Introduction
Large-cell neuroendocrine carcinomas (LCNECs) of the
lung are aggressive tumors. Patients with LCNEC have an extremely
poor prognosis since the biological behavior of LCNECs is similar
to that of small cell lung carcinomas and LCNECs also have
characteristics of high-grade neuroendocrine tumors (1–9).
However, the treatment for patients with LCNEC has been based on
non-small cell carcinomas. To improve the outcomes for patients
with LCNEC, a better understanding of its clinicopathological
characteristics, including preoperative diagnoses, the
effectiveness of adjuvant chemotherapy, tumor recurrence rates and
the prognosis of different stages, is required. In addition, a new
edition (7th) of the TNM classification of malignant tumors was
introduced in 2007 by the International Association for the Study
of Lung Cancer (IASLC) to replace the 6th edition (10). At present, the prognosis of LCNEC
tumors staged according to the newer edition of the TNM
classification is unknown. The aim of this study was to describe
the clinicopathological features of LCNECs in detail and compare
outcomes between stages determined by the guidelines of the 6th and
7th editions. We also discuss the role of adjuvant chemotherapy and
the optimal management of patients with LCNEC.
Patients and methods
Ethics
The Institutional Review Board of Kitasato
University Hospital approved the protocols and procedures used in
the study. As this was a retrospective study, treatments varied,
particularly adjuvant chemotherapies.
Patients
Clinical data from 42 patients diagnosed with
primary LCNEC who underwent treatment at the Kitasato University
Hospital between 1991 and 2009 were retrospectively analyzed. LCNEC
was diagnosed in resected surgical specimens showing evidence of
neuroendocrine differentiation detected by immunohistochemistry and
neuroendocrine morphologic features according to the World Health
Organization International Histological Classification of Tumors
(1).
Immunohistochemical staining used the following
antibodies: polyclonal anti-chromogranin A (Dako, Glostrup,
Denmark), polyclonal antisynaptophysin (Dako) and anti-NCAM (Nippon
Kayaku Co., Tokyo, Japan). Positive staining for chromogranin A,
synaptophysin or NCAM indicated neuroendocrine differentiation
(1,11).
The following data were gathered from the medical
records: patient gender, age, smoking index, clinical staging,
preoperative symptoms, presence of paraneoplastic syndrome, tumor
sites, definitive preoperative diagnosis, preoperative serum tumor
marker levels [carcinoembryonic antigen (CEA), squamous cell
carcinoma antigen (SCC-A), neuron-specific enolase (NSE),
cytokeratin 19 fragment (CYFRA) and progastrin- releasing peptide
(ProGRP)], surgical procedure, pathological findings (tumor size,
mitotic rate, surgical margin, immunohistochemical findings),
pathological TNM stages according to the 6th and 7th editions,
adjuvant chemotherapy, time of recurrence, time of mortality, date
of last follow-up, recurrence site(s), occurrence of metachronous
or synchronous primary cancer and patient outcome.
Treatments and evaluation
Preoperative chest computed tomography (CT) was
performed to evaluate the primary tumor, lymph node metastases,
pulmonary metastases and pleural effusion. To evaluate distant
metastases, magnetic resonance imaging (MRI) or CT were used for
brain lesions, abdominal CT for liver and adrenal gland metastases,
bone scintigraphy for bone metastases and in certain cases,
positron emission tomography was used for distant metastases.
The response of LCNEC tumors to induction
chemotherapy was based on the Response Evaluation Criteria in Solid
Tumors (RECIST) Guidelines (12).
Postoperative follow-ups of patients with LCNEC were routinely
performed 3 or 4 times per year and consisted of screening for
symptoms and chest roentgenography. When abnormal findings were
identifed, the patient underwent chest CT and if recurrences were
suspected, the patient also underwent abdominal CT, brain MRI or
CT, bone scintigraphy or positron emission tomography.
Statistical analysis
Survival time was calculated from the date of
surgery to the time of recurrence or mortality or date of last
follow-up and was evaluated using the Kaplan-Meier method. Survival
curves were compared using the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The 42 patients with LCNEC were predominantly male,
predominantly smokers and none of the patients manifested
paraneoplastic syndrome (Table I).
With regard to preoperative serum tumor markers, CEA was elevated
in 50% of patients, whereas NSE and ProGRP, specific neuroendocrine
tumor markers, were infrequently elevated (Table II). There were 6 patients whose
preoperative diagnoses were definitive or suspicious for LCNEC.
Limited resection was performed in only 2 cases and intraoperative
pleural lavage cytology was positive in only 1 case (Table III).
 | Table IPreoperative characteristics of 42
patients with pulmonary large-cell neuroendocrine carcinoma
(LCNEC). |
Table I
Preoperative characteristics of 42
patients with pulmonary large-cell neuroendocrine carcinoma
(LCNEC).
| Variable | N (%) |
|---|
| Total | 42 (100) |
| Age (years) | |
| Mean (range) ±
SD | 64.4 (50–85)±8.4 |
| Gender | |
| Male | 38 (90.5) |
| Female | 4 (9.5) |
| Clinical stage | |
| IA | 11 (26.2) |
| IB | 13 (31.0) |
| IIA | 1 (2.4) |
| IIB | 8 (19.0) |
| IIIA | 8 (19.0) |
| IIIB | 0 (0.0) |
| IV | 1 (2.4) |
| Smoking | |
| Smoker | 40 (95.2) |
| Non-smoker | 2 (4.8) |
| Symptomatic | |
| + | 15 (35.7) |
| − | 27 (64.3) |
| Paraneoplastic
syndrome | |
| + | 0 (0.0) |
| − | 42 (100) |
| Primary site | |
| Right | 29 (69.0) |
| Left | 13 (31.0) |
| Definitive or
suspicious preoperative diagnosis | |
| LCNEC | 6 (14.3) |
| Not LCNEC | 30 (71.4) |
| Unknown | 6 (14.3) |
 | Table IIPreoperative serum tumor markers. |
Table II
Preoperative serum tumor markers.
| Tumor marker | N (%) |
|---|
| CEA (38 informative
cases) | |
| Elevated | 19 (50.0) |
| Not | 19 (50.0) |
| CYFRA (18 informative
cases) | |
| Elevated | 5 (27.8) |
| Not | 13 (72.2) |
| NSE (35 informative
cases) | |
| Elevated | 9 (25.7) |
| Not | 26 (74.3) |
| ProGRP (21
informative cases) | |
| Elevated | 4 (19.0) |
| Not | 17 (81.0) |
| SCC-A (34 informative
cases) | |
| Elevated | 4 (11.8) |
| Not | 30 (88.2) |
 | Table IIISurgical procedures. |
Table III
Surgical procedures.
| Variable | N (%) |
|---|
| Total | 42 (100.0) |
| Surgery | |
|
Pneumonectomy | 5 (11.9) |
| Bilobectomy | 3 (7.1) |
| Lobectomy | 32 (76.2) |
| Wedge
resection | 2 (4.8) |
| Plasty | |
| Pulmonary
artery | 1 (2.4) |
| Carina | 1 (2.4) |
| Intraoperative
pleural lavage cytology (26 informative cases) | |
| Positive | 1 (3.8) |
| Negative | 25 (96.2) |
| Combined
resection | |
| Chest wall | 3 (7.1) |
| Parietal
pleura | 2 (4.8) |
| Carina +
esophagus | 1 (2.4) |
| Pericardium | 1 (2.4) |
Pathological findings
Pathological findings revealed that 37 patients
underwent complete resection, with negative tumor margins. There
were 11 patients whose tumors were diagnosed as combined LCNEC with
components of adenocarcinoma in 7, squamous cell carcinoma in 3 and
large cell carcinoma in 1 case. The mean number of mitoses per 10
high-power fields was 63.7 (Tables
IV and V). Immunohistochemical
staining revealed that no neuroendocrine marker was expressed in
every LCNEC case and there were 12 cases that were only positive
for 1 marker (Table IV).
 | Table IVPostoperative characteristics of 42
patients with pulmonary large-cell neuroendocrine carcinoma
(LCNEC). |
Table IV
Postoperative characteristics of 42
patients with pulmonary large-cell neuroendocrine carcinoma
(LCNEC).
| Variable | N (%) |
|---|
| Total | 42 (100.0) |
| Surgical
margin | |
| Negative | 37 (88.1) |
|
Microscopic-positive | 4 (9.5) |
|
Macroscopic-positive | 1 (2.4) |
| Pure or
combined | |
| LCNEC | 31 (73.8) |
| Combined
LCNEC | 11 (26.2) |
|
Adenocarcinoma | 7 |
| Squamous cell
ca | 3 |
| Large cell
ca | 1 |
| Tumor size
(cm) | |
| Mean (range) ±
SD | 4.0
(1.4–9.0)±1.8 |
| Mitoses/10 hpf | |
| Mean (range) ±
SD | 63.7
(16–141)±30.7 |
| Neuroendocrine
marker | |
| Chromogranin | |
| Positive | 21 (50.0) |
| Negative | 21 (50.0) |
| Synaptophysin | |
| Positive | 35 (83.3) |
| Negative | 7 (16.7) |
| NCAM | |
| Positive | 34 (81.0) |
| Negative | 8 (19.0) |
| Number of positive
markers | |
| 1 | 12 (28.6) |
| 2 | 12 (28.6) |
| All 3 | 18 (42.9) |
 | Table VMitoses in 40 patients with
large-cell neuroendocrine carcinoma. |
Table V
Mitoses in 40 patients with
large-cell neuroendocrine carcinoma.
| Mitoses/10 hpf | No. of cases |
|---|
| 11 to 20 | 3 |
| 21 to 30 | 1 |
| 31 to 40 | 5 |
| 41 to 50 | 7 |
| 51 to 60 | 3 |
| 61 to 70 | 5 |
| 71 to 80 | 5 |
| 81 to 90 | 4 |
| 91 to 100 | 1 |
| >100 | 6 |
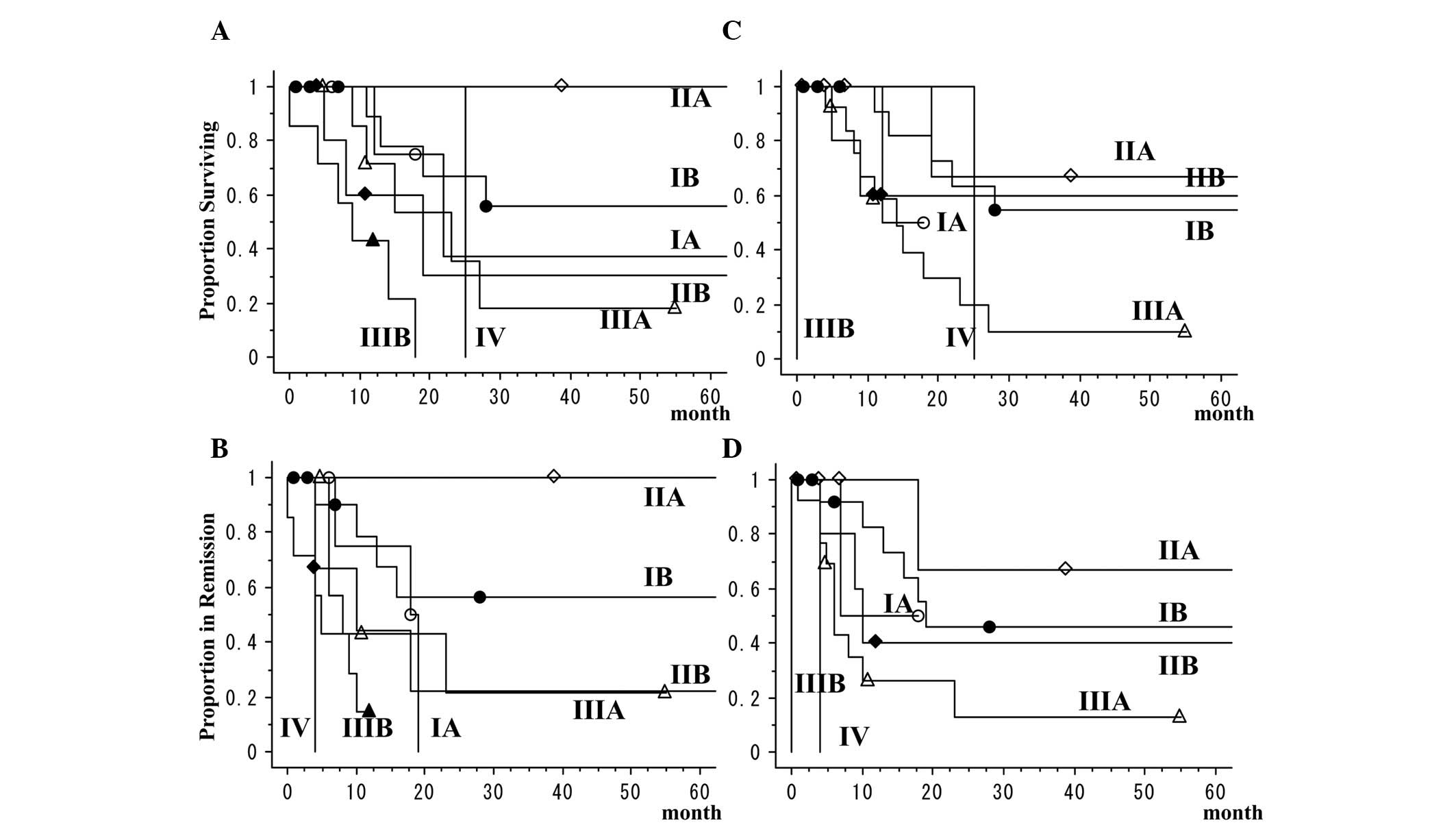
TNM classifications according to the 6th
and 7th editions and prognosis
Comparison of the pathological classifications of
the 7th and 6th edition of the TNM classification revealed that in
the newer classification, 6 cases were reclassified from stage IIIB
of the older classification to stage IIB or IIIA, 4 from stage IA
to stage IB, 3 from stage IIB to stage IIA or IIIA and 2 from stage
IB to stage IIA. For the patients, the 5-year overall survival rate
was 34.7% and the 5-year disease-free survival rate was 32.9%. The
5-year overall survival rates of patients with stage I cancers
according to the 6th and 7th staging editions was 51.3%. In the two
classifications, patients with LCNEC had extremely poor outcomes,
even for stage I cancers (Table VI
and Fig. 1). The 3-year overall
survival rate of patients with pure LCNEC was 38.5% and that of
patients with combined LCNEC was 17.9%. There was no significant
difference between the survival rates of pure LCNEC and combined
LCNEC (P=0.7546).
 | Table VIPathological staging according to the
6th and 7th editions of the TNM classification. |
Table VI
Pathological staging according to the
6th and 7th editions of the TNM classification.
| Variable | N (%) | 5-year overall
survival rate (%) | 5-year disease-free
survival rate (%) |
|---|
| 6th edition
pathological stage | | | |
| IA | 6 (14.3) | 37.5 | 0 |
| IB | 12 (28.6) | 55.6 | 56.3 |
| IIA | 2 (4.8) | 100 | 100 |
| IIB | 6 (14.3) | 30.0 | 22.2 |
| IIIA | 8 (19.0) | - | - |
| IIIB | 7 (16.7) | 0 | - |
| IV | 1 (2.4) | 0 | 0 |
| 7th edition
pathological stage | | | |
| IA | 2 (4.8) | - | - |
| IB | 14 (33.3) | 54.5 | 45.8 |
| IIA | 6 (14.3) | 66.7 | 66.7 |
| IIB | 5 (11.9) | 60.0 | 40.0 |
| IIIA | 13 (31.0) | - | - |
| IIIB | 1 (2.4) | 0 | 0 |
| IV | 1 (2.4) | 0 | 0 |
Induction and adjuvant therapy
Five patients underwent platinum-based induction
chemotherapy and 3 patients achieved partial responses (response
rate, 60%). Nine patients underwent adjuvant chemotherapy,
consisting of platinum-based chemotherapy in 5 and
non-platinum-based chemotherapy in 4 cases (Table VII). Three of the latter patients,
who were staged IB and IIB according to the 6th edition, received
uracil and tegafur (UFT).
 | Table VIIAdjuvant therapies and
recurrence. |
Table VII
Adjuvant therapies and
recurrence.
| Variable | N (%) |
|---|
| Total | 42 (100.0) |
| Therapy | |
| Induction
therapy | |
|
Chemotherapy | 5 (11.9) |
| Adjuvant
therapy | 14 (33.3) |
|
Chemotherapy | 9 (21.4) |
|
Platinum-based | 5 (11.9) |
|
Non-platinum-based | 4 (9.5) |
| Radiation
therapy | 5 (11.9) |
| Postoperative
recurrence | |
| Positive | 22 (52.4) |
| Negative | 20 (47.6) |
| Site of
recurrence | |
| Local | 3 (7.1) |
| Local +
distant | 6 (14.3) |
| Distant | 13 (31.0) |
Recurrences and other cancers
Postoperative recurrences were observed in 22 cases
(52.4%). Recurrences consisted of local mediastinal lymph node
recurrences in 3, mediastinal lymph node recurrences with distant
metastases in 5, localized recurrence in the diaphragm with distant
metastases in 1 and distant metastases only in 13 patients
(Table VII). Distant metastases
were frequently observed in the brain, bone, liver, lung and
adrenal glands (Table VIII).
 | Table VIIIDistant metastases in patients with
large-cell neuroendocrine carcinoma. |
Table VIII
Distant metastases in patients with
large-cell neuroendocrine carcinoma.
| Site | No. of cases |
|---|
| Brain | 8 |
| Bone | 8 |
| Live | 7 |
| Lung | 5 |
| Adrenal gland | 4 |
Of the 37 patients with complete tumor resection, 18
(48.6%) had postoperative recurrences and 16 of those had distant
metastases. In 15 patients with pathological stage I tumors
according to the 7th edition of the TNM classification, 7 (46.7%)
had distant metastases without local recurrences. In 26 patients
undergoing surgery alone without induction or adjuvant therapy, 13
(50.0%) had postoperative recurrences, of which 12 had distant
metastases. Of the 9 patients undergoing adjuvant chemotherapy,
only 2 who had undergone UFT adjuvant chemotherapy had distant
metastases. Four of 5 patients undergoing platinum-based adjuvant
chemotherapy had no recurrences. Of the 22 patients with
postoperative recurrences, 21 developed recurrences <2 years
after surgery (Table IX).
 | Table IXDuration from date of surgery until
tumor recurrence. |
Table IX
Duration from date of surgery until
tumor recurrence.
| Duration
(months) | No. of cases |
|---|
| ≤3 | 1 |
| 4–6 | 9 |
| 7–12 | 5 |
| 13–24 | 6 |
| 25–36 | 0 |
| ≥37 | 1 |
Of 42 patients, 13 (31.0%) had metachronous or
synchronous primary cancers and 3 patients had primary cancers at
>1 site with the exception of LCNEC tumors (Table X). Of the 13 cases, only 3 cases
had undergone induction or adjuvant chemotherapy.
 | Table XMetachronous or synchronous primary
cancers in patients with large cell neuroendocrine carcinoma. |
Table X
Metachronous or synchronous primary
cancers in patients with large cell neuroendocrine carcinoma.
| Metachronous or
synchronous primary cancers | N |
|---|
| Lung ca | 3 |
| Gastric ca | 2 |
| Esophageal ca | 1 |
| Ca of the uterine
cervix | 1 |
| Ca of the uterine
body | 1 |
| Colon ca | 1 |
| Urinary bladder
ca | 1 |
| Pharyngeal ca +
lung ca | 1 |
| Colon ca + lung
ca | 1 |
| Esophageal ca +
prostate ca | 1 |
Discussion
In 2007, IASLC published a new staging system;
however, no studies are available on the correlations between the
new staging system and the prognoses of LCNEC stages. In the
present study, we have demonstrated that LCNEC patients had
extremely poor outcomes, even for stage I disease according to the
new staging system. Studies published prior to 2007 have also
reported poor outcomes for patients with LCNEC, with 5-year
survival rates ranging from 15 to 57% (2–4,13–19).
Even LCNEC patients at pathological stage I had poor outcomes, with
5-year survival rates of 27–67%. Therefore, according to the new
TNM staging system, the prognoses of patients with different stages
of LCNEC have not changed.
In patients with LCNEC who have had surgery as
initial treatment, knowledge of the frequent sites of recurrence is
required for treatment planning. In this study, we revealed that 21
of 22 patients with recurrent tumors developed their recurrences
within 2 years after surgery. There has been limited information
published on treatments for patients with LCNEC, particularly with
regard to recurrent LCNEC tumors and sites of recurrence. In a
study of LCNEC patients who were followed over time, the majority
of recurrent tumors occurred within 3 years after surgery; patients
frequently developed brain metastases and a number of patients with
recurrence had extremely poor outcomes. However, certain cases with
recurrence achieved good responses to treatment, particularly
treatment with platinum-based chemotherapy, radiation therapy or a
combination thereof (20).
Findings of another study demonstrated that LCNEC tumors responded
well to chemotherapy (21). These
results indicate that it is necessary to treat recurrences as early
as possible after the initial surgery and recurrent tumors should
be treated using platinum-based chemotherapy, radiation therapy or
chemoradiotherapy with platinum-based agents.
As a number of patients with recurrent LCNEC tumors
had an extremely poor outcome, preventing recurrent tumors is
crucial. Promising results of previous studies indicate that the
efficacy of adjuvant chemotherapy for patients with LCNEC should be
examined (15,22,23).
In the prospective study initiated in 2000 by Iyoda et
al(23), treatment using
cisplatin plus etoposide was evaluated, since it is similar to
therapy used for small cell lung carcinoma, which has
clinicopathological and biological features resembling LCNEC. The
results revealed that patients undergoing
cisplatin-plus-etoposide-based adjuvant chemotherapy after complete
surgical resection achieved good outcomes. In their retrospective
study, Rossi et al(15)
also reported that cisplatin-plus-etoposide-based adjuvant
chemotherapy was effective for patients with LCNEC. Thus, adjuvant
chemotherapy following complete resection of LCNEC tumors appears
promising for achieving good outcomes. Moreover, platinum-based
adjuvant chemotherapy for LCNEC patients following surgery
significantly prevented recurrence (20). Multivariate analyses revealed that
platinum-based adjuvant chemotherapy was a significant, good
prognostic factor in patients with LCNEC, although propensity score
analyses did not confirm this observation. In the present study,
platinum-based adjuvant chemotherapy appeared to protect patients
from recurrences.
Although it is important to check for LCNEC tumor
recurrence, we must also be aware of additional new cancer lesions,
including those at other sites. The incidence of a second primary
digestive cancer following resection of lung cancer has been
reported to be 1–2% (24). The
proportion of patients successfully treated for their initial
non-small cell lung carcinoma and at risk of developing a second
non-small cell lung carcinoma has been reported to be 1–2%
(25,26). A previous study has also revealed a
high rate of postoperative secondary cancers following surgery for
LCNEC tumors (20). Our results
demonstrated that 31% of LCNEC patients had metachronous or
synchronous primary cancer. These results indicate that a high
proportion of LCNEC patients develop a second primary cancer.
Although LCNEC tumors are intrinsically aggressive, development of
second primary cancers may be one of the reasons that patients with
LCNEC have poor outcomes. We need to consider the risk of the
occurrence of second primary cancers when following postoperative
LCNEC patients and continue to investigate the mechanisms involved
in the frequent development of metachronous or synchronous primary
cancers in these patients.
In conclusion, our results have shown that the
histological classifications of LCNEC were reproducible and
patients with LCNEC had poor outcomes, even for stage I disease in
the new TNM staging system. Frequent recurrences and metachronous
or synchronous primary cancers in patients with LCNEC should be
treated. Adjuvant chemotherapy with platinum-based regimens may be
effective in preventing recurrent tumors.
Acknowledgements
The authors thank Isao Okayasu,
Noriyuki Masuda, Kazushige Hayakawa, Hidenori Hara, Yoshio Matsui
and Kenji Nezu for their helpful and excellent contributions. This
study was supported in part by a Grant-in-aid for Scientific
Research (C) 24592098 of the Japanese Ministry of Education,
Culture, Sports, Science and Technology.
References
|
1.
|
Travis WD, Colby TV, Corrin B, Shimosato Y
and Brambilla E: Histological Typing of Lung and Pleural Tumours.
World Health Organization International Histological Classification
of Tumors, XIII. 3rd edition. Springer-Verlag; Berlin/Heidelberg:
1999
|
|
2.
|
Iyoda A, Hiroshima K, Nakatani Y and
Fujisawa T: Pulmonary large cell neuroendocrine carcinoma: its
place in the spectrum of pulmonary carcinoma. Ann Thorac Surg.
84:702–707. 2007.PubMed/NCBI
|
|
3.
|
Iyoda A, Hiroshima K, Toyozaki T, Haga Y,
Fujisawa T and Ohwada H: Clinical characterization of pulmonary
large cell neuroendocrine carcinoma and large cell carcinoma with
neuroendocrine morphology. Cancer. 91:1992–2000. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Asamura H, Kameya T, Matsuno Y, et al:
Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin
Oncol. 24:70–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Iyoda A, Hiroshima K, Moriya Y, et al:
Pulmonary large cell neuroendocrine carcinoma demonstrates high
proliferative activity. Ann Thorac Surg. 77:1891–1895. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Iyoda A, Hiroshima K, Baba M, Saitoh Y,
Ohwada H and Fujisawa T: Pulmonary large cell carcinomas with
neuroendocrine features are high-grade neuroendocrine tumors. Ann
Thorac Surg. 73:1049–1054. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Onuki N, Wistuba II, Travis WD, et al:
Genetic changes in the spectrum of neuroendocrine lung tumors.
Cancer. 85:600–607. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jones MH, Virtanen C, Honjoh D, et al: Two
prognostically significant subtypes of high-grade lung
neuroendocrine tumours independent of small-cell and large-cell
neuroendocrine carcinomas identified by gene expression profiles.
Lancet. 363:775–781. 2004. View Article : Google Scholar
|
|
9.
|
Hiroshima K, Iyoda A, Shibuya K, et al:
Genetic alterations in early-stage pulmonary large cell
neuroendocrine carcinoma. Cancer. 100:1190–1198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Goldstraw P, Crowley J, Chansky K, et al:
International Association for the Study of Lung Cancer
International Staging Committee. The IASLC Lung Cancer Staging
Project: proposals for the revision of the TNM stage groupings in
the forthcoming (seventh) edition of the TNM Classification of
malignant tumours. J Thorac Oncol. 2:706–714. 2007.
|
|
11.
|
Jiang SX, Kameya T, Shoji M, Dobashi Y,
Shinada J and Yoshimura H: Large cell neuroendocrine carcinoma of
the lung. A histologic and immunohistochemical study of 22 cases.
Am J Surg Pathol. 22:526–537. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
13.
|
Veronesi G, Morandi U, Alloisio M, et al:
Large cell neuroendocrine carcinoma of the lung: A retrospective
analysis of 144 surgical cases. Lung Cancer. 53:111–115. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Takei H, Asamura H, Maeshima A, et al:
Large cell neuroendocrine carcinoma of the lung: a
clinicopathologic study of eighty-seven cases. J Thorac Cardiovasc
Surg. 124:285–292. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Rossi G, Cavazza A, Marchioni A, et al:
Role of chemotherapy and the receptor tyrosine kinases KIT,
PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine
carcinoma of the lung. J Clin Oncol. 23:8774–8785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Travis WD, Rush W, Flieder DB, et al:
Survival analysis of 200 pulmonary neuroendocrine tumors with
clarification of criteria for atypical carcinoid and its separation
from typical carcinoid. Am J Surg Pathol. 22:934–944. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Paci M, Cavazza A, Annessi V, et al: Large
cell neuroendocrine carcinoma of the lung: a 10-year
clinicopathologic retrospective study. Ann Thorac Surg.
77:1163–1167. 2004.PubMed/NCBI
|
|
18.
|
Battafarano RJ, Fernandez FG, Ritter J, et
al: Large cell neuroendocrine carcinoma: an aggressive form of
non-small cell lung cancer. J Thorac Cardiovasc Surg. 130:166–172.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Skuladottir H, Hirsch FR, Hansen HH and
Olsen JH: Pulmonary neuroendocrine tumors: incidence and prognosis
of histological subtypes. A population-based study in Denmark. Lung
Cancer. 37:127–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Iyoda A, Hiroshima K, Moriya Y, et al:
Postoperative recurrence and the role of adjuvant chemotherapy in
patients with pulmonary large-cell neuroendocrine carcinoma. J
Thorac Cardiovasc Surg. 138:446–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yamazaki S, Sekine I, Matsuno Y, et al:
Clinical responses of large cell neuroendocrine carcinoma of the
lung to cisplatin-based chemotherapy. Lung Cancer. 49:217–223.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Iyoda A, Hiroshima K, Toyozaki T, et al:
Adjuvant chemotherapy for large cell carcinoma with neuroendocrine
features. Cancer. 92:1108–1112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Iyoda A, Hiroshima K, Moriya Y, et al:
Prospective study of adjuvant chemotherapy for pulmonary large cell
neuroendocrine carcinoma. Ann Thorac Surg. 82:1802–1807. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kamiyama H, Ikeya T, Suda K, Murai K,
Aoyama K and Hoshi E: Second primary digestive cancer after
resection of lung cancer. Surg Today. 34:577–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Battafarano RJ, Force SD, Meyers BF, et
al: Benefits of resection for metachronous lung cancer. J Thorac
Cardiovasc Surg. 127:836–842. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Johnson BE: Second lung cancers in
patients after treatment for an initial lung cancer. J Natl Cancer
Inst. 90:1335–1345. 1998. View Article : Google Scholar : PubMed/NCBI
|