Introduction
The standard treatment for muscle-invasive urinary
bladder cancer is radical cystectomy followed by urinary diversion;
however, this procedure negatively affects the quality of life of
patients (1). Several studies
reported promising results using combined trimodality therapy in
invasive bladder cancer with transurethral resection (TUR),
radiation therapy and platinum-based systemic chemotherapy
(1–4). Those studies demonstrated 5-year
survival rates of 50–65% and approximately three-quarters of the
surviving patients maintained their own bladders. However, combined
trimodality therapy is potentially toxic and may also decrease
survival due to delayed cystectomy in patients with non-responding
tumors (5). Therefore, it may be
beneficial to identify predictors of response and prognosis in
order to select appropriate patients for this type of therapy.
The nucleotide excision repair (NER) pathway is
essential for maintaining genomic stability and the main mechanism
in mammalian cells for removal of bulky, helix-distorting DNA
adducts produced by platinum agents (6,7).
Excision repair cross-complementing group 1 (ERCC1) is considered a
key molecule in this pathway (8).
This enzyme plays a rate-limiting role in the NER pathway,
recognizing and removing cisplatin-induced DNA adducts (9). ERCC1 is also important in the repair
of interstrand cross-links in DNA and in recombination processes.
Ionizing radiation causes more prominent DNA base damage and
single-strand rather than double-strand breaks (10). These lesions are repaired by the
base excision repair (BER) pathway. BER proteins include X-ray
repair cross-complementing group 1 (XRCC1) and
apurinic/apyrimidinic endonuclease 1 (APE1). XRCC1 plays an
important role in BER and acts as a scaffolding intermediate,
interacting with ligase III, DNA polymerase β and poly(ADP-ribose)
polymerase in the C-terminal, N-terminal and central regions of
XRCC1, respectively (11). XRCC1
mutant cells exhibit increased sensitivity to ionizing radiation,
ultraviolet light, hydrogen peroxide and mitomycin C (12). APE1 is the rate-limiting enzyme in
the BER pathway (11,13) and cleaves the 5′-terminal of DNA
abasic sugar residues generated by exogenous factors, such as
ionizing radiation and environmental carcinogens, as well as by
endogenous agents from normal cellular metabolism. Previous studies
demonstrated that amino acid substitution variants of the
APE1 and XRCC1 genes are associated with sensitivity
to ionizing radiation (11,14).
These DNA repair proteins may also be involved in reducing the
aggressive nature of tumors by inhibiting the accumulation of
genetic alterations in tumor cells of patients treated only by
surgical resection. Therefore, ERCC1, XRCC1 and APE1 expression in
tumor cells may affect response and survival in bladder cancer
patients receiving TUR and platinum-based chemoradiotherapy (CRT).
Previous studies revealed significant associations between ERCC1
expression and survival in lung, esophageal, gastric, pancreatic
and head and neck cancer patients treated by surgery and/or
platinum-based (radio)chemotherapy (8,9,15).
In addition, XRCC1 and APE1 expression has been strongly associated
with survival following radical radiotherapy in bladder cancer
(10).
There are no reports thus far on the association
between DNA repair protein expression and survival in patients with
locally invasive bladder cancer treated with combined trimodality
therapy, including TUR and platinum-based CRT. We investigated the
association of ERCC1, XRCC1 and APE1 expression with response and
survival in bladder cancer patients treated with combined
trimodality therapy and determined the predictive value of the
expression of these DNA repair proteins in patient selection for
therapy.
Materials and methods
Patients
This retrospective cohort study included 186
patients who underwent combined trimodality therapy including TUR
and CRT for locally advanced muscle-invasive (T2-4N0M0) or
high-risk non-muscle-invasive (T1G3) (16) urothelial carcinoma of the bladder
at Yamaguchi University Hospital between November, 1994 and July,
2009. We included a total of 157 patients for whom
clinicopathological information and immunohistochemistry (IHC) of
the tumor was assessable. The study was approved by the
Institutional Ethics Review Committee of the Graduate School of
Medicine, Yamaguchi University and written informed consent was
obtained from each patient. The patients were native Japanese and
their clinical characteristics are presented in Table I. The median age was 70 years
(range, 29–89 years) and the cohort included 118 males (75.2%) and
39 females (24.8%). Prior to treatment, all patients underwent
computed tomography (CT) of the chest, abdomen and pelvis, bone
scans, as well as transurethral tumor and random mucosal biopsies
of the bladder. In the majority of the patients, bladder tumors
were treated with TUR to reduce tumor volume as much as
possible.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Age, years | |
| Median | 70 |
| Range | 29–89 |
| Gender, no. (%) | |
| Male | 118 (75.2) |
| Female | 39 (24.8) |
| Performance status,
no. (%) | |
| 0 | 65 (41.4) |
| 1 | 50 (31.8) |
| 2 | 14 (8.9) |
| Unknown | 28 (17.8) |
| Tumor stage, no.
(%) | |
| T1G3 | 27 (17.2) |
| T2 | 59 (37.6) |
| T3 | 61 (38.9) |
| T4 | 10 (6.4) |
| Tumor grade, no.
(%) | |
| 2 | 28 (17.8) |
| 3 | 128 (81.5) |
| Histopathology, no.
(%) | |
| Pure UC | 145 (92.4) |
| UC with SCC | 5 (3.2) |
| UC with
adenocarcinoma | 7 (4.5) |
| Total cisplatin
dose, mg | |
| Median | 240 |
| Range | 30–406 |
| Total radiation
dose, Gy | |
| Median | 48.6 |
| Range | 18–63 |
Patients were staged according to the TNM system of
the International Union Against Cancer (UICC; 1997) as follows: 27
patients (17.2%) were stage T1G3; 59 (37.6%) were stage T2; 61
(38.9%) were stage T3; and 10 (6.4%) were stage T4. All bladder
tumors were histopathologically confirmed as urothelial carcinomas.
Of these, 145 (92.4%) showed evidence of urothelial carcinoma
alone, 5 (3.2%) included squamous differentiation and 7 (4.5%)
included an adenocarcinomatous component. The tumors were graded
according to the WHO classification as follows: 128 tumors (81.5%)
were grade 3 and the remaining 28 (17.8%) were grade 2.
CRT
The patients received combined platinum-based
systemic CRT. In the majority of patients, one cycle of the regimen
(based on Shipley’s method with slight modification) (17) included administration of cisplatin
(70 mg/m2) on day 1, followed by radiation at 1.8 Gy per
fraction on days 2–5 in the first week and every 5 days
consecutively in the second week (18–20).
Radiotherapy involved 10-MV photons with a 4-field technique,
treating the bladder and pelvic lymph nodes to 32.4 Gy during 2
cycles, followed by a CT-planned whole-bladder boost of 16.2 Gy for
an additional cycle. Although we aimed for 3 cycles of CRT, the
treatment was discontinued after 2 cycles in 48 (30.6%) patients
who exhibited persistent toxicity or refused to continue with CRT.
The median total doses of cisplatin and radiation were 240 mg
(range, 30–406 mg) and 48.6 Gy (range, 18–63 Gy), respectively
(Table I).
Four weeks after completion of CRT, we assessed the
patient response with CT scan, random mucosal biopsies and TUR. A
complete response (CR) was defined as no pathologically detected
residual tumor and non-CR as any pathologically detected residual
tumor. CR was observed in 56 (35.7%) and non-CR in 97 (61.8%)
patients. Non-CR patients with residual non-muscle-invasive tumors
underwent complete resection of the residual tumor by TUR and
residual carcinoma in situ was treated with intravesical
instillation of bacillus Calmette-Guérin. Non-CR patients with
residual muscle-invasive tumors were referred for radical
cystectomy and salvage cystectomy was performed in 22 patients
(14.0%). In addition, 6 patients underwent cystectomy due to
recurrence of muscle-invasive bladder cancer during the follow-up
period; therefore, a total of 28 patients (17.8%) underwent
cystectomy during this study. We performed cystoscopic examination
followed by washing cytology every 3 months for the first 5 years
and every 6 months thereafter. Complementary examinations,
including chest X-ray and/or CT scan, were performed every 6
months. The median duration of the follow-up was 39 months (range,
1–193 months). The incidence of bladder cancer-related mortality
during follow-up was 18.4% (29 patients).
IHC
Biopsy specimens were selected from the main bladder
tumors prior to CRT. IHC was performed on routinely-processed,
formalin-fixed paraffin-embedded sections using the avidin-biotin
complex immunoperoxidase technique, as previously described
(19,21). Briefly, serial 5-μm sections
were mounted on poly-L-lysine-coated slides, baked at 50°C for 1 h,
dewaxed using xylene and rehydrated with graded alcohols to water.
The sections were then immersed in a 10 mM citrate buffer (pH 6.0)
and heated in a water bath at 98°C for 30 min. Following antigen
retrieval, endogenous peroxidase activity was blocked by 3%
hydrogen peroxide in methanol. Tumor sections were incubated with
mouse monoclonal antibody for ERCC1 (1:100; Neomarkers Inc.,
Fremont, CA, USA), XRCC1 (1:10; Abcam, Cambridge, UK), or APE1
(1:5000; Abcam, Cambridge, UK) at 4°C overnight, followed by
immunostaining using Vectastain Universal Quick kit (Vector
Laboratories Inc., Burlingame, CA, USA). Sections were
counterstained with hematoxylin. A duplicate section without
primary antibody from the IHC procedure was used as negative
control. The number of cells with nuclei positive for ERCC1, XRCC1
and APE1 was determined by scoring 10 microscopic fields of 100
tumor cells each. Staining intensities of each protein were graded
on a scale of 0–3. The percentage of positive tumor nuclei was
calculated for each specimen, with 0 indicating 0% staining; 0.1,
1–9% staining; 0.5, 10–49% staining and 1, 50–100% staining. The
proportion score was multiplied by the staining intensity to obtain
a semiquantitative H-score (0–3) (9). All measurements were performed by the
same investigator (S.O.), using coded samples without prior
knowledge of the clinical data. The median H-score for each
monoclonal antibody was chosen a priori as the cut-off point
for separating positive from negative tumors.
Statistical analysis
The associations between the IHC results (ERCC1,
XRCC1 and APE1 expression) and clinicopathological data or response
to CRT were assessed using the Chi-square test with an odds ratio
(OR) or risk ratio (RR) with 95% confidence interval (CI). The
primary endpoint was disease-specific survival, defined as the time
from the initiation of CRT to the date of death from bladder
cancer. Disease-specific survival was analyzed by plotting
Kaplan-Meier curves and the survival probability distributions were
compared using the log-rank test. Categorical variables influencing
disease-specific mortality were compared using Cox proportional
hazards regression models. Variables with P<0.05 in univariate
analysis were also assessed for their association with
disease-specific mortality in multivariate analysis. JMP software
(SAS Institute Inc., Cary, NC, USA) was used for all analyses, with
P<0.05 (two-sided) indicating a statistically significant
difference.
Results
The median H-scores obtained from the IHC slides
were 1.0 for ERCC1, XRCC1 and APE1; therefore, an H-score ≥1.0 was
considered positive for each monoclonal antibody. Seventy-seven out
of the 148 (52.0%), 91 out of the 146 (62.3%) and 89 out of the 146
(61.0%) tumors were positive for ERCC1, XRCC1 and APE1,
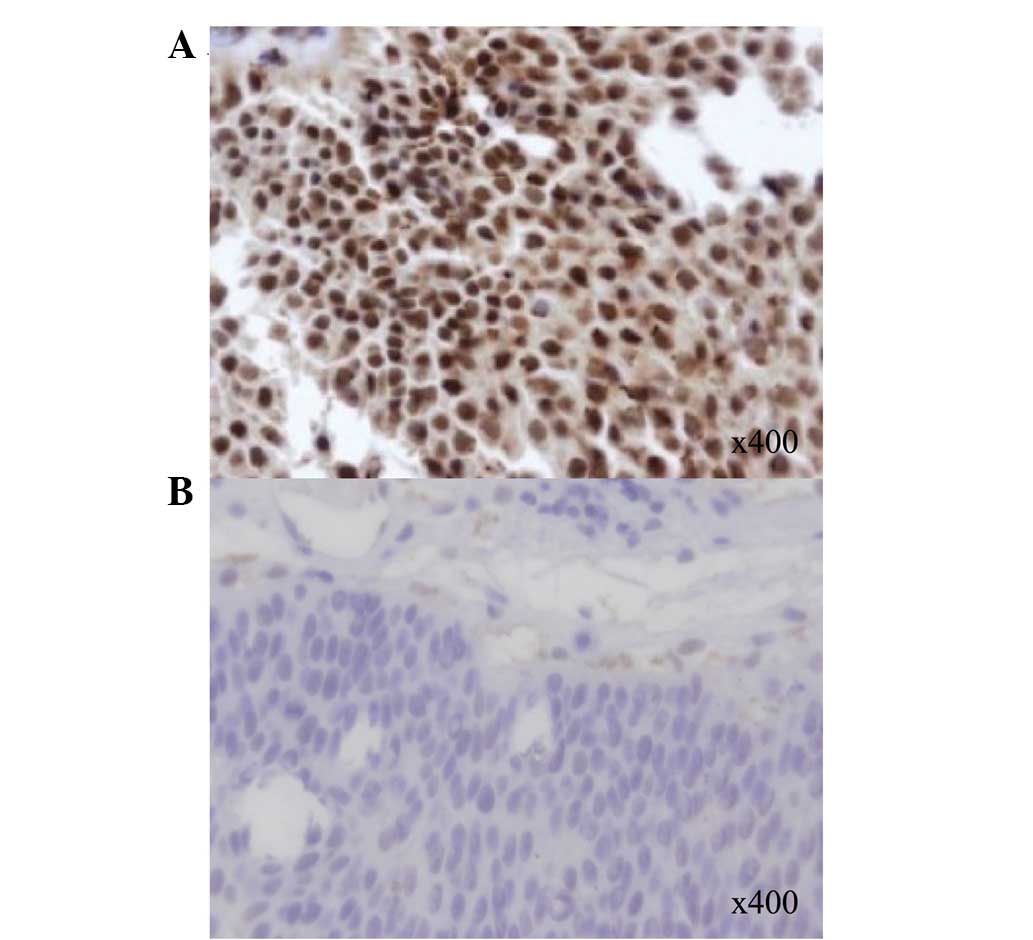
respectively. Representative analyses for ERCC1 IHC are shown in
Fig. 1. The associations between
ERCC1, XRCC1 and APE1 protein expression are shown in Table II. We observed a significant
correlation between ERCC1 and XRCC1 expression (P<0.001;
Chi-square test). No significant correlation was observed between
the expression of ERCC1 and APE1, or between XRCC1 and APE1.
 | Table IIAssociations between ERCC1, XRCC1 and
APE1 protein expression. |
Table II
Associations between ERCC1, XRCC1 and
APE1 protein expression.
| | | OR (95% CI) | P-valuea |
|---|
| XRCC1 expression
(no.)
| | |
| Negative | Positive | | |
| ERCC1
expression | | | | |
| Negative | 35 | 32 | Reference | |
| Positive | 18 | 55 | 3.34
(1.63–6.84) | <0.001b |
| APE1 expression
(no.)
| | |
| Negative | Positive | | |
| ERCC1
expression | | | | |
| Negative | 25 | 39 | Reference | |
| Positive | 29 | 46 | 1.02
(0.51–2.02) | 0.96 |
| APE1 expression
(no.)
| | |
| Negative | Positive | | |
| XRCC1
expression | | | | |
| Negative | 20 | 33 | Reference | |
| Positive | 29 | 54 | 1.13
(0.55–2.31) | 0.74 |
There was no significant correlation between the
expression of ERCC1, XRCC1 or APE1 and tumor stage or grade in
bladder cancer treated with combined trimodality therapy
(Chi-square test, Table III). The
associations of the expression of ERCC1, XRCC1 or APE1 with
response to CRT at 4 weeks after evaluation are also shown in
Table III. No DNA repair protein
expression was significantly associated with response to CRT using
the Chi-square test.
 | Table IIIAssociations of ERCC1, XRCC1 and APE1
expression with tumor stage, tumor grade and response to
chemoradiotherapy. |
Table III
Associations of ERCC1, XRCC1 and APE1
expression with tumor stage, tumor grade and response to
chemoradiotherapy.
| Tumor stage
(no.) | | | Tumor grade
(no.) | | | Response (no.) | | |
|---|
|
|
|
|---|
| T1G3/T2 | T3/T4 | OR (95% CI) | P-valuea | G2 | G3 | OR (95% CI) | P-valuea | CR | Non-CR | RR (95% CI) | P-valuea |
|---|
| ERCC1
expression | | | | | | | | | | | | |
| Negative | 35 | 36 | Reference | | 11 | 60 | Reference | | 24 | 47 | Reference | |
| Positive | 46 | 31 | 0.66
(0.34–1.26) | 0.20 | 14 | 62 | 0.81
(0.34–1.93) | 0.64 | 30 | 43 | 0.85
(0.60–1.22) | 0.37 |
| XRCC1
expression | | | | | | | | | | | | |
| Negative | 31 | 24 | Reference | | 9 | 46 | Reference | | 20 | 34 | Reference | |
| Positive | 50 | 41 | 1.06
(0.54–2.08) | 0.87 | 18 | 72 | 0.78
(0.32–1.89) | 0.59 | 32 | 56 | 1.02
(0.66–1.57) | 0.94 |
| APE1
expression | | | | | | | | | | | | |
| Negative | 33 | 24 | Reference | | 11 | 46 | Reference | | 19 | 35 | Reference | |
| Positive | 45 | 44 | 1.34
(0.69–2.63) | 0.39 | 14 | 74 | 1.26
(0.53–3.02) | 0.60 | 33 | 55 | 0.94
(0.60–1.46) | 0.78 |
The associations between the expression of ERCC1,
XRCC1 or APE1 and disease-specific mortality in bladder cancer
patients treated with combined trimodality therapy are shown in
Table IV. In univariate analysis
using the Cox proportional hazards regression model, ERCC1
expression, XRCC1 expression and combined ERCC1 and XRCC1
expression were significantly associated with disease-specific
mortality (RR: 0.65; 95% CI, 0.43–0.94 and P=0.023 for ERCC1; RR:
0.65; 95% CI, 0.44–0.95 and P=0.028 for XRCC1; RR: 0.61; 95% CI,
0.42–0.91 and P=0.016 for combined ERCC1 and XRCC1). Thus, patients
who were positive for ERCC1, XRCC1 and either ERCC1 or XRCC1,
exhibited improved disease-specific survival rates. In addition,
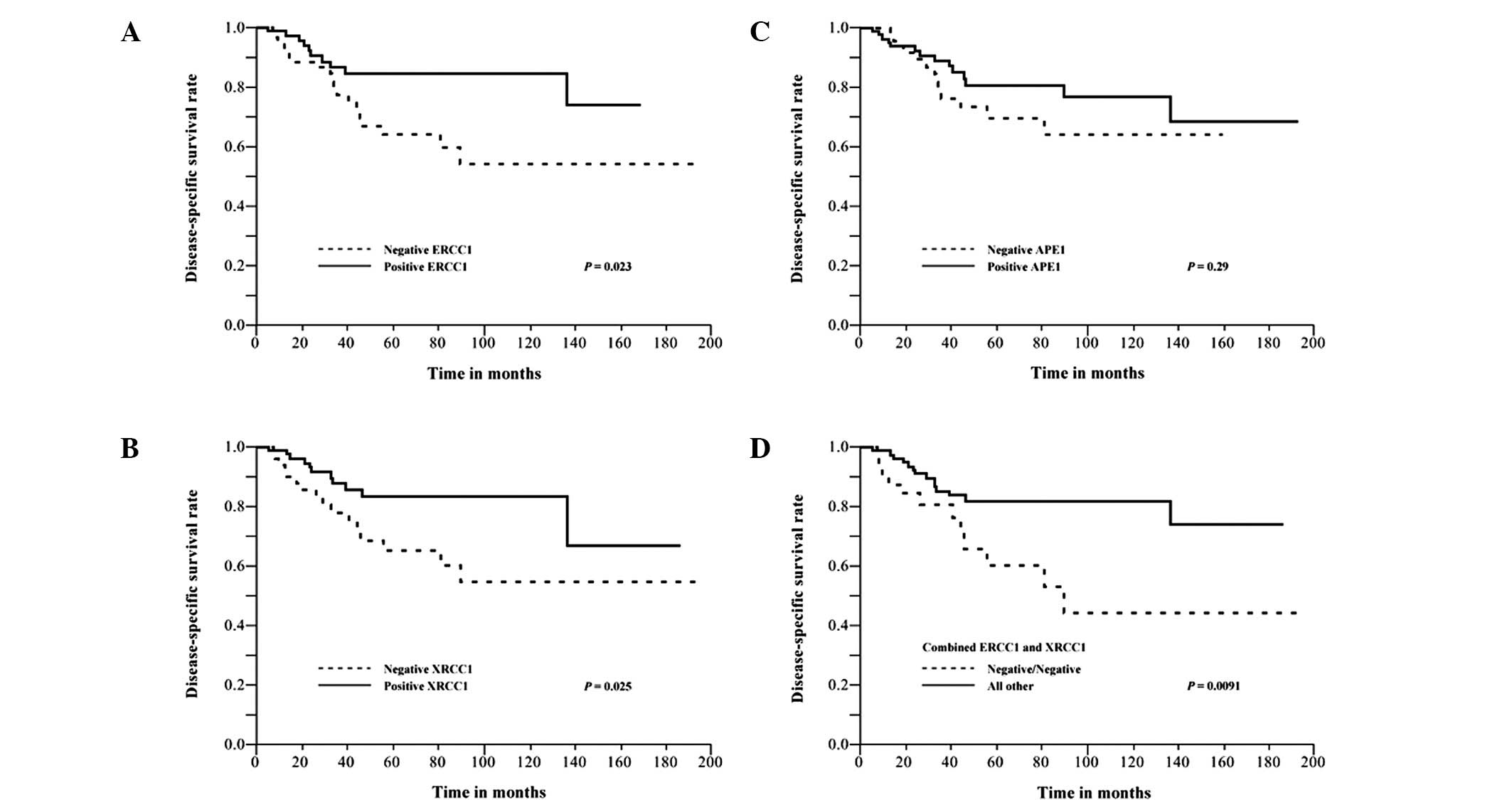
disease-specific survival rates were plotted for the protein
expression using Kaplan-Meier survival curves (P=0.023 for ERCC1,
P=0.025 for XRCC1 and P=0.0091 for combined ERCC1 and XRCC1;
log-rank test; Fig. 2). APE1
expression was not significantly associated with disease-specific
survival (P=0.29). Clinical variables were also assessed for their
association with disease-specific mortality (Table IV). Patients who did not achieve a
CR to CRT exhibited a significant association with unfavorable
outcome using univariate analysis (RR: 1.65; 95% CI, 1.09–2.72 and
P=0.017).
 | Table IVUnivariate and multivariate
regression analyses for predicting disease-specific mortality. |
Table IV
Univariate and multivariate
regression analyses for predicting disease-specific mortality.
| Univariate
analysis | Multivariate model
one | Multivariate model
two |
|---|
|
|
|
|---|
| Variable (no.) | RR (95% CI) | P-value | RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| Age, years | | | | | | |
| <70 (74) | Reference | | | | | |
| ≥70 (80) | 1.11
(0.77–1.61) | 0.57 | | | | |
| Gender | | | | | | |
| Male (117) | Reference | | | | | |
| Female (37) | 1.02
(0.64–1.52) | 0.93 | | | | |
| Tumor stage | | | | | | |
| T1G3/T2 (85) | Reference | | | | | |
| T3/T4 (69) | 1.86
(0.89–3.99) | 0.097 | | | | |
| Tumor grade | | | | | | |
| G2 (26) | Reference | | | | | |
| G3 (127) | 0.99
(0.41–2.95) | 0.98 | | | | |
| Histopathology | | | | | | |
| Pure UC
(141) | Reference | | | | | |
| UC + other
element (13) | 1.42
(0.69–2.40) | 0.30 | | | | |
| Cisplatin dose,
mg | | | | | | |
| ≤240 (64) | Reference | | | | | |
| >240 (64) | 1.21
(0.82–1.80) | 0.33 | | | | |
| Radiation dose,
Gy | | | | | | |
| <48.6
(51) | Reference | | | | | |
| ≥48.6 (94) | 1.50
(0.99–2.42) | 0.055 | | | | |
| Response to
chemoradiotherapy | | | | | | |
| CR (56) | Reference | | Reference | | Reference | |
| Non-CR (94) | 1.65
(1.09–2.72)a | 0.017a | 1.55
(1.01–2.57)a | 0.047a | 1.55
(1.02–2.56)a | 0.042a |
| ERCC1
expression | | | | | | |
| Negative
(69) | Reference | | Reference | | | |
| Positive
(76) | 0.65
(0.43–0.94)a | 0.023a | 0.75
(0.48–1.13) | 0.17 | | |
| XRCC1
expression | | | | | | |
| Negative
(54) | Reference | | Reference | | | |
| Positive
(89) | 0.65
(0.44–0.95)a | 0.028a | 0.74
(0.48–1.12) | 0.15 | | |
| APE1
expression | | | | | | |
| Negative
(57) | Reference | | | | | |
| Positive
(87) | 0.82
(0.56–1.20) | 0.30 | | | | |
| Combined ERCC1 and
XRCC1 expression | | | | | | |
| Negative/Negative
(34) | Reference | | | | Reference | |
| Other (111) | 0.61
(0.42–0.91)a | 0.016a | | | 0.64
(0.43–0.94)a | 0.024a |
In multivariate analysis (model one) of response to
CRT, ERCC1 and XRCC1 expression, the response to CRT was the only
factor independently associated with disease-specific mortality
(RR: 1.55; 95% CI, 1.01–2.57 and P=0.047; Table IV). When combined ERCC1 and XRCC1
expression was used instead of individual ERCC1 or XRCC1 expression
in multivariate analysis (model two), the response to CRT and the
combined ERCC1 and XRCC1 expression were independently associated
with disease-specific mortality (RR: 1.55; 95% CI, 1.02–2.56 and
P=0.042 for response to CRT; RR: 0.64; 95% CI, 0.43–0.94 and
P=0.024 for combined ERCC1 and XRCC1). Thus, patients who were
positive for either ERCC1 or XRCC1 exhibited significantly more
favorable disease-specific survival rates compared to those
negative for both ERCC1 and XRCC1.
Discussion
To the best of our knowledge, this is the first
study on the association between DNA repair protein expression and
survival in patients with locally advanced bladder cancer treated
with combined trimodality therapy, including TUR and platinum-based
CRT. We demonstrated a significant association between positive
expression for ERCC1 or XRCC1 and longer disease-specific survival.
Our data indicated that combined ERCC1 and XRCC1 expression is
useful as an independent prognostic marker for survival in bladder
cancer patients receiving combined trimodality therapy. ERCC1 and
XRCC1 are not involved in the same DNA repair pathway. However,
both ERCC1 and XRCC1 genes are located in close
proximity to each other at 19q13.2–13.3 (22) and they may be simultaneously
altered by deletion and other changes. A significant correlation
was observed between ERCC1 and XRCC1 expression in bladder tumors
(Table II). Weakened expression of
both ERCC1 and XRCC1 may affect the NER and BER pathways and,
therefore, affect the aggressiveness of the tumor or reduce the
responce to CRT. Liang et al(23) reported that polymorphisms of
ERCC1 and XRCC1, in combination but not individually,
were independent predictors for clinical responce to
oxaliplatin-based chemotherapy in metastatic colorectal cancer.
Previous studies have demonstrated significant
associations between ERCC1 expression and the prognosis of lung,
upper gastrointestinal and head and neck cancer patients who
received platinum-based chemotherapy, with or without radiotherapy
(8,9,15).
Olaussen et al(9)
demonstrated that patients with completely resected non-small cell
lung cancer (NSCLC) and ERCC1-negative tumors, appear to benefit
from adjuvant cisplatin-based chemotherapy. The authors suggested
that ERCC1 is the limiting factor in the NER pathway, which removes
platinum-DNA adducts and contributes to cisplatin resistance.
Although these results appear to contradict our findings, the
authors also reported that, among patients who did not receive
adjuvant chemotherapy, those with ERCC1-positive tumors survived
longer compared to those with ERCC1-negative tumors.
A previous study by Bamias et al(24) reported findings similar to those of
the present study, suggesting that patients with radically resected
ERCC1-positive gastric cancer who received adjuvant
platinum/docetaxel chemotherapy, with or without radiation therapy,
exhibited significantly higher overall and disease-free survival
rates compared to ERCC1-negative patients. The authors hypothesized
that the prognostic role of ERCC1 expression was more significant
compared to its predictive value. In addition, Simon et
al(25) reported that patients
with resected NSCLC with high ERCC1 expression exhibited better
survival compared to those with low ERCC1 expression. The majority
of our patients were treated with combined trimodality therapy,
including surgery, potentially affecting our finding that positive
ERCC1 or XRCC1 expression was associated with longer survival,
since recent evidence suggested that intraoperative tumor
manipulation results in detachment of tumor cells that may lead to
metastases, particularly tumor cells of high malignant potential
(26,27). Additionally, the postoperative
phase is characterized by transient changes in the immune system,
hampering the antitumor response and rendering the host more
susceptible to metastasis.
De Castro et al(28) also demonstrated that the prognostic
value of ERCC1 may be dependent on the treatment modality. In
surgically treated head and neck squamous cell carcinoma patients,
a high expression of ERCC1 was associated with better prognosis,
whereas the opposite effect was observed in patients treated with
(chemo)radiation, with or without prior induction chemotherapy. The
underlying mechanism may be related to the dual nature of ERCC1,
favoring reduced mutagenesis and associated with less aggressive
tumors, or counteracting cisplatin-induced cell death (29).
Previous studies investigated ERCC1 expression in
bladder cancer treated with (chemo)radiation therapy (30–33).
Bellmunt et al(30)
demonstrated that survival was significantly higher in individuals
with low ERCC1 mRNA levels among 57 patients with advanced and
metastatic bladder cancer treated with cisplatin-based
chemotherapy. Matsumura et al(31) reported no significant differences
in survival between high- and low-ERCC1 expression in 40 metastatic
bladder cancer patients treated with gemcitabine-cisplatin-based
combination chemotherapy. Hoffmann et al(32) indicated that high-ERCC1 mRNA
expression was associated with inferior outcomes following
cisplatin-based adjuvant chemotherapy in 108 patients with locally
advanced bladder cancer. Those results are not in concordance with
our findings. However, the patients in those studies were not
treated with combined trimodality therapy and the sample sizes were
relatively small. Kawashima et al(33) demonstrated that resistance to
irradiation, but not to cisplatin, was eliminated by suppressing
ERCC1 using siRNA in Cl8-2 and CDDP10-3 cells. The authors also
indicated that negative IHC nuclear staining for ERCC1 correlated
with efficacy of CRT using cisplatin in 22 patients with
muscle-invasive bladder cancer. However, they did not investigate
the association of ERCC1 expression with survival in patients with
muscle-invasive bladder cancer treated with CRT.
Sak et al(10) reported that a high expression of
APE1 or XRCC1 was associated with improved cancer-specific survival
following radical radiotherapy in bladder cancer. The authors
suggested that a reduced expression of XRCC1 and APE1 reflects the
poorly differentiated nature of tumor cells in more aggressive
tumors and that cells from aggressive tumors with extensive genomic
instability may harbor chromosomal aberrations that result in
failure of gene transcription, including DNA repair genes,
resulting in lower protein expression of the gene products. Further
investigation of the relationship between ERCC1 and XRCC1
expression and outcomes in bladder cancer patients treated by other
modalities, such as cystectomy, is required to determine whether
the expression of these proteins is a general prognostic factor
reflecting tumor aggressiveness or a predictive factor specific to
CRT (10). We observed no
significant correlation between ERCC1 or XRCC1 expression and tumor
grade, since the majority of the patients included in this study
had high-grade disease. ERCC1 and XRCC1 expression may be a
surrogate marker for stratification within the poorly
differentiated group of bladder tumors. The conflicting results
underline the complexity of DNA repair pathways, with
cross-functionality existing between pathways that may exhibit
variations in regulation and activation among different tissues or
resulting from different drugs, radiation and their combinations
(34,35).
In conclusion, the combined pattern of ERCC1 and
XRCC1 expression was independently associated with disease-specific
mortality in patients with locally advanced bladder cancer treated
with combined trimodality therapy. Our results suggested that ERCC1
and XRCC1 expression may predict disease-specific survival in
bladder cancer patients treated with combined trimodality therapy.
However, our results are limited by the small sample size and allow
only preliminary conclusions. Prospective studies including a
larger sample size are required to confirm the predictive
significance of the expression of these DNA repair proteins.
Acknowledgements
This study was supported, in part, by
a Grant-in-Aid for Scientific Research (C) (24592393) from the
Japan Society for the Promotion of Science.
References
|
1.
|
Rödel C, Grabenbauer GG, Kühn R, et al:
Combined-modality treatment and selective organ preservation in
invasive bladder cancer: long-term results. J Clin Oncol.
20:3061–3071. 2002.PubMed/NCBI
|
|
2.
|
Tester W, Caplan R, Heaney J, et al:
Neoadjuvant combined modality program with selective organ
preservation for invasive bladder cancer: results of Radiation
Therapy Oncology Group phase II trial 8802. J Clin Oncol.
14:119–126. 1996.
|
|
3.
|
Kachnic LA, Kaufman DS, Heney NM, et al:
Bladder preservation by combined modality therapy for invasive
bladder cancer. J Clin Oncol. 15:1022–1029. 1997.PubMed/NCBI
|
|
4.
|
Dunst J, Rödel C, Zietman A, Schrott KM,
Sauer R and Shipley WU: Bladder preservation in muscle-invasive
bladder cancer by conservative surgery and radiochemotherapy. Semin
Surg Oncol. 20:24–32. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lee CT, Madii R, Daignault S, et al:
Cystectomy delay more than 3 months from initial bladder cancer
diagnosis results in decreased disease specific and overall
survival. J Urol. 175:1262–1267. 2006. View Article : Google Scholar
|
|
6.
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: the role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Metzger R, Bollschweiler E, Hölscher AH
and Warnecke-Eberz U: ERCC1: impact in multimodality treatment of
upper gastrointestinal cancer. Future Oncol. 6:1735–1749. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Olaussen KA, Dunant A, Fouret P, et al:
DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sak SC, Harnden P, Johnston CF, Paul AB
and Kiltie AE: APE1 and XRCC1 protein expression levels predict
cancer-specific survival following radical radiotherapy in bladder
cancer. Clin Cancer Res. 11:6205–6211. 2005. View Article : Google Scholar
|
|
11.
|
Hu JJ, Smith TR, Miller MS, Mohrenweiser
HW, Golden A and Case LD: Amino acid substitution variants of APE1
and XRCC1 genes associated with ionizing radiation sensitivity.
Carcinogenesis. 22:917–922. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Thompson LH and West MG: XRCC1 keeps DNA
from getting stranded. Mutat Res. 459:1–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ramana CV, Boldogh I, Izumi T and Mitra S:
Activation of apurinic/apyrimidinic endonuclease in human cells by
reactive oxygen species and its correlation with their adaptive
response to genotoxicity of free radicals. Proc Natl Acad Sci USA.
95:5061–5066. 1998. View Article : Google Scholar
|
|
14.
|
Chang-Claude J, Popanda O, Tan XL, et al:
Association between polymorphisms in the DNA repair genes, XRCC1,
APE1, and XPD and acute side effects of radiotherapy in breast
cancer patients. Clin Cancer Res. 11:4802–4809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jun HJ, Ahn MJ, Kim HS, et al: ERCC1
expression as a predictive marker of squamous cell carcinoma of the
head and neck treated with cisplatin-based concurrent
chemoradiation. Br J Cancer. 99:167–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Weiss C, Wolze C, Engehausen DG, et al:
Radiochemotherapy after transurethral resection for high-risk T1
bladder cancer: an alternative to intravesical therapy or early
cystectomy? J Clin Oncol. 24:2318–2324. 2006. View Article : Google Scholar
|
|
17.
|
Shipley WU, Prout GR Jr, Einstein AB, et
al: Treatment of invasive bladder cancer by cisplatin and radiation
in patients unsuited for surgery. JAMA. 258:931–935. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sakano S, Wada T, Matsumoto H, et al:
Single nucleotide polymorphisms in DNA repair genes might be
prognostic factors in muscle-invasive bladder cancer patients
treated with chemoradiotherapy. Br J Cancer. 95:561–570. 2006.
View Article : Google Scholar
|
|
19.
|
Shinohara A, Sakano S, Hinoda Y, et al:
Association of TP53 and MDM2 polymorphisms with survival in bladder
cancer patients treated with chemoradiotherapy. Cancer Sci.
100:2376–2382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sakano S, Hinoda Y, Sasaki M, et al:
Nucleotide excision repair gene polymorphisms may predict acute
toxicity in patients treated with chemoradiotherapy for bladder
cancer. Pharmacogenomics. 11:1377–1387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sakano S, Matsumoto H, Yamamoto Y, et al:
Association between DNA repair gene polymorphisms and p53
alterations in Japanese patients with muscle-invasive bladder
cancer. Pathobiology. 73:295–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mohrenweiser HW, Carrano AV, Fertitta A,
et al: Refined mapping of the three DNA repair genes, ERCC1, ERCC2,
and XRCC1, on human chromosome 19. Cytogenet Cell Genet. 52:11–14.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liang J, Jiang T, Yao RY, Liu ZM, Lv HY
and Qi WW: The combination of ERCC1 and XRCC1 gene polymorphisms
better predicts clinical outcome to oxaliplatin-based chemotherapy
in metastatic colorectal cancer. Cancer Chemother Pharmacol.
66:493–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Bamias A, Karina M, Papakostas P, et al: A
randomized phase III study of adjuvant platinum/docetaxel
chemotherapy with or without radiation therapy in patients with
gastric cancer. Cancer Chemother Pharmacol. 65:1009–1021. 2010.
View Article : Google Scholar
|
|
25.
|
Simon GR, Sharma S, Cantor A, Smith P and
Bepler G: ERCC1 expression is a predictor of survival in resected
patients with non-small cell lung cancer. Chest. 127:978–983. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Coffey JC, Wang JH, Smith MJ,
Bouchier-Hayes D, Cotter TG and Redmond HP: Excisional surgery for
cancer cure: therapy at a cost. Lancet Oncol. 4:760–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Sergeant G, Roskams T, van Pelt J,
Houtmeyers F, Aerts R and Topal B: Perioperative cancer cell
dissemination detected with a real-time RT-PCR assay for EpCAM is
not associated with worse prognosis in pancreatic ductal
adenocarcinoma. BMC Cancer. 11:472011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
De Castro G Jr, Pasini FS, Siqueira SA, et
al: ERCC1 protein, mRNA expression and T19007C polymorphism as
prognostic markers in head and neck squamous cell carcinoma
patients treated with surgery and adjuvant cisplatin-based
chemoradiation. Oncol Rep. 25:693–699. 2011.PubMed/NCBI
|
|
29.
|
Gazdar AF: DNA repair and survival in lung
cancer - the two faces of Janus. N Engl J Med. 356:771–773. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Bellmunt J, Paz-Ares L, Cuello M, et al:
Gene expression of ERCC1 as a novel prognostic marker in advanced
bladder cancer patients receiving cisplatin-based chemotherapy. Ann
Oncol. 18:522–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Matsumura N, Nakamura Y, Kohjimoto Y, et
al: The prognostic significance of human equilibrative nucleoside
transporter 1 expression in patients with metastatic bladder cancer
treated with gemcitabine-cisplatin-based combination chemotherapy.
BJU Int. 108:E110–E116. 2011. View Article : Google Scholar
|
|
32.
|
Hoffmann AC, Wild P, Leicht C, et al: MDR1
and ERCC1 expression predict outcome of patients with locally
advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia.
12:628–636. 2010.PubMed/NCBI
|
|
33.
|
Kawashima A, Nakayama M, Kakuta Y, et al:
Excision repair cross-complementing group 1 may predict the
efficacy of chemoradiation therapy for muscle-invasive bladder
cancer. Clin Cancer Res. 17:2561–2569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Zhang Y, Rohde LH and Wu H: Involvement of
nucleotide excision and mismatch repair mechanisms in double strand
break repair. Curr Genomics. 10:250–258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Herrera M, Dominguez G, Garcia JM, et al:
Differences in repair of DNA cross-links between lymphocytes and
epithelial tumor cells from colon cancer patients measured in vitro
with the comet assay. Clin Cancer Res. 15:5466–5472. 2009.
View Article : Google Scholar : PubMed/NCBI
|