes | SD | 1a | R0 |
| 6 | 1 | T3N1M0 | No | PR | 3b | R0 |
Discussion
Conventional chemoradiotherapy using 5-FU has been
the standard treatment for locally advanced rectal cancer, even
after several active agents, such as oxaliplatin, irinotecan and
molecular-targeted agents, have been developed. To improve
oncological outcome, more synergistically active and safe
chemotherapy is required for administration in combination with
preoperative radiotherapy, for the treatment of advanced rectal
cancer. Among the established agents for recurrent or metastatic
colorectal cancer, VEGF-targeted chemotherapy appears appropriate
for inclusion in new chemoradiotherapeutic regimens for rectal
cancer, since the radiation-enhancing effects of VEGF-targeted
therapy may occur through several mechanisms, such as the
normalization of tumor vascularity and subsequent reoxygenation,
which results in the enhancement of the radiosensitivity of cancer
cells; the killing of endothelial cells in the tumor vasculature,
which results in cancer cell death; and the reduction in the number
of blood endothelial and progenitor cells. However, the role of
molecular-targeted agents, including bevacizumab, in preoperative
chemoradiotherapy for rectal cancer remains to be elucidated.
Several lines of evidence have suggested that the
inhibition of VEGF may also be effective in chemoradiotherapy for
locally advanced rectal cancer. Anti-VEGF antibody has been
reported to enhance the effectiveness of radiotherapy by reducing
tumor vascular density and interstitial fluid pressure (IFP) in
xenografts (14). It has also been
reported that a single infusion of bevacizumab decreased tumor
perfusion, vascular volume, microvascular density, IFP and the
number of viable circulating endothelial and progenitor cells and
increased the fraction of vessels with pericyte coverage in rectal
cancer patients (15). Clinically,
Giralt et al(16) have
demonstrated that VEGF overexpression is an indicator of poor
clinical outcome following preoperative chemoradiotherapy, whereas
Nozue et al(17) described
an association between post-treatment VEGF overexpression and
distant metastases. As regards pre-treatment elevated VEGF
expression, Toiyama et al(11) demonstrated that analyzing VEGF
expression levels in rectal cancer may be of benefit in estimating
the effects of preoperative chemoradiotherapy and predicting
systemic recurrence following rectal cancer surgery.
Although several trials of bevacizumab with
chemoradiotherapy have shown promising results (18–20),
there is no available randomized study and there is bias associated
with these single-arm studies. Willett et al(15) reported on a phase I/II study, in
which 32 patients were treated with bevacizumab and 5-FU (at a dose
of 225 mg/m2 per 24 h). Radiation was delivered to the
pelvis (50.4 Gy in 28 fractions over 5.5 weeks) and surgery was
performed 7–10 weeks following completion of the preoperative
therapy. In the phase I portion of the study, two out of every five
patients treated with 10 mg/kg of bevacizumab developed DLT
(diarrhea and colitis) and the phase II dose was reduced to 5
mg/kg. The majority of the adverse effects of this regimen were
mild (Grade 1/2); however, certain patients experienced Grade 3
toxicities, including diarrhea, hypertension and radiation
dermatitis. Among these patients, the pCR rate was 16% and ∼40% of
patients developed postoperative complications (18,19).
Recently, Crane et al(20)
treated 25 advanced rectal cancer patients with radiotherapy at a
total dose of 50.4 Gy, capecitabine and bevacizumab (at a dose of 5
mg/kg, administered every 2 weeks) followed ∼7 weeks later by total
mesorectal excision. No patient experienced Grade 3 hand-foot
syndrome, gastrointestinal toxicity or significant hematological
toxicity. The patients received surgery and the pCR rate was
32%.
In our study, we used a sLV5F2 plus bevacizumab
regimen as concurrent chemotherapy. The sLV5FU2 plus bevacizumab
regimen has been established as the initial therapy for patients
not considered appropriate for intensive therapy, in accordance
with the National Comprehensive Cancer Network guidelines, since
the addition of bevacizumab to 5-FU/LV has improved the survival of
patients with metastatic colorectal cancer, without the severe
adverse effects that result from the use of the irinotecan- or
oxaliplatin-based regimens (21).
The concurrent administration of sLV5FU2 and bevacizumab causes
less cytotoxicity, as well as greater oncological benefit.
Therefore, this regimen seems to be appropriate for administration
in combination with preoperative radiotherapy for the treatment of
locally advanced rectal cancer. We infused bevacizumab at a fixed
dose of 5 mg/kg, according to a previous report by Willett et
al(18), then evaluated the
MTD and RD of 5-FU administered by continuous i.v. infusion
initiated at a dose of 2,000 mg/m2, with a planned dose
escalation. Japanese patients, who received preoperative
chemoradiotherapy with the combination regimen of sLV5FU2 and
bevacizumab, exhibited good compliance without compromising their
quality of life and were able to receive treatment according to the
planned protocol. In addition, two out of the six patients (33.3%)
exhibited pCR, despite one patient experiencing DLT, thus the final
course of chemotherapy was cancelled. These results suggest that
bevacizumab exerted an additional beneficial effect on positive
oncological outcome in patients who underwent standard preoperative
chemoradiotherapy using 5-FU-based chemotherapy.
In conclusion, the combination of 5-FU, LV,
bevacizumab and radiation therapy for the treatment of locally
advanced rectal cancer, as evaluated by our study, resulted in
acceptable toxicity and promising effectiveness. A recommended
phase II dose of sLV5FU2/bevacizumab based on our findings would
consist of 200 mg/m2 of LV, administered by continuous
i.v. infusion over 2 h, followed by 400 mg/m2 of 5-FU,
administered by i.v. bolus injection at a total dose of 2,000
mg/m2 over 46 h and 5 mg/kg of bevacizumab on Days 1, 15
and 29, in combination with radiation therapy. A phase II study
should be performed to examine the efficacy and safety of our
regimen in patients with locally advanced rectal cancer.
References
|
1.
|
Douglass HO Jr, Moertel CG, Mayer RJ, et
al: Survival after postoperative combination treatment of rectal
cancer. N Engl J Med. 315:1294–1295. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Cammà C, Giunta M, Fiorica F, et al:
Preoperative radiotherapy for resectable rectal cancer: a
meta-analysis. JAMA. 284:1008–1015. 2000.PubMed/NCBI
|
|
3.
|
Bosset JF, Collette L, Calais G, et al:
Chemotherapy with preoperative radiotherapy in rectal cancer. N
Engl J Med. 355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gérard JP, Conroy T, Bonnetain F, et al:
Preoperative radiotherapy with or without concurrent fluorouracil
and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin
Oncol. 24:4620–4625. 2006.
|
|
5.
|
Sato T, Ozawa H, Hatate K, et al: A phase
II trial of neoadjuvant preoperative chemoradiotherapy with S-1
plus irinotecan and radiation in patients with locally advanced
rectal cancer: clinical feasibility and response rate. Int J Radiat
Oncol Biol Phys. 79:677–683. 2011. View Article : Google Scholar
|
|
6.
|
Rödel C, Liersch T, Hermann RM, et al:
Multicenter phase II trial of chemoradiation with oxaliplatin for
rectal cancer. J Clin Oncol. 25:110–117. 2007.PubMed/NCBI
|
|
7.
|
Mohiuddin M, Winter K, Mitchell E, et al:
Randomized phase II study of neoadjuvant combined-modality
chemoradiation for distal rectal cancer: Radiation Therapy Oncology
Group Trial 0012. J Clin Oncol. 24:650–655. 2006. View Article : Google Scholar
|
|
8.
|
Cancer and Leukemia Group B 89901; Ryan
DP, Niedzwiecki D, Hollis D, et al: Phase I/II study of
preoperative oxaliplatin, fluorouracil, and external-beam radiation
therapy in patients with locally advanced rectal cancer: Cancer and
Leukemia Group B 89901. J Clin Oncol. 24:2557–2562. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wadlow RC and Ryan DP: The role of
targeted agents in preoperative chemoradiation for rectal cancer.
Cancer. 116:3537–3548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Inoue Y, Ojima E, Watanabe H, et al: Does
preoperative chemoradiotherapy enhance the expression of vascular
endothelial growth factor in patients with rectal cancer? Oncol
Rep. 18:369–375. 2007.PubMed/NCBI
|
|
11.
|
Toiyama Y, Inoue Y, Saigusa S, et al: Gene
expression profiles of epidermal growth factor receptor, vascular
endothelial growth factor and hypoxia-inducible factor-1 with
special reference to local responsiveness to neoadjuvant
chemoradiotherapy and disease recurrence after rectal cancer
surgery. Clin Oncol (R Coll Radiol). 22:272–280. 2010.
|
|
12.
|
Dipetrillo T, Pricolo V, Lagares-Garcia J,
et al: Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and
radiation for rectal cancer. Int J Radiat Oncol Biol Phys.
82:124–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kennecke H, Berry S, Wong R, et al:
Pre-operative bevacizumab, capecitabine, oxaliplatin and radiation
among patients with locally advanced or low rectal cancer: a phase
II trial. Eur J Cancer. 48:37–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kozin SV, Boucher Y, Hicklin DJ, et al:
Vascular endothelial growth factor receptor-2-blocking antibody
potentiates radiation-induced long-term control of human tumor
xenografts. Cancer Res. 61:39–44. 2001.
|
|
15.
|
Willett CG, Boucher Y, di Tomaso E, et al:
Direct evidence that the VEGF-specific antibody bevacizumab has
antivascular effects in human rectal cancer. Nat Med. 10:145–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Giralt J, Navalpotro B, Hermosilla E, et
al: Prognostic significance of vascular endothelial growth factor
and cyclooxygenase-2 in patients with rectal cancer treated with
preoperative radiotherapy. Oncology. 71:312–319. 2006. View Article : Google Scholar
|
|
17.
|
Nozue M, Isaka N and Fukao K:
Over-expression of vascular endothelial growth factor after
preoperative radiation therapy for rectal cancer. Oncol Rep.
8:1247–1249. 2001.PubMed/NCBI
|
|
18.
|
Willett CG, Boucher Y, Duda DG, et al:
Surrogate markers for antiangiogenic therapy and dose-limiting
toxicities for bevacizumab with radiation and chemotherapy:
continued experience of a phase I trial in rectal cancer patients.
J Clin Oncol. 23:8136–8139. 2005. View Article : Google Scholar
|
|
19.
|
Willett CG, Duda DG, di Tomaso E, et al:
Efficacy, safety, and biomarkers of neoadjuvant bevacizumab,
radiation therapy, and fluorouracil in rectal cancer: a
multidisciplinary phase II study. J Clin Oncol. 27:3020–3026. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Crane CH, Eng C, Feig BW, et al: Phase II
trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy
for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys.
76:824–830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kabbinavar F, Hambleton J, Mass RD, et al:
Combined analysis of efficacy: the addition of bevacizumab to
fluorouracil/leucovorin improves survival for patients with
metastatic colorectal cancer. J Clin Oncol. 23:3706–3712. 2005.
View Article : Google Scholar : PubMed/NCBI
|