Introduction
Squamous cell carcinoma (SCC) of the buccal mucosa
is a common malignant tumor in the Chinese mainland, Taiwan and
India; however, it is rarely encountered in Europe and North
America. Risk factors associated with SCC include betel quid
chewing, tobacco and alcohol consumption (1–3). Due
to the differences in etiology and species, there are significant
differences in pathology, clinical presentation, treatment outcomes
and survival between Western and Southeastern countries. Several
studies on buccal SCC have been conducted in Western countries
(4), India (5) and Taiwan (6). However, available data on the
treatment and survival outcome of buccal SCC patients in the
Chinese mainland are limited.
Surgery or radiotherapy as a single modality is
currently considered a suitable method for the treatment of
early-stage buccal SCC, whereas postoperative radiation combined
with surgical excision is recommended for advanced tumors (7).
The aim of this study was to present our clinical
experience with this tumor over a 7-year period and to focus our
analysis of clinical presentation, outcome and prognostic factors
on a homogeneous patient population, by including only previously
untreated buccal SCC patients with tumors restricted to or
originating from the buccal mucosa. We also evaluated the role of
neck dissection (ND) in the treatment of buccal SCC staged as
cT1-2N0.
Materials and methods
This retrospective chart review was authorized and
approved by the China Medical University Review Board.
Patient selection
A search was conducted for medical records of
patients diagnosed with buccal SCC between September, 2005 and May,
2011. A total of 67 patients (33 male and 34 female) were included
in our study. The mean age was 65 years (range, 25–86 years).
Exclusion criteria included lesions originating from adjacent
intraoral structures with extension into the buccal mucosa and a
pathological diagnosis of adenoid cystic carcinoma.
Statistical analysis
Follow-up time was defined as the time period
between the first appointment at the Oral Maxillofacial Head and
Neck Tumor Center and the date of last contact or death. The
Kaplan-Meier method was used to analyze the factors affecting
survival. The Cox logistic regression model (uni- and multivariate)
was used to analyze the risk factors for recurrence. P<0.05 was
considered to indicate a statistically significant difference and
P<0.1 indicated a trend toward significance (4).
Results
Patients and treatments
A total of 67 patients (33 male and 34 female) were
included in our study. The mean age was 65 years (range, 25–86
years) the mean follow-up time was 34 months (range, 7–84 months).
Forty-one (61.3%) out of the 67 patients had a history of smoking
and 26 (38.8%) had a history of alcohol consumption. pTNM stage,
tumor and nodal stage were classified according to UICC, 2002.
Fifty-nine patients underwent a selective or modified radical ND,
whereas the remaining patients refused the ND due to their concerns
regarding the complications associated with this procedure. Sixteen
patients received postoperative radiation, 30 patients presented
with bone involvement and underwent resection of either the maxilla
or the mandible, while through-and-through skin resection was
performed in 7 patients. Thirty-six tumors were pathologically
confirmed as well-differentiated, 23 were moderately differentiated
and 8 were poorly differentiated. There were no positive resection
margins in any of the patients (Table
I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Age | | | | | Tumor
differentiation |
|---|
|
|
|---|
| Stage | Mean | Range | ND | PR | CR | Skin resection | High | Moderate | Poor |
|---|
| I (n=8) | 73.5 | 55–86 | 5 | 0 | 2 | 0 | 4 | 4 | 0 |
| II (n=21) | 64.7 | 25–83 | 18 | 3 | 6 | 1 | 13 | 4 | 4 |
| III (n=20) | 65.2 | 44–84 | 19 | 7 | 9 | 1 | 11 | 7 | 2 |
| IV (n=18) | 63.2 | 43–79 | 17 | 6 | 13 | 5 | 8 | 8 | 2 |
Recurrence occurred in 32 (47.8%) out of the 67
patients. The longest and shortest time period to first recurrence
was 43 and 3 months, respectively (average, 14.7 months).
Recurrence risk factors
Statistical analysis was performed to determine the
recurrence risk factors. In the univariate model, regional lymph
node metastasis was associated with an increased risk of recurrence
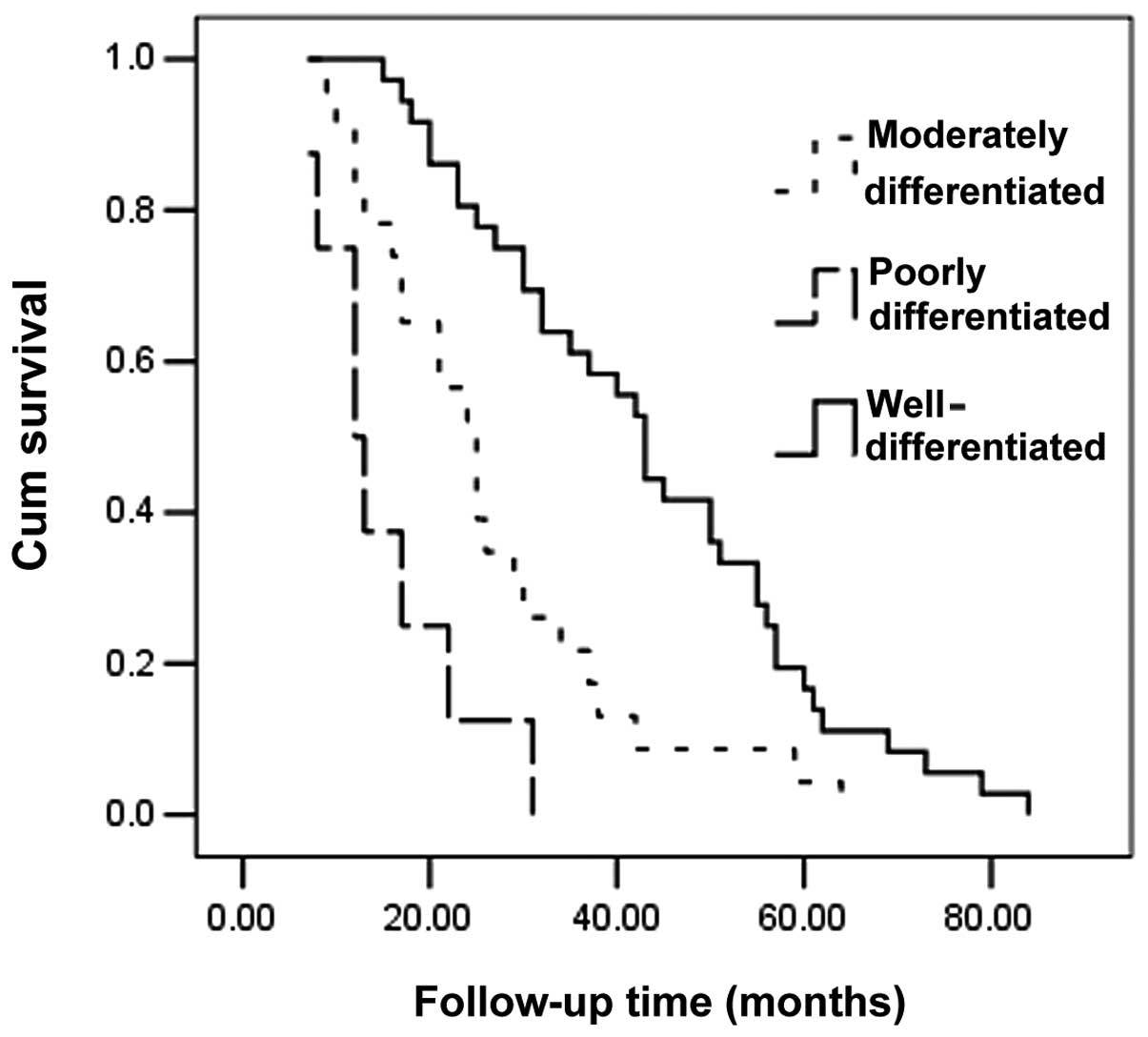
(P=0.067), whereas high tumor differentiation and composite
resection were associated with a decreased risk of recurrence
(P<0.001 and P=0.073, respectively). Multivariate analysis
identified high tumor differentiation as being protective against
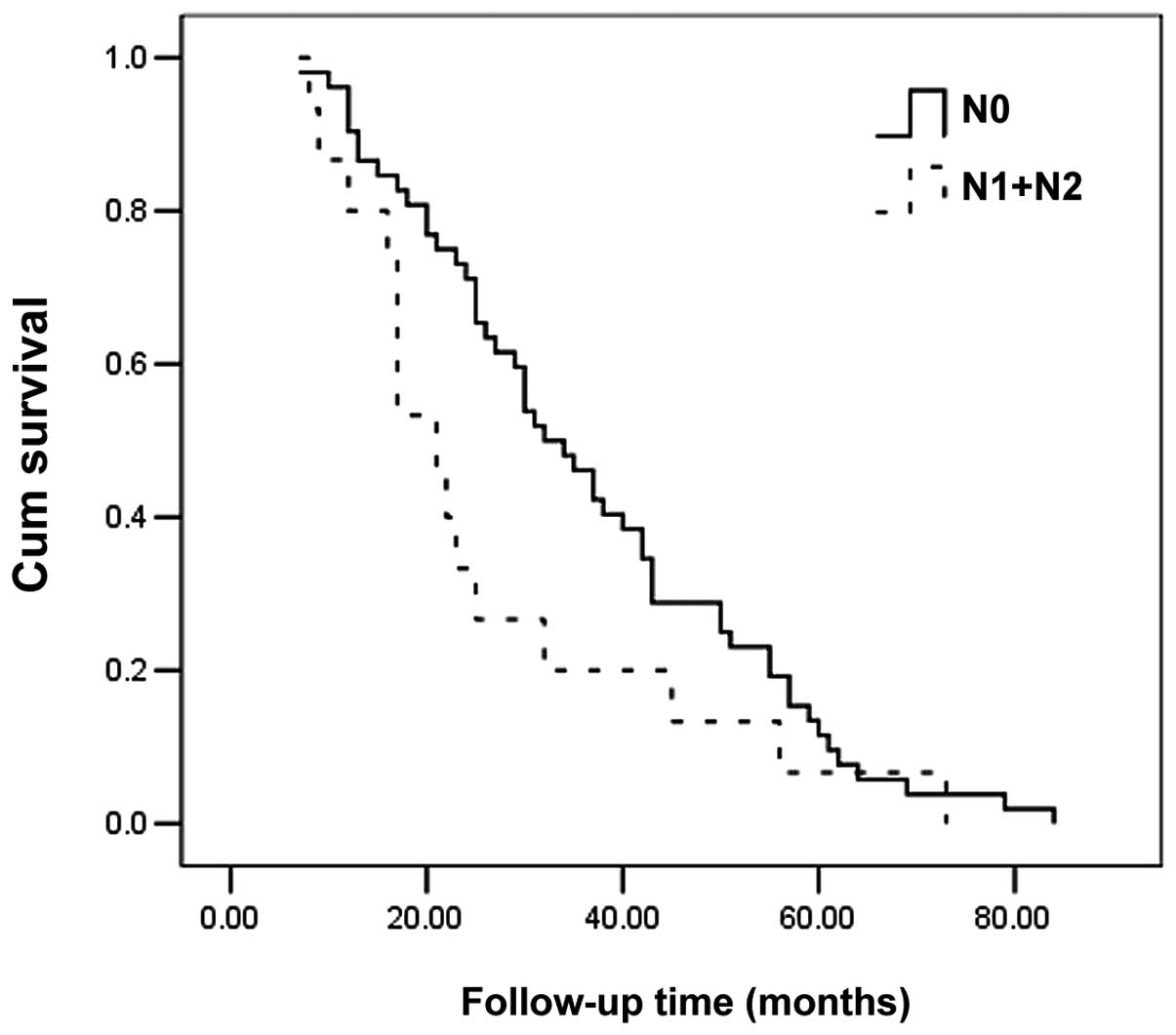
disease recurrence (P<0.001). The Kaplan-Meier method
demonstrated that poorly differentiated tumors, regional lymph node
metastasis and recurrence may exert a negative effect on survival
(P<0.001, P=0.082 and P<0.001, respectively) (Table II, Figs. 1–3).
 | Table IIRecurrence risk factors. |
Table II
Recurrence risk factors.
| Variable | P-value (UVA) | P-value (MVA) |
|---|
| Gender | 0.210 | 0.439 |
| Age | 0.194 | 0.602 |
| Postoperative
radiation | 0.912 | 0.202 |
| Tumor stage | 0.303 | 0.240 |
| Nodal stage | 0.067 | 0.299 |
| History of
tobacco | 0.555 | 0.965 |
| History of
alcohol | 0.105 | 0.969 |
| Neck dissection | 0.863 | 0.680 |
| Differentiation | <0.001 | <0.001 |
| Skin resection | 0.857 | 0.195 |
| Composite
resection | 0.073 | 0.470 |
| Clinical stage | 0.105 | 0.313 |
Discussion
Alcohol, tobacco and betel quid chewing are
well-recognized risk factors for buccal SCC development (1,2).
Markopoulos (8) reported that
older males, ethnic minority groups and lower socioeconomic groups
were more commonly affected by buccal SCC. In the present study, we
demonstrated that 38.8% of the patients had a history of alcohol
consumption, 61.3% had a history of tobacco product consumption and
all patients belonged to low socioeconomic groups.
Buccal SCC commonly occurs in people aged 50–80
years. The findings of a previous study conducted by Diaz et
al(9) demonstrated that the
mean age of the patients was 66.0 years. In the present study, the
mean age was 65.0 years; however, one patient was diagnosed at the
age of 25 years. This is a rare finding, which may be partially
attributed to HPV infection and unhealthy life habits. In addition,
we demonstrated that the male:female ratio was ∼1:1, which was not
consistent with the findings of Lin et al(6) and Huang et al(1). A possible explanation is that the
main cause of the disease in Taiwan is betel quid chewing, which is
more commonly encountered among men (1,10).
Of note, there is an increasing prevalence of smokers among the
female population in China.
Previous studies demonstrated that regional lymph
node metastases in buccal carcinoma occurred less commonly compared
to other oral cavity subsites. Coppen et al(7) reported that the prevalence of neck
lymph node metastasis was 25.0%, whereas Diaz et al(9) suggested that the prevalence was
27.8%. In our study, the prevalence was 23.4%. However, in a study
published by DeConde et al(4), 54% of the patient sample was positive
for neck lymph node metastasis. These findings may be explained by
the differences in distribution according to tumor
differentiation.
The recurrence rate of buccal SCC was relatively
high according to previously published studies (4,9,11).
Diaz et al(9) reported that
54 (45%) out of 119 patients with buccal SCC presented with
recurrences, whereas DeConde et al(4) reported tumor recurrence in 21 (44%)
out of 48 patients. In the present study, out of the 67 patients
with negative resection margins, 32 (47.8%) presented with
recurrences. Possible explanations are as follows: first, the only
barrier to the spread of buccal malignant tumor was the buccinator
muscle and its overlying fascia (9) and there was no reliable anatomic
barrier to prevent invasion once the carcinoma encroached upon the
buccal fat pad; second, radiation therapy may improve locoregional
control (12), however, only 16
(23.2%) patients received postoperative radiation in our study;
third, during our follow-up, the majority of the patients with
recurrences remained addicted to tobacco and/or alcohol. Several
recurrent tumors were advanced and unresectable and only a few
patients could be successfully salvaged (10). We demonstrated that recurrence
exerted a significant negative effect on survival, which was
consistent with the findings of Yanamoto et al(13). Therefore, aggressive treatment of
the tumor in its early stages is critical. Thirty patients
underwent resection of either the mandible or the maxilla and in
the univariate model we identified composite resection as a
positive prognostic factor. Pathak et al(14) reported that involvement of the
maxillary bone was a prognostic factor affecting disease-free
survival. Ghoshal et al(2)
concluded that most locoregional recurrences of buccal SCC occurred
within the first 2 years. In our study, the rate of recurrence
within 2 years after surgical resection was 94.1%. Therefore, we
recommend that the follow-up time not be shorter than 2 years and
it should be at least 5 years in patients treated for SCC of the
head and neck; however, the value of routine follow-up is
controversial, since a previous study published by Coppen et
al(7) reported that continuous
follow-up visits had little value in the detection of local
recurrences after 5 years and even less value after 3 years.
However, the patient sample included in that study was small and
there were differences among different species; thus, a follow-up
period of at least 5 years remains our recommendation.
Neck lymph node metastasis was considered as a
negative factor for recurrence, which was inconsistent with the
findings of previous studies (1,15).
We attributed this inconsistency to our limited patient sample. In
the case of pathologically proven lymph node metastasis, a
selective or radical ND is necessary. There remains the issue of
contralateral neck treatment. Koo et al(16) reported that 2 out of 8 patients
staged as T3 presented with contralateral neck metastasis and
suggested prophylactic neck treatment if the lesion was staged as
higher than T3. However, Lin et al(6) reported that bilateral treatment was
performed on patients staged as N2, but provided no additional
benefit compared to unilateral treatment; therefore, the authors
concluded that there was little lymphatic drainage in the neck that
crossed the midline and metastasis of buccal cancer to the
contralateral side was a rare finding. In our clinical experience,
there has been no report of contralateral neck disease recurrence
and ipsilateral ND should suffice, unless the tumor crosses the
midline.
It remains debatable whether to perform an ND on cN0
patients, particularly cT1-2N0 patients. In the present study, 29
patients were staged as cT1-2N0 and 23 underwent ND, following
which no patients were pathologically confirmed as N1 or N2. During
follow-up, 2 out of 23 patients with ND and 3 out of 6 patients
without ND presented with neck recurrence and although our patient
sample was relatively small, we considered the difference as
significant (P=0.046). Diaz et al(9) demonstrated that the rate of neck
recurrence in cN0 patients with or without ND was 10 and 25%,
respectively. Liao et al(15) reported that the rate of neck
recurrence in cN0 patients with or without ND was 5 and 18%,
respectively. In a risk-benefit evaluation regarding the indication
of ND, factors that need to be considered include the possible
prognostic influence of delayed diagnosis of metastasis during
follow-up, the probability of neck metastasis and the probability
of complications associated with ND. In the case of low probability
of neck node metastasis, certain studies (17) suggested ND would be an
overtreatment; however, there are no reliable methods, such as
palpation, CT and MRI, which may predict the risk of metastasis.
Therefore, it remains our policy to perform an ND in order to
improve locoregional control.
In this study, we demonstrated that tumor
differentiation was intimately associated with recurrence in
univariate and multivariate models and affected survival time. We
hypothesized that tumor differentiation was inversely correlated
with aggressiveness. Seven out of 8 patients with poorly
differentiated tumor presented with recurrences, which may be
related to the fact that these patients remained addicted to
tobacco and alcohol postoperatively and only 1 patient received
postoperative radiation therapy. Lin et al(6) conducted a retrospective study
including 145 patients diagnosed with buccal SCC and demonstrated
that tumor differentiation was the most significant prognostic
factor. The authors suggested that in the case of a poorly
differentiated carcinoma, an effective systemic treatment was
required in order to achieve a better outcome. Pathak et
al(14) demonstrated that the
degree of tumor differentiation was a prognostic factor affecting
the disease-free survival. However, Fang et al(10) assessed the prognostic factors on
locoregional control of buccal SCC and reported that histological
grading was of no prognostic value.
In conclusion, buccal SCC is an aggressive malignant
tumor, with its degree of differentiation being the most important
factor affecting prognosis and survival. In case of poorly
differentiated tumors, an adequate systemic treatment is necessary.
ND exerts a positive effect on the locoregional control of buccal
SCC staged as cT1-2N0. In the case of identification of positive
lymph nodes during surgery, postoperative radiation is recommended
in order to improve locoregional control.
References
|
1.
|
Huang CH, Chu ST, Ger LP, Hou YY and Sun
CP: Clinicopathologic evaluation of prognostic factors for squamous
cell carcinoma of the buccal mucosa. J Chin Med Assoc. 70:164–170.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ghoshal S, Mallick I, Panda N and Sharma
SC: Carcinoma of the buccal mucosa: analysis of clinical
presentation, outcome and prognostic factors. Oral Oncol.
42:533–539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Freedman ND, Abnet CC, Leitzmann MF,
Hollenbeck AR and Schatzkin A: Prognostic investigation of the
cigarette smoking-head and neck cancer association by sex. Cancer.
110:1593–1601. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
DeConde A, Miller ME, Palla B, Lai C,
Elashoff D, Chhetri D and St John MA: Squamous cell carcinoma of
buccal mucosa: a 40-year review. Am J Otolaryngol. 33:673–677.
2012.PubMed/NCBI
|
|
5.
|
Pandey M, Bindu R and Soumithran CS:
Results of primary versus salvage surgery in carcinomas of the
buccal mucosa. Eur J Surg Oncol. 35:362–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lin CY, Lee LY, Huang SF, et al: Treatment
outcome of combined modalities for buccal cancers: unilateral or
bilateral neck radiation? Int J Radiat Onco Biol Phys.
70:1373–1381. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Coppen C, de Wilde PC, Pop LA, van den
Hoogen FJ and Merkx MA: Treatment results of patients with a
squamous cell carcinoma of the buccal mucosa. Oral Oncol.
42:795–799. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Markopoulos AK: Current aspects on oral
squamous cell carcinoma. Open Dent J. 6:126–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Diaz EM Jr, Holsinger FC, Zuniga ER,
Roberts DB and Sorensen DM: Squamous cell carcinoma of the buccal
mucosa: one institution’s experience with 119 previously untreated
patients. Head Neck. 25:267–273. 2003.
|
|
10.
|
Fang FM, Leung SW, Huang CC, et al:
Combined-modality therapy for squamous carcinoma of the buccal
mucosa: treatment results and prognostic factors. Head Neck.
19:506–512. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liao CT, Huang SF, Chen IH, et al: Tongue
and buccal mucosa carcinoma: is there a difference in outcome? Ann
Surg Oncol. 17:2984–2991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bumpous J: Metastatic cutaneous squamous
cell carcinoma to the parotid and cervical lymph nodes: treatment
and outcomes. Curr Opin Otolaryngol Head Neck Surg. 17:122–125.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yanamoto S, Yamada S, Takahashi H, et al:
Clinicopathological risk factors for local recurrence in oral
squamous cell carcinoma. Int J Oral Maxillofac Surg. 41:1195–1200.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Pathak KA, Mathur N, Talobe S, et al:
Squamous cell carcinoma of the superior gingival-buccal complex.
Oral Oncol. 43:774–779. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Liao CT, Wang HM, Ng SH, et al: Good tumor
control and survivals of squamous cell carcinoma of buccal mucosa
treated with radical surgery with or without neck dissection in
Taiwan. Oral Oncol. 42:800–809. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Koo BS, Lim YC, Lee JS and Choi EC:
Management of contra-lateral N0 neck in oral cavity squamous cell
carcinoma. Head Neck. 28:896–901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Weiss MH, Harrison LB and Isaacs RS: Use
of decision analysis in planning a management strategy for the
stage N0 neck. Arch Otolaryngol Head Neck Surg. 120:699–702. 1994.
View Article : Google Scholar : PubMed/NCBI
|