Introduction
Esophageal carcinoma is highly malignant and the
prognosis of patients with locally advanced tumors is poor.
Preoperative chemoradiotherapy (CRT) has been shown to
significantly improve survival in patients with advanced esophageal
carcinoma. However, this survival benefit is limited to patients
with a major pathological response (complete or subtotal tumor
regression) (1). Prediction of the
CRT response prior to or early during the course of CRT may be
beneficial in avoiding or discontinuing this type of treatment in
non-responders and may also help responders avoid invasive surgery
through the initiation or continuation of CRT. Therefore, the
identification of biomarkers that predict the CRT response is
critical in multimodality treatment for advanced esophageal
carcinoma.
Elevated serum C-reactive protein (CRP) levels have
been shown to be associated with disease progression and poor
prognosis in patients with esophageal carcinoma and preoperative
serum CRP levels have been shown to be an independent prognostic
factor in patients with resectable esophageal carcinoma (2–5).
However, the clinical significance of serum CRP levels in patients
with unresectable tumors in need of chemotherapy or CRT as an
initial treatment has not been fully elucidated in relation to
treatment response and prognosis.
In the present study, we investigated the
association between pathological response and survival and the time
course of serum CRP levels during induction CRT in patients with
clinical T3–T4 esophageal squamous cell carcinoma, all of whom
underwent subsequent esophagectomy. We also verified the usefulness
of serum CRP levels as a potential biomarker in the prediction of
the CRT response early in the course of induction CRT.
Patients and methods
Patients
Thirty-four patients with clinical T3–T4 esophageal
squamous cell carcinoma, who received induction CRT followed by
esophagectomy at the Kyoto Prefectural University of Medicine
Hospital between 2001 and 2008, were analyzed in this retrospective
study. Induction CRT was indicated for unresectable or marginally
resectable tumors, i.e., T4 or bulky T3 tumors that were considered
difficult to completely resect in the absence of induction therapy.
If a clinical response was observed and complete resection was thus
considered possible, the patient was scheduled for surgery.
Clinical and pathological staging was performed according to the
tumor-node-metastasis (TNM) classification of the International
Union Against Cancer (UICC) (6).
Esophagography, endoscopy, computed tomography and/or bronchoscopy
were routinely performed to determine pretreatment clinical staging
and treatment response. Endoscopic ultrasonography was occasionally
performed. From 2004 onward, PET scans were performed prior to and
following CRT. Written informed consent was obtained from all the
patients.
Induction CRT
The induction CRT regimen consisted of radiation and
concurrent administration of 5-fluorouracil (5-FU) and cisplatin,
as previously described (7).
Briefly, 5-FU was administered intravenously at 200–250
mg/m2/day on days 1–5, 8–12, 15–19 and 22–26 and
cisplatin was administered at a dose of 5–7 mg/m2/day by
drip infusion for 1 h on days 1–5, 8–12, 15–19 and 22–26. In total,
40 Gy of radiation for 4 weeks at 2 Gy daily (5 days/week) was
delivered. Treatment responses were evaluated 2–3 weeks following
completion of CRT. Surgery was scheduled 4–6 weeks after the last
day of CRT in patients for whom complete resection was considered
feasible.
Surgical therapy and pathological
response evaluation
The patients underwent subtotal esophagectomy with
regional lymphadenectomy through right thoracotomy and laparotomy,
followed by reconstruction using the stomach via a retrosternal
route with cervical anastomosis through a neck incision. The
pathological response to CRT was evaluated by the grade of response
of the primary tumor. The complete tumor bed was cut into sections
including the entire esophageal wall and grading of the response to
CRT was as follows (8): grade 3,
complete disappearance of viable cancer cells in the tumor bed;
grade 2, >2/3 disappearance of viable cancer cells; and grade 1,
<2/3 disappearance of viable cancer cells. CRT was considered
effective in patients with grade 2 or 3 histological response
(responders) and ineffective in patients with grade 1 histological
response (non-responders).
Measurement of serum CRP
Serum CRP levels were measured in blood samples
collected from each patient at the following six time points: prior
to CRT (preCRT); 1, 2, 3 and 4 [-weeks after initiation of CRT
(CRT1W, CRT2W, CRT3W and CRT4W, respectively); and prior to surgery
(PreOP). Serum CRP levels were measured with a latex agglutination
turbidimetric immunoassay (LZ-TEST ‘Eiken’ CRP-HG, Tokyo, Japan);
normal serum levels were ≤0.3 mg/dl.
Statistical analysis
In the correlation analysis between serum CRP levels
and pathological response, continuous data were compared using the
Mann-Whitney U test and ordinal data using the χ2 or
Fisher’s exact test. In survival analysis, data are shown as mean
and range [95% confidence interval (CI)]. Univariate survival
analysis was performed using the Kaplan-Meier method with the
log-rank test. Multivariate survival analysis and calculation of
the odds ratios with 95% CI was performed using a Cox proportional
hazard regression model, including all covariates that were
identified as significant by univariate analysis. Statistical
analyses were performed using software JMP version 8 for Macintosh
(SAS Institute Inc., Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics of the study
patients
The 34 patients analyzed in the present study were
the same as those analyzed in our previous study (7). The background characteristics of the
patients are listed in Table I.
The patient sample consisted of 28 males and 6 females with a
median age of 66.5 years. At pretreatment diagnosis, all primary
tumors were classified as T3 (47.1%) or T4 (52.9%) and the majority
of the patients (79.4%) were classified as stage III. All M1 cases
had distant lymph node metastases at the supraclavicular and celiac
regions. At postoperative diagnosis, 8 primary tumors (23.5%) were
evaluated as grade 3 and pathological responses of primary tumors
(grades 3 and 2) were observed in 18 patients (52.9%). Complete
resection was performed in 29 patients (85.3%). No infective
complications, such as pneumonia, were detected during the
pretreatment evaluation and no infective adverse events, resulting
in the discontinuation of CRT or the administration of antibiotics,
were observed during CRT.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Patient no. (total,
34) |
|---|
| Total | 34 |
| Gender | |
| Male | 28 |
| Female | 6 |
| Age (years) | |
| <65 | 14 |
| ≥65 | 20 |
| Tumor location | |
| Ce | 4 |
| Ut | 8 |
| Mt | 18 |
| Lt | 4 |
| cStage | |
| II | 3 |
| III | 27 |
| IV | 4 |
| cT | |
| T3 | 16 |
| T4 | 18 |
| cN | |
| N0 | 9 |
| N1 | 25 |
| cM | |
| M0 | 30 |
| M1 (lym) | 4 |
| cCRT response | |
| CR | 6 |
| PR | 18 |
| SD | 10 |
| pCRT responsea | |
| Grade 1 | 16 |
| Grade 2 | 10 |
| Grade 3 | 8 |
| ypStage | |
| 0 | 7 |
| I | 2 |
| II | 10 |
| III | 11 |
| IV | 4 |
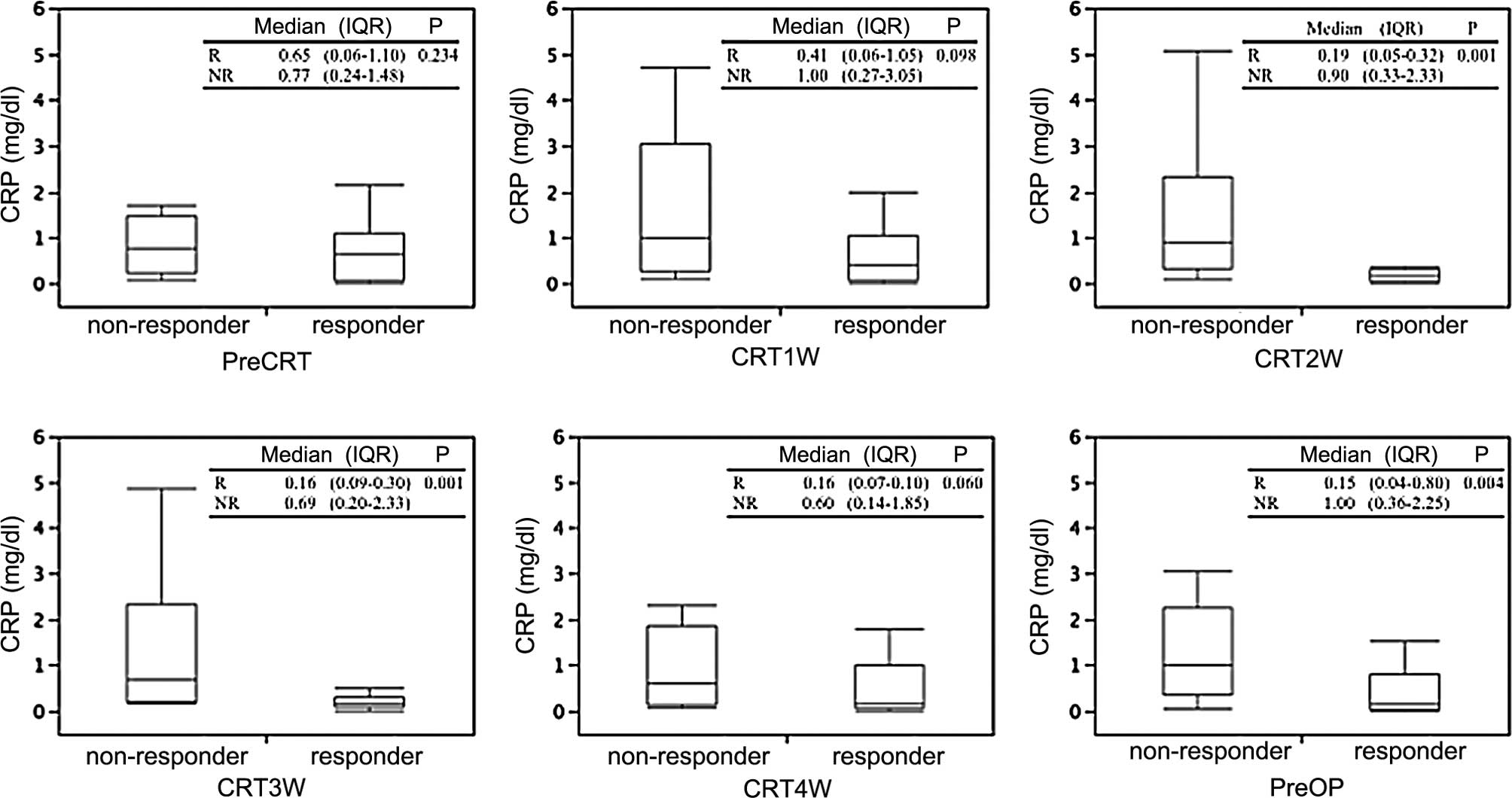
Association of serum CRP levels with
pathological response
First, absolute values of CRP at each of the six
time points during the course of induction CRT were compared
according to the primary tumor response. No significant difference
in pretreatment CRP values was observed between responders and
non-responders, whereas responders exhibited significantly lower
CRP values compared to those of non-responders at CRT2W, CRT3W and
PreOP. There were no significant differences between CRP values at
CRT1W and CRT4W (Fig. 1). Although
pretreatment CRP values showed a tendency to decrease 2–3 weeks
after the initiation of CRT in responders compared to
non-responders, no significant differences were observed between
CRP values prior to CRT and those at each subsequent time point.
Subsequently, CRP levels (>0.3 vs. ≤0.3) at each of the 6 time
points during the course of induction CRT were compared according
to the primary tumor response (Table
IIA). Pretreatment serum CRP levels were elevated (CRP >0.3)
in 23 out of the 34 patients (67.6%). Prior to CRT, no significant
difference in CRP levels was observed between responders and
non-responders, whereas responders exhibited significantly lower
CRP levels (≤0.3) compared to non-responders at CRT2W, CRT3W and
PreOP. CRP levels at CRT1W and CRT4W were not different between the
2 groups. In addition, CRP levels (>0.3 vs. ≤0.3) were compared
according to the primary tumor response in patients with elevated
pretreatment CRP levels (CRP >0.3 prior to CRT) (Table IIB). Responders exhibited
significantly lower CRP levels (≤0.3) compared to non-responders at
CRT2W and CRT3W, whereas no significant difference was detected in
CRP levels at CRT1W, CRT4W and PreOP between the 2 groups.
 | Table IIAssociation of pathological CRT
response with serum CRP levels. |
Table II
Association of pathological CRT
response with serum CRP levels.
| A, All patients
(n=34). |
|
| Parameter | Responders
(n=18) | Non-responders
(n=16) | P-value |
|
| PreCRT | | | |
| CRP ≤0.3 | 7 | 4 | 0.477 |
| CRP >0.3 | 11 | 12 | |
| CRT1W | | | |
| CRP ≤0.3 | 8 | 6 | 0.681 |
| CRP >0.3 | 10 | 10 | |
| CRT2W | | | |
| CRP ≤0.3 | 14 | 4 | 0.005 |
| CRP >0.3 | 4 | 12 | |
| CRT3W | | | |
| CRP ≤0.3 | 14 | 5 | 0.014 |
| CRP >0.3 | 4 | 11 | |
| CRT4W | | | |
| CRP ≤0.3 | 10 | 6 | 0.291 |
| CRP >0.3 | 8 | 10 | |
| PreOP | | | |
| CRP ≤0.3 | 12 | 3 | 0.007 |
| CRP >0.3 | 6 | 13 | |
|
| B, Patients with
pretreatment elevated CRP levels (n=23). |
|
| Parameter | Responders
(n=11) | Non-responders
(n=12) | P-value |
|
| CRT1W | | | |
| CRP ≤0.3 | 2 | 2 | >0.999 |
| CRP >0.3 | 9 | 10 | |
| CRT2W | | | |
| CRP ≤0.3 | 9 | 1 | 0.001 |
| CRP >0.3 | 2 | 11 | |
| CRT3W | | | |
| CRP ≤0.3 | 9 | 4 | 0.036 |
| CRP >0.3 | 2 | 8 | |
| CRT4W | | | |
| CRP ≤0.3 | 5 | 5 | 0.855 |
| CRP >0.3 | 6 | 7 | |
| PreOP | | | |
| CRP ≤0.3 | 5 | 1 | 0.069 |
| CRP >0.3 | 6 | 11 | |
Prediction of pathological response by
serum CRP levels
Prediction accuracies for pathological response by
CRP ≤0.3 at CRT2W, CRT3W and PreOP are shown in Table III. Among all 34 patients, CRP ≤0.3
at CRT2W, CRT3W and PreOP predicted responders with accuracies of
76.5, 73.5 and 73.5%, respectively. Furthermore, among patients
with CRP >0.3 at PreCRT and CRP ≤0.3 at CRT2W and CRT3W,
predicted responders with accuracies of 87.0 and 73.9%,
respectively.
 | Table IIIPrediction accuracies of pathological
CRT response by serum CRP levels. |
Table III
Prediction accuracies of pathological
CRT response by serum CRP levels.
| Prediction
responses | All patients (n=34)
| CRP >0.3
(PreCRT) patients (n=23)
|
|---|
| Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | Accuracy |
|---|
| CRP ≤0.3 | | | | | | |
| CRT2W | 77.8 | 75 | 76.5 | 81.8 | 91.7 | 87 |
| CRT3W | 77.8 | 68.8 | 73.5 | 81.8 | 66.7 | 73.9 |
| PreOP | 66.7 | 81.3 | 73.5 | 45.5 | 91.7 | 69.6 |
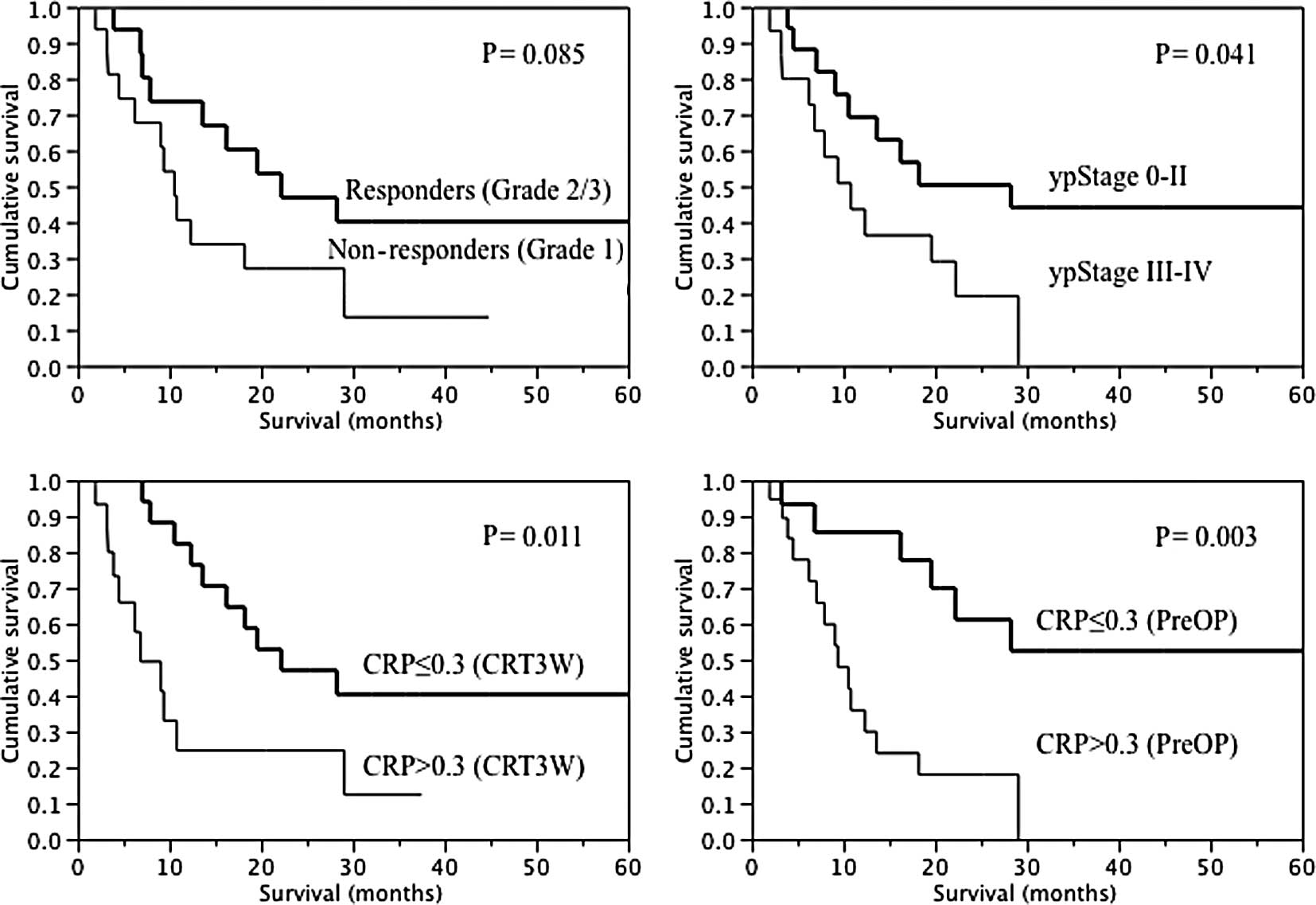
Association of serum CRP levels with
survival
Results of univariate analysis of overall survival
are shown in Table IV and Fig. 2. The mean survival time in all 34
patients was 17.2 months, with a 3-year survival rate of 28.2%.
There were no significant differences in overall survival in terms
of gender, age, tumor location, cTNM classification, or clinical
response to CRT. Regarding the grade of the primary tumor response,
although the result was not significant, responders tended to
survive longer compared to non-responders. Significant differences
were observed in ypStage (0–II vs. III–IV) (Table IVA). Furthermore, in relation to
serum CRP levels, significantly longer survival was observed in CRP
≤0.3 patients compared to CRP >0.3 patients at CRT3W and PreOP.
Although the result was not significant at PreCRT and CRT4W, CRP
≤0.3 patients tended to survive longer compared to CRP >0.3
patients. No significant difference was observed at CRT1W and CRT2W
(Table IVB). In multivariate
analysis, preoperative CRP ≤0.3 was identified as the only
independent prognostic factor (Table
V).
 | Table IVUnivariate analysis of overall
survival according to clinicopathological parameters and serum CRP
levels. |
Table IV
Univariate analysis of overall
survival according to clinicopathological parameters and serum CRP
levels.
| Parameter | Patients
(n=34) | Mean survival (95%
CI) | Mean survival (95%
CI) | P-value |
|
| A,
Clinicopathological parameters and overall survival. |
|
| Overall | 34 | 17.2 | 12.1–22.3 | |
| Gender | | | | |
| Male | 28 | 16.1 | 10.5–21.7 | 0.148 |
| Female | 6 | 22.2 | 5.3–39.0 | |
| Age (years) | | | | |
| <65 | 14 | 18.4 | 9.5–27.3 | 0.622 |
| ≥65 | 20 | 16.1 | 9.7–22.6 | |
| Tumor location | | | | |
| Supracarinal | 12 | 14.1 | 5.7–22.5 | 0.378 |
| Infracarinal | 22 | 18.9 | 12.0–25.7 | |
| cT | | | | |
| T3 | 16 | 18.4 | 13.1–23.7 | 0.401 |
| T4 | 18 | 16.1 | 7.1–25.1 | |
| cN | | | | |
| N0 | 9 | 19.3 | 1.8–36.9 | 0.730 |
| N1 | 25 | 16.4 | 11.9–20.9 | |
| cStage | | | | |
| II+III | 30 | 17.9 | 12.2–23.6 | 0.128 |
| IV | 4 | 12.0 | 1.0–24.9 | |
| cCRT response | | | | |
| CR+PR | 18 | 18.8 | 12.5–25.1 | 0.104 |
| SD | 16 | 13.3 | 3.4–23.2 | |
| ypStage | | | | |
| 0–II | 24 | 21.0 | 12.9–29.4 | 0.041 |
| III–IV | 10 | 12.2 | 7.2–17.1 | |
| pCRT response | | | | |
| Responders
(grades 2+3) | 18 | 20.6 | 12.5–28.8 | 0.085 |
| Non–responders
(grade 1) | 16 | 13.3 | 7.1–19.6 | |
|
| B, CRP levels and
overall survival. |
|
| Parameter | Patients
(n=34) | Mean survival
(months) | Mean survival (95%
CI) | P-value |
|
| PreCRT | | | | |
| CRP ≤0.3 | 11 | 23.1 | 10.8–35.4 | 0.054 |
| CRP >0.3 | 23 | 14.3 | 9.2–19.5 | |
| CRT1W | | | | |
| CRP ≤0.3 | 14 | 18.9 | 8.9–29.4 | 0.194 |
| CRP >0.3 | 20 | 16.0 | 10.0–21.9 | |
| CRT2W | | | | |
| CRP ≤0.3 | 18 | 19.8 | 11.9–27.6 | 0.256 |
| CRP >0.3 | 16 | 14.3 | 7.3–21.3 | |
| CRT3W | | | | |
| CRP ≤0.3 | 19 | 22.6 | 15.2–30.0 | 0.011 |
| CRP >0.3 | 15 | 10.3 | 4.5–16.1 | |
| CRT4W | | | | |
| CRP ≤0.3 | 16 | 23.5 | 14.9–32.1 | 0.066 |
| CRP >0.3 | 18 | 11.6 | 6.2–17.0 | |
| PreOP | | | | |
| CRP≤0.3 | 15 | 25.0 | 15.5–34.5 | 0.003 |
| CRP>0.3 | 19 | 11.0 | 6.9–15.1 | |
 | Table VMultivariate analysis of overall
survival. |
Table V
Multivariate analysis of overall
survival.
| P-value | Odds ratio | 95% CI |
|---|
| ypStage | | | |
| 0–II/III–IV | 0.659 | 0.80 | 0.28–2.20 |
| CRP (CRT3W) | | | |
| ≤0.3/>0.3 | 0.124 | 0.46 | 0.17–1.24 |
| CRP (PreOP) | | | |
| ≤0.3/>0.3 | 0.021 | 0.31 | 0.10–0.84 |
Discussion
In the present study, we investigated the
association between serum CRP levels, pathological CRT response and
prognosis in patients with unresectable or marginally resectable
tumors who underwent induction CRT followed by esophagectomy, with
a focus on temporal changes in CRP levels during CRT (not prior to
or after CRT) and demonstrated that serum CRP levels measured
shortly after CRT initiation correlated with CRT response and
prognosis.
Elevated serum CRP levels have been shown to be
associated with disease progression and poor prognosis in patients
with esophageal carcinoma (2–5,9,10).
However, the underlying mechanisms by which CRP levels are
associated with disease progression or prognosis have not been
fully elucidated. In a previous study, we included the same
patients as the present study and demonstrated that elevated serum
CRP levels after CRT completion (prior to surgery), but not prior
to CRT initiation, correlated with a poor CRT response and
prognosis in close association with IL-6 levels in serum and local
tumor tissues and that IL-6 and other cytokines or growth factors
secreted by tumor tissues through tumor-stromal or tumor-host
interactions may play a critical role in the elevation of serum CRP
levels and poor clinical outcome (7).
The significant correlation between postoperative
pathological tumor parameters and prognosis has been well
characterized and the grade of the primary tumor response has a
prognostic significance (10,11),
as does pathological stage (1,12–14).
In the present study, among postoperative parameters, pathological
stage was significantly correlated with prognosis, whereas the
primary tumor response was not identified as a significant
prognostic factor. Several factors, such as the limited patient
sample and the nodal status may have affected the results. It is
noteworthy that preoperative serum CRP levels, after CRT completion
(prior to surgery), as well as during CRT (3 weeks after CRT
initiation), have a superior prognostic impact over postoperative
pathological tumor parameters in multivariate analysis.
As regards the correlation between CRP and the CRT
response, pretreatment elevated CRP values exhibited a clear
tendency to decrease 2–3 weeks following CRT initiation in
responders, which strongly suggests that CRP levels may decrease
according to CRT-induced tumor reduction in responders.
Furthermore, CRP values exhibited a poor tendency to decrease in
non-responders. In this regard, CRT-induced increases in activated
NF-κB in tumor tissues have been shown to be associated with a poor
CRT response and prognosis in patients who underwent preoperative
CRT (15,16). NF-κB is a transcription factor that
is highly involved in cancer progression and inflammatory response
regulation (17). CRP may reflect
the tumor status associated with CRT resistance in
non-responders.
Significant differences between responders and
non-responders were observed in both absolute CRP values and
relative CRP levels (CRP ≤0.3 vs. CRP >0.3) at identical time
points (CRT2W, CRT3W and PreOP). From the viewpoint of a simple and
easy evaluation, the comparison of relative CRP levels and not
absolute CRP values is considered suitable for predicting the CRT
response. In addition, we investigated the relationship between CRP
levels and the CRT response according to pretreatment CRP levels.
As a result, among all patients, the prediction accuracy of CRP
levels for responders was not as significant (76.5% at CRT2W),
whereas in patients with pretreatment CRP >0.3, the prediction
accuracy for responders increased to up to 87%. By contrast, CRP
≤0.3 at PreOP was a significant predictor for responders in all
patients, whereas it was not in patients with pretreatment CRP
>0.3. The presence of responders among patients with
pretreatment CRP ≤0.3 is considered to have affected prediction
accuracy. Pretreatment baseline CRP levels induced by tumor
progression and the CRP-mediated tumor response to CRT may
determine the ability of serum CRP levels to predict the CRT
response.
Furthermore, no significant correlation was observed
between CRP levels at CRT4W and the CRT response. The frequency of
adverse events, such as bone marrow suppression and
radiation-induced esophagitis, increased during the time course of
CRT among the patients assessed in this study (data not shown).
Tumor-derived decreases in CRP levels may have been masked by
adverse event-derived inflammation, resulting in a reduction in the
predictive accuracy of CRP at CRT4W.
In conclusion, the present study has demonstrated
that serum CRP levels during CRT were closely associated with the
pathological CRT response and prognosis and, particularly in
patients with elevated CRP prior to CRT, a decrease in CRP within
normal ranges 2–3 weeks following CRT initiation predicted a
favorable pathological response with the highest accuracy. Despite
the small patient sample and retrospective nature of the present
study, our results suggested that serum CRP may provide additional
predictive information to conventional imaging studies for early
modification of the treatment protocol during CRT in advanced
esophageal squamous cell carcinoma. The utility of serum CRP for
early prediction of the CRT response may be determined by a
well-designed prospective study.
References
|
1.
|
Gebski V, Burmeister B, Smithers BM, Foo
K, Zalcberg J and Simes J: Survival benefits from neoadjuvant
chemoradiotherapy or chemotherapy in oesophageal carcinoma: a
meta-analysis. Lancet Oncol. 8:226–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nozoe T, Saeki H and Sugimachi K:
Significance of preoperative elevation of serum C-reactive protein
as an indicator of prognosis in esophageal carcinoma. Am J Surg.
182:197–201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ikeda M, Natsugoe S, Ueno S, Baba M and
Aikou T: Significant host- and tumor-related factors for predicting
prognosis in patients with esophageal carcinoma. Ann Surg.
238:197–202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Shimada H, Nabeya Y, Okazumi S, et al:
Elevation of preoperative serum C-reactive protein level is related
to poor prognosis in esophageal squamous cell carcinoma. J Surg
Oncol. 83:248–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Crumley AB, McMillan DC, McKernan M, Going
JJ, Shearer CJ and Stuart RC: An elevated C-reactive protein
concentration, prior to surgery, predicts poor cancer-specific
survival in patients undergoing resection for gastro-oesophageal
cancer. Br J Cancer. 94:1568–1571. 2006.
|
|
6.
|
Sobin LH and Wittekind CH: UICC TNM
Classification of Malignant Tumors. 6th edition. Wiley-Liss; New
York, NY: pp. 60–64. 2002
|
|
7.
|
Fujiwara H, Suchi K, Okamura S, et al:
Elevated serum CRP levels after induction chemoradiotherapy reflect
poor treatment response in association with IL-6 in serum and local
tumor site in patients with advanced esophageal cancer. J Surg
Oncol. 103:62–68. 2011. View Article : Google Scholar
|
|
8.
|
Japan Esophageal Society: Japanese
Classification of Esophageal Cancer. 10th edition. Kanehara, Tokyo:
2008
|
|
9.
|
Deans DA, Wigmore SJ, Gilmour H,
Paterson-Brown S, Ross JA and Fearon KC: Elevated tumour
interleukin-1beta is associated with systemic inflammation: a
marker of reduced survival in gastro-oesophageal cancer. Br J
Cancer. 95:1568–1575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kobayashi T, Teruya M, Kishiki T, et al:
Inflammation-based prognostic score, prior to neoadjuvant
chemoradiotherapy, predicts postoperative outcome in patients with
esophageal squamous cell carcinoma. Surgery. 144:729–735. 2008.
View Article : Google Scholar
|
|
11.
|
Yano M, Tsujinaka T, Shiozaki H, et al:
Concurrent chemotherapy (5-fluorouracil and cisplatin) and
radiation therapy followed by surgery for T4 squamous cell
carcinoma of the esophagus. J Surg Oncol. 70:25–32. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chirieac LR, Swisher SG, Ajani JA, et al:
Posttherapy pathologic stage predicts survival in patients with
esophageal carcinoma receiving preoperative chemoradiation. Cancer.
103:1347–1355. 2005. View Article : Google Scholar
|
|
13.
|
Berger AC, Farma J, Scott WJ, et al:
Complete response to neoadjuvant chemoradiotherapy in esophageal
carcinoma is associated with significantly improved survival. J
Clin Oncol. 23:4330–4337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
de Manzoni G, Pedrazzani C, Pasini F, et
al: Chemoradiotherapy followed by surgery for squamous cell
carcinoma of the thoracic esophagus with clinical evidence of
adjacent organ invasion. J Surg Oncol. 95:261–266. 2007.PubMed/NCBI
|
|
15.
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Izzo JG, Malhotra U, Wu TT, et al:
Association of activated transcription factor nuclear factor kappaB
with chemoradiation resistance and poor outcome in esophageal
carcinoma. J Clin Oncol. 24:748–754. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Izzo JG, Wu X, Wu TT, et al:
Therapy-induced expression of NF-kappaB portends poor prognosis in
patients with localized esophageal cancer undergoing preoperative
chemoradiation. Dis Esophagus. 22:127–132. 2009. View Article : Google Scholar
|