Introduction
The mechanisms underlying the development of ascites
in patients with liver cirrhosis are associated with several
factors, without reaching any definitive conclusions. The
underfilling theory (1) suggests
that water and sodium retention may be a secondary phenomenon that
results from the formation of ascites and the decreased effective
circulating blood volume. With the progression of liver cirrhosis,
the sinusoidal hydro-static pressure and lymph formation in the
liver are increased; when lymph formation exceeds the amount of
flow to lymphatic vessels, it is retained as ascites. This
subsequently decreases the effective circulating blood volume and
causes retention of water and sodium in the renal tubules through
neurohumoral factors. According to the underfilling theory,
patients with cirrhotic ascites are treated with a low-salt diet
and rest, with the addition of diuretic treatment for patients with
cirrhotic ascites (2). Diuretic
therapy improves ascites and edema by actively excreting retained
water and sodium. Recent guidelines published in Japan reported the
administration of human serum albumin (HSA) to patients with
diuretic-resistant cirrhotic ascites who did not show improvement
following administration of a diuretic alone (2,3). The
main purpose of HSA administration in the treatment of cirrhotic
ascites is to increase the effective circulating blood volume. A
previous article on the ‘proper use of albumin’ in the Japanese
Guidelines for Blood Product Use and Blood Transfusion Therapy
Practice states that hypertonic albumin (Alb) may be used as a
concomitant drug in the short-term (maximum of 1 week) treatment of
diuretic-resistant ascites (3).
The following actions of HSA were previously
established: First, HSA was shown to prevent circulatory failure
following abdominal paracentesis of a large amount of ascitic
fluid. It was reported that 8–10 g of Alb administered per 1 liter
of drainage can prevent circulatory failure (4). Second, HSA was shown to prevent renal
failure due to spontaneous bacterial peritonitis. Sort et al
(5) reported that patients
administered antibiotics plus HSA exhibited a more significant
improvement in renal function and survival compared to those who
received antibiotics alone. Finally, HSA was shown to improve renal
function when used with terlipressin for the treatment of
hepatorenal syndrome. A recovery of renal function was reported
following combination therapy with terlipressin and intravenous HSA
(6). In light of this evidence,
Gentilini et al (7)
conducted a randomized, controlled trial to investigate the
efficacy of HSA in patients with cirrhotic ascites. Their results
demonstrated that the co-administration of diuretic and Alb
resulted in a better control of all parameters, including
disappearance of ascites, duration of hospital stay, recurrence of
ascites and hospital readmission due to ascites exacerbation,
compared to the use of diuretics alone. The Clinical Practice
Guidelines for the Management of Liver Cirrhosis by the Japanese
Society of Gastroenterology state that ‘HSA is useful in treating
ascites associated with liver cirrhosis’ (2). However, there are certain limitations
to using HSA in patients with cirrhotic ascites, including the high
treatment cost and the risk of infectious disease transmission with
HSA, as it is a derivative of human blood. Therefore, HSA therapy
is not currently widely used in an international setting.
We conducted a phase II (multicenter, randomized,
open-label, parallel-controlled) clinical trial of KD-294, a
recombinant HSA, in patients with cirrhotic ascites, initiated in
2005. Our results suggested that there may be a specific factor
that is correlated with the improving effect of HSA on cirrhotic
ascites. In particular, it was demonstrated that HSA may exert
additional effects on diuretic therapy in patients with high plasma
renin concentration (PRC) (8). In
the present observational cohort study, which was conducted
following the completion of the phase II study mentioned above, we
aimed to investigate each parameter considered to be associated
with the treatment of cirrhotic ascites with diuretics and HSA.
Materials and methods
Study drug
HSA, the study drug, was derived from human plasma
and is currently marketed in Japan. The use of diuretics and
concomitant drugs was not specified, nor was the use of a low-salt
diet.
Patients
The study population comprised hospitalized patients
diagnosed with liver cirrhosis and ascites, with a serum Alb
concentration of <3.5 g/dl. Patients with severe renal disease
(serum creatinine levels ≥2.0 mg/dl or proteinuria ≥3.5 g/day),
patients scheduled to undergo drainage of ascites and patients
considered unsuitable for HSA administration, were excluded from
this study. The patients were recruited from the following: Kurume
University Hospital, Showa University Hospital, Gifu University
Hospital, Tottori University Hospital, Nagasaki Medical Center and
NTT West Kyushu Hospital.
Informed consent and patient
registration
Prior to enrollment, each patient provided written
informed consent to the investigator or sub-investigators after
receiving a sufficient explanation of the study protocol and
objective. This study was reviewed and approved by the local Ethics
Committee of each study site and conducted in accordance with the
principles of the Declaration of Helsinki.
Study design
This study was conducted as a prospective cohort
study between November, 2007 and January, 2009. The HSA dose was
tailored to each patient's requirements for the treatment of
ascites and the treatment duration was set to a maximum of 7 days,
according to the Japanese Guidelines for Blood Product Use and
Blood Transfusion Therapy Practice (3). The type of the diuretic administered
was not specified, whereas oral administration and injection were
both permitted. The recorded parameters included body weight,
ascites severity, hematological tests [hematocrit (Ht)], laboratory
serum test (serum Alb concentration) and endocrine tests [PRC and
aldosterone (ALD)]. It was mandatory that these parameters were
measured on days 1, 4 and 6. If HSA was used for >5 days, a
measurement was also performed on the day after the last day of
administration. PRC and ALD were measured by the Mitsubishi
Chemical Medience Corporation (Tokyo, Japan). HSA safety was not
investigated, since it was previously established and was not
considered necessary as this study was conducted while HSA was in
use.
Methods of analysis
The mean value and standard deviation (SD) at each
observation time point for Alb concentration, body weight and other
laboratory parameters, as well as the mean value and 95% confidence
interval (CI) (two-sided) for the changes from baseline to each
observation time point, were calculated. The Ht ratio was
calculated as the change from baseline to the observation time
point by using the value at day 1 as the denominator and the values
at each observation time point as the numerators. Logarithmic
transformation was applied for PRC and ALD and the values were
analyzed with normal distribution. The frequencies for the category
of ascites status and the proportion of change in that status were
calculated at each observation time point. The descriptive
statistics were calculated for the weight change from baseline to
the observation time point, in order to investigate the effect of
the HSA dose and diuretic use on weight change by stratifying the
total amount of HSA and that of the diuretic. In addition, a
multiple linear regression analysis was performed, using an
objective variable, such as weight change from baseline, as well as
explanatory variables, such as the total amount of HSA and
diuretics administered and the values of PRC and ALD. All the
statistical analyses were performed using SAS software, version
9.2, or JMP software, version 8 (SAS Institute Japan Ltd., Tokyo,
Japan). Two-sided tests at the 0.05 level were considered to be
statistically significant.
Results
Study population
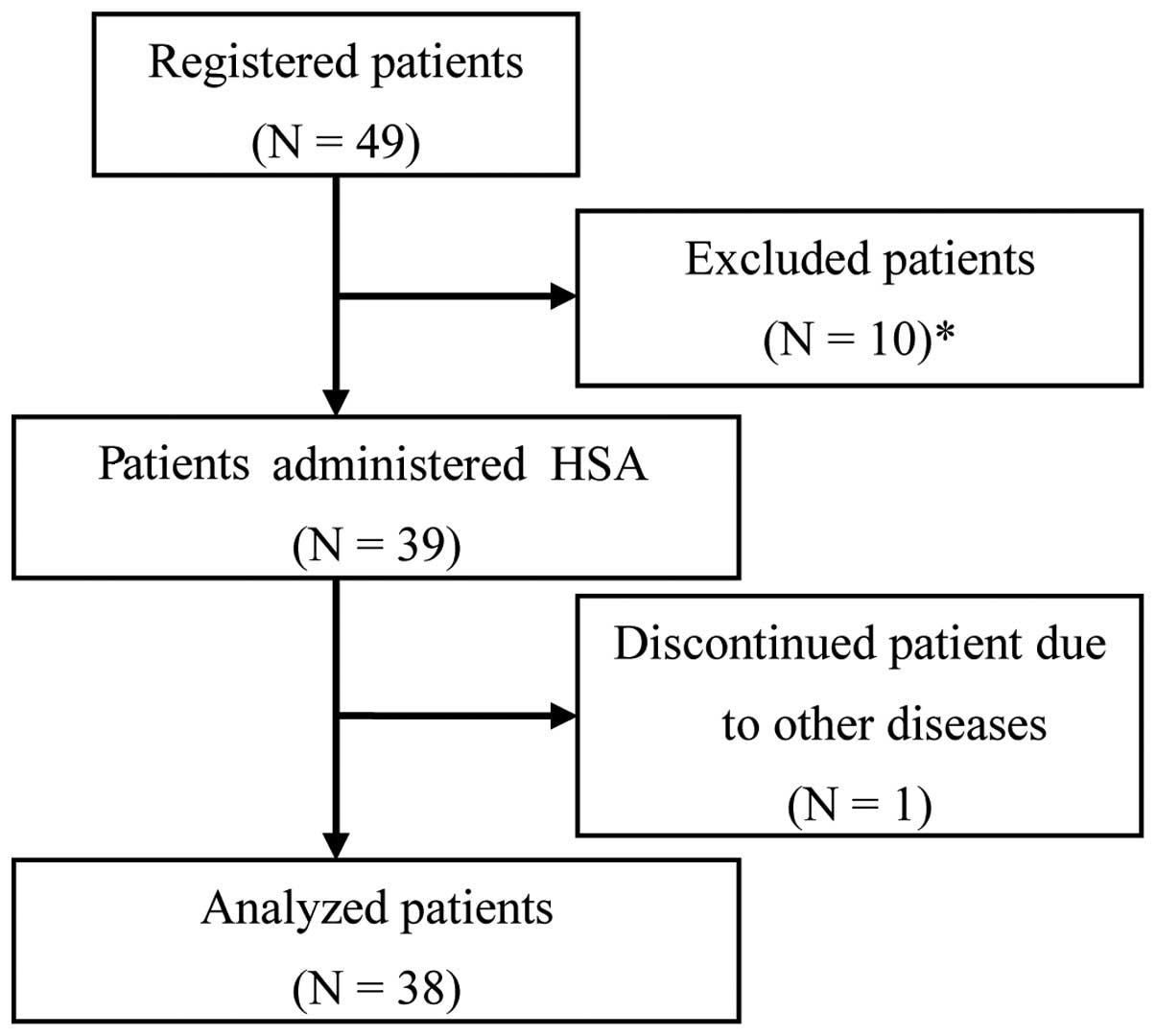
Of the 49 registered patients, 11 were considered
unsuitable for the analysis and were excluded from this study. The
exclusion criteria were as follows: patients considered to have
ascites due to portal vein embolism; patients with a history of
abdominal paracentesis; patients who were administered HSA within 1
week prior to the study initiation; patients without ascites; and
patients who discontinued treatment due to another disease
(dementia) from day 2. The remaining 38 patients constituted the
study cohort (Fig. 1) and their
clinical characteristics are presented in Table I.
 | Table I.Clinical characteristics. |
Table I.
Clinical characteristics.
| Variables | Category | Patient no. (%) |
|---|
| Gender | Male | 24 (63.2) |
| Female | 14 (36.8) |
| Age, years
(n=38) | <65 | 15 (39.5) |
| 66–74 | 13 (34.2) |
| ≥75 | 10 (26.3) |
| Mean ± SD | 66.1±11.0 | |
| BW, kg (n=36) | <50 | 11 (30.6) |
| 50–59 | 9 (25.0) |
| 60–69 | 9 (25.0) |
| ≥70 | 7 (19.4) |
| Mean ± SD | 59.37±15.36 | |
| Etiology of liver
cirrhosis | HBV infection | 5 (13.2) |
| HCV infection | 20 (52.6) |
| ALC | 5 (13.2) |
| Mixeda | 5 (13.2) |
| Othersb | 3 (7.9) |
| Alb, g/dl (n=38) | <1.5 | 0 (0.0) |
| 1.5–1.9 | 7 (18.4) |
| 2.0–2.4 | 20 (52.6) |
| 2.5–2.9 | 10 (26.3) |
| ≥3.0 | 1 (2.6) |
| Mean ± SD | 2.29±0.34 | |
| Ascitesc | + | 8 (21.1) |
| 2+ | 18 (47.4) |
| 3+ | 12 (31.6) |
| Edema | − | 13 (34.2) |
| + | 25 (65.8) |
| Ht, % (n=38) | <25 | 4 (10.5) |
| 25–29 | 14 (36.8) |
| 30–34 | 14 (36.8) |
| ≥35 | 6 (15.8) |
| Mean ± SD | 30.23±4.55 | |
| T-BIL, mg/dl
(n=33) | ≤1.2 | 7 (21.2) |
| 1.2–1.9 | 11 (33.3) |
| 2.0–2.9 | 8 (24.2) |
| 3.0–3.9 | 3 (9.1) |
| 4.0–4.9 | 2 (6.1) |
| ≥5.0 | 2 (6.1) |
| Mean ± SD | 2.18±1.38 | |
| Cr, mg/dl (n=36) | ≤1.1 | 31 (86.1) |
| 1.1–1.9 | 5 (13.9) |
| ≥2.0 | 0 (0.0) |
| Mean ± SD | 0.899±0.354 | |
| BUN, mg/dl
(n=31) | ≤23 | 22 (71.0) |
| 23–50 | 9 (29.0) |
| >50 | 0 (0.0) |
| Mean ± SD | 19.95±9.82 | |
Use of HSA and diuretics
An HSA dose was administered in one or two vials per
day (containing 12.5–25.0 g of HSA) for a maximum of 7 days. Four
patients received a total dose of one vial of HSA for ≤3 days (37.5
g); 9 patients received >3 but <10 vials (>37.5 but
<125.0 g) and 25 patients received ≥10 vials (≥125.0 g).
As regards the use of diuretics, 28 patients
received a diuretic for ≥7 days prior to HSA administration and 10
patients received diuretics for <7 days or did not receive any
diuretics prior to the initiation of HSA administration. As regards
the use of anti-ALD diuretics, 23 patients received anti-ALD for ≥7
days prior to the administration of HSA and 15 patients received
anti-ALD for <7 days or not at all prior to the administration
of HSA. The actual dose of diuretics was within the range
recommended on the package insert.
HSA treatment decreases body weight, Ht
ratio and PRC and increases serum Alb levels
The weight changes from baseline (day 1) were −1.49
kg (95% CI: −2.05 to −0.94) on day 4 and −2.24 kg (95% CI: −3.06 to
−1.43) on day 6 (Table II). As
regards the change in the ascites status, an improvement was
observed in 14 of the 38 patients on day 4 and in 22 patients on
day 6 (Table III).
 | Table II.Changes in the laboratory values from
baseline (day 1) to observation time points. |
Table II.
Changes in the laboratory values from
baseline (day 1) to observation time points.
| Variables | Day 1 | Day 4 | Day 6 |
|---|
| Body weight, kg
(n) | 36 | 36 | 36 |
| Mean (SD) | 59.37 (15.36) | 57.88 (15.13) | 57.13 (14.61) |
| Mean change | - | −1.49 | −2.24 |
| 95% CI
(two-sided) | - | (−2.05, −0.94) | (−3.06, −1.43) |
| Serum Alb, g/dl
(n) | 38 | 37 | 38 |
| Mean (SD) | 2.29 (0.34) | 3.00 (0.50) | 3.26 (0.58) |
| Mean change | - | 0.70 | 0.97 |
| 95% CI
(two-sided) | - | (0.59, 0.81) | (0.83, 1.11) |
| Ht ratioa, % (n) | 38 | 37 | 38 |
| Mean (SD) | 30.23 (4.55) | 28.75 (4.63) | 28.97 (4.70) |
| Mean change | - | 0.95 | 0.96 |
| 95% CI
(two-sided) | - | (0.93, 0.97) | (0.94, 0.98) |
| PRCb, pg/ml (n) | 38 | 38 | 38 |
| Geometric mean
(SD) | 38.33 (6.78,
216.59) | 26.96 (5.30,
137.03) | 30.77 (6.84,
138.37) |
| Geometric mean
fold changec | - | −0.1528 | −0.0953 |
| 95% CI
(two-sided) | - | (−0.2510,
−0.0545) | (−0.1972,
0.0066) |
| ALDd, pg/ml (n) | 38 | 38 | 38 |
| Geometric mean
(SD) | 90.32 (20.68,
394.52) | 78.67 (25.35,
244.13) | 82.44 (28.09,
241.97) |
| Geometric mean
fold changec | - | −0.0599 | −0.0396 |
| 95% CI
(two-sided) | - | (−0.1925,
0.0726) | (−0.1790,
0.0998) |
 | Table III.Change in ascites status over
time. |
Table III.
Change in ascites status over
time.
| Item | Day 1 (%) | Day 4 (%) | Day 6 (%) |
|---|
| Ascites
statusa | | | |
| − | 0 (0.0) | 2 (5.3) | 2 (5.3) |
| + | 8 (21.1) | 12 (31.6) | 21 (55.3) |
| 2+ | 18 (47.4) | 18 (47.4) | 11 (28.9) |
| 3+ | 12 (31.6) | 6 (15.8) | 4 (10.5) |
| Total | 38 (100.0) | 38 (100.0) | 38 (100.0) |
| Change in ascites
status | | | |
| −2 | - | 0 (0.0) | 4 (10.5) |
| −1 | - | 14 (36.8) | 18 (47.4) |
| 0 | - | 24 (63.2) | 15 (39.5) |
| +1 | - | 0 (0.0) | 1 (2.6) |
| Total | - | 38 (100.0) | 38 (100.0) |
As regards the change in laboratory values from
baseline (day 1), the Alb concentration was significantly increased
on day 4 (0.70 g/dl; 95% CI: 0.59–0.81) and on day 6 (0.97 g/dl;
95% CI: 0.83–1.11) (Table II),
whereas the Ht ratio was significantly decreased on day 4 (0.95;
95% CI: 0.93–0.97) and on day 6 (0.96; 95% CI: 0.94–0.98) (Table II). The PRC was significantly
decreased on day 4 compared to that at baseline (day 1) (geometric
mean fold change: −0.1528, 95%CI: −0.2510 to −0.0545) (Table II). The change in ALD was not found
to be statistically significant.
Weight loss correlates with the total
amount of infused HSA
An exploratory analysis was performed to identify
the correlation between weight loss and total dose of HSA, anti-ALD
and loop diuretics and on the combined total amount of anti-ALD and
loop diuretics. The results revealed that the mean weight loss on
day 4 was −0.29, −0.97 and −2.13 in the HSA low-dose (HSA ≤37.5 g),
middle-dose (37.5<HSA≤60 g) and high-dose group (60<HSA≤75
g), respectively and the mean weight loss on day 6 was −0.55, −1.27
and −3.24 kg in the HSA low-dose (HSA ≤62.5 g), middle-dose
(62.5<HSA≤100 g) and high-dose group (100<HSA≤125 g),
respectively. This finding suggested that weight loss was dependent
on the total amount of HSA on days 4 and 6 (Table IV). Since there are several types
of diuretics, each diuretic was classified as anti-ALD or loop
diuretics prior to the investigation. The value of each diuretic
that was classified as anti-ALD was converted to the value of
spironolactone, i.e., 400 mg of potassium canrenoate was equivalent
to 100 mg of spironolactone. The value of each diuretic that was
classified as a loop diuretic was converted to intravenous
furosemide, i.e., 40 mg of oral furosemide and 60 mg of azosemide
were equivalent to 20 mg of intravenous furosemide. The results
demonstrated that weight loss did not clearly depend on the
combined total amount of the two types of diuretics, but rather on
the total amount of each diuretic on days 4 and 6 (Tables V–VII).
 | Table IV.Mean weight loss by total HSA. |
Table IV.
Mean weight loss by total HSA.
A, Mean weight loss
(kg) on day 4 by total HSA
|
|---|
| Total amount of HSA
(g) (3 days completed) | No. | Mean | Median | SD |
|---|
| HSA≤37.5 | 8 | −0.29 | 0.05 | 1.00 |
| 37.5<HSA≤60 | 7 | −0.97 | −0.6 | 1.04 |
| 60<HSA≤75 | 21 | −2.13 | −2.0 | 1.70 |
B, Mean weight loss
(kg) on day 6 by total HSA
|
|---|
| Total amount of HSA
(g) (5 days completed) | No. | Mean | Median | SD |
|---|
| HSA≤62.5 | 4 | −0.55 | −0.1 | 1.27 |
|
62.5<HSA≤100 | 7 | −1.27 | −0.8 | 1.95 |
| 100<HSA≤125 | 17 | −3.24 | −2.8 | 2.71 |
 | Table V.Mean weight loss by total diuretic
(anti-ALD). |
Table V.
Mean weight loss by total diuretic
(anti-ALD).
A, Mean weight loss
(kg) on day 4 by total diuretic (anti-ALD)
|
|---|
| Total amount of
anti-ALDa (mg) (3
days completed) | No. | Mean | Median | SD |
|---|
| Anti-ALD=0 | 3 | 0.10 | 0.1 | 0.30 |
|
0<anti-ALD≤150 | 24 | −1.36 | −1.25 | 1.49 |
|
Anti-ALD>150 | 9 | −2.39 | −1.9 | 1.81 |
B, Mean weight loss
(kg) on day 6 by total diuretic (anti-ALD)
|
|---|
| Total amount of
anti-ALD (mg) (5 days completed) | No. | Mean | Median | SD |
|---|
| Anti-ALD=0 | 3 | 0.27 | 0.3 | 0.15 |
|
0<anti-ALD≤250 | 24 | −2.11 | −1.5 | 2.30 |
|
Anti-ALD>250 | 9 | −3.42 | −3.2 | 2.49 |
 | Table VII.Mean weight loss by combined total
diuretic (anti-ALD and loop diuretic). |
Table VII.
Mean weight loss by combined total
diuretic (anti-ALD and loop diuretic).
A, Mean weight loss
(kg) on day 4 by combined total diuretic (anti-ALD and loop
diuretic)
|
|---|
| Total amount of
anti-ALD (mg) (3 days completed) | Total amount of
loop diuretic (mg) (3 days completed) | No. | Mean | Median | SD |
|---|
| Anti-ALD=0 | Loop
diuretic=0 | 0 | - | - | - |
| 0<loop
diuretic≤60 | 2 | −0.05 | −0.05 | 0.21 |
| Loop
diuretic>60 | 1 | 0.40 | 0.4 | - |
|
0<anti-ALD≤150 | Loop
diuretic=0 | 1 | −0.10 | −0.1 | - |
| 0<loop
diuretic≤60 | 13 | −1.31 | −0.9 | 1.74 |
| Loop
diuretic>60 | 10 | −1.55 | −1.7 | 1.21 |
|
Anti-ALD>150 | Loop
diuretic=0 | 0 | - | - | - |
| 0<loop
diuretic≤60 | 2 | −2.35 | −2.35 | 3.61 |
| Loop
diuretic>60 | 7 | −2.40 | −1.9 | 1.49 |
B, Mean weight loss
(kg) on day 6 by combined total diuretic (anti-ALD and loop
diuretic)
|
|---|
| Total amount of
anti-ALD (mg) (5 days completed) | Total amount of
loop diuretic (mg) (5 days completed) | No. | Mean | Median | SD |
|---|
| Anti-ALD=0 | Loop
diuretic=0 | 0 | - | - | - |
| 0<loop
diuretic≤100 | 2 | 0.35 | 0.35 | 0.07 |
| Loop
diuretic>100 | 1 | 0.10 | 0.1 | - |
|
0<anti-ALD≤250 | Loop
diuretic=0 | 1 | −0.20 | −0.2 | - |
| 0<loop
diuretic≤100 | 12 | −1.68 | −0.85 | 2.48 |
| Loop
diuretic>100 | 11 | −2.75 | −2.8 | 2.09 |
|
Anti-ALD>250 | Loop
diuretic=0 | 0 | - | - | - |
| 0<loop
diuretic≤100 | 2 | −3.85 | −3.85 | 4.60 |
| Loop
diuretic>100 | 7 | −3.30 | −3.2 | 2.16 |
In addition, to identify the factors that affect
weight loss, a multiple linear regression analysis was performed in
the patients who completed the HSA treatment for 3 and 5 days using
an objective variable (weight loss) and explanatory variables
(total amount of HSA, anti-ALD and loop diuretic and PRC and ALD
values at the initiation of HSA treatment). The results
demonstrated that only the total amount of HSA was significantly
associated with weight loss (PRC, P=0.0012 on day 4 and P=0.0229 on
day 6; ALD, P=0.0016 on day 4 and P=0.0200 on day 6).
Discussion
HSA treatment is used in patients with
diuretic-resistant cirrhotic ascites in order to control the
decreased colloidal osmotic pressure due to hypoalbuminemia and to
excrete the excess water that was ultimately retained as ascites or
edema in the urine. Several factors, including hormones, are
considered to be associated with the process of improvement in the
colloidal osmotic pressure and the reduction of the ascites.
However, the precise mechanism underlying the decrease in the
ascites is complicated and has not yet been fully elucidated
(7,9). We previously compared the
administration of diuretics alone with the combined administration
of KD-294 (recombinant HSA) and diuretics, in order to investigate
the association between the predicted parameters for the decrease
of ascites and weight loss, which was a surrogate marker for the
decrease of ascites in the phase II clinical trial of KD-294. As a
result, we focused on PRC at the time of initiation of treatment as
a factor affecting weight loss (8,10,11).
In this clinical study, the use of diuretics was not
restricted and the HSA dose was tailored to each patient's
requirements for the treatment of ascites, with the treatment
duration set to a maximum of 7 days. Thus, this study was conducted
to assess the correlation between each laboratory parameter and
weight loss to allow physicians to select the appropriate treatment
course for cirrhotic ascites in the clinical setting. The actual
doses of HSA were one or two vials per day and a maximum treatment
duration of 7 days.
Regarding the change in each laboratory value, the
serum Alb concentration was significantly increased and the Ht
ratio was significantly decreased on days 4 and 6 following HSA
administration, compared to those at baseline. Weight loss, which
was a surrogate marker of decreased ascites, was found to be
significantly decreased on days 4 and 6. The increased serum Alb
concentration and decreased Ht ratio were attributed to the
improved colloidal osmotic pressure due to the increased Alb
concentration following HSA administration, thereby resulting in an
increased effective circulating blood volume. Identical results
were reported in the phase II clinical trial of KD-294, confirming
the reproducibility of the improving effect of HSA on effective
circulating blood volume (8).
PRC, on which we focused in light of the results of
our previous phase II clinical trial (8), was found to be significantly
decreased on day 4. Although the mean concentration was decreased
on day 6 compared to that on day 1, the change was not
statistically significant (geometric mean fold change, −0.0953; 95%
CI: −0.1972 to 0.0066). The reason for this finding may be the fact
that, from the data on day 4, all 38 patients received HSA for 3
days, whereas from the data on day 6, 8 of the 38 patients
discontinued the study on or after day 4. Five of these 8 patients
exhibited an increased PRC on day 6 compared to that on day 4.
Therefore, no significant decrease was observed on day 6 (data not
shown). The levels of ALD were not significantly decreased on days
4 and 6, which was consistent with the results from our previous
phase II clinical trial of KD-294 (8).
In addition to the abovementioned results, an
exploratory analysis was performed to identify the factors that
significantly affect weight loss in patients with cirrhotic
ascites. We analyzed the mean weight loss on days 4 and 6 by total
HSA, total anti-ALD, total loop diuretics and combined total
anti-ALD and loop diuretics, among several other factors that
affect weight loss. A dose-dependent increase in the mean weight
loss was observed on days 4 and 6 by total HSA, total anti-ALD and
total loop diuretics. One of the dose groups included only one
patient, therefore, the result of anti-ALD and loop diuretics on
weight loss was not reliable (Table
VII). Thus, it was suggested that the results may not clearly
reflect the increase in weight loss dependent on the total
administration amount. However, the results generally suggested
that there may be a correlation between total diuretic or HSA
amount and weight loss.
In addition to these factors, we measured the PRC
and ALD levels at the time of HSA administration and performed
multiple linear regression analyses to identify the factors
affecting weight loss. The results indicated that only total HSA
was significantly associated with weight loss. This may have been
due to the dose-response of HSA on weight loss, i.e., the efficacy
of HSA in patients with cirrhotic ascites was assessed based on the
clinical practice data. Therefore, if the patient did not respond
adequately after 3 days of HSA administration, the 5-day
administration was considered appropriate.
In this clinical study, we were unable to confirm
that the PRC at the initiation of HSA administration affects weight
loss, as was reported in our KD-294 phase II controlled clinical
trial (8). This may have been due
to the fact that the targeted population of this study consisted of
patients who were co-administered HSA and diuretics. In addition,
in the phase II clinical trial, the additional effect of HSA on
diuretic therapy was observed in patients with a PRC of >200
pg/ml at the initiation of HSA administration. However, the effect
of HSA in the group of patients with lower PRC levels (≤200 pg/ml)
was not confirmed (8). The mean
PRC [139.52 pg/ml, 10(Mean-SD); 35.92 pg/ml,
10(Mean+SD); 541.94 pg/ml] of the patients at the time
of the initiation of HSA administration in the phase II clinical
trial was higher compared to that of the patients in the present
clinical study (8). The mean PRC
of the patients in this study was also lower compared to that of
the patients in the phase II clinical trial, with a value of ≤200
pg/ml in ≥75% of the patients (29/38) (data not shown). Therefore,
the results of the previous and the present study taken together
suggest that we may expect HSA administration to decrease ascites
in patients with a PRC of ≤200 pg/ml in clinical practice. We were
unable to directly compare the results of this study and that of
the previous phase II clinical trial, as the physicians were
allowed to select the precise combination of HSA and diuretics.
However, we consider the conclusion that HSA was not effective due
to a low PRC to be rather simplistic.
The multiple linear regression analysis results were
not statistically significant. However, due to the fact that the
P-value of the loop diuretic was near 0.1, significant results may
be dependent on sample size. We hypothesized that the total loop
diuretics may be a significant factor, next to total HSA, in the
combination therapy of HSA and diuretics.
The dosage of HSA used in this study was within the
recommended range of actual dosages used in the medical setting. As
a clearly significant effect of HSA was demonstrated in the
multiple linear regression analysis of the 3-day administration
(the shortest allowed treatment duration), we consider the observed
dose-response of HSA reported in this study to be reliable.
However, the prescription of diuretics based on a patient's
condition may affect the result. A limitation of this study was
that its statistical power was low due to the small sample size.
Thus, clinical studies with larger sample sizes are required to
reconfirm the efficacy of HSA.
Acknowledgements
This observational study was conducted
upon request from Kaketsuken (The Chemo-Sero-Therapeutic Research
Institute, Kumamoto, Japan). The authors would like to thank Mr
Fujio Matsuo of Statcom Company Ltd. for his statistical
advice.
References
|
1.
|
Uemura M, Yamano J and Fukui H: The
condition and treatment of intractable ascites. Shoukakika
(Gastroenterology). 35:448–458. 2002.(In Japanese).
|
|
2.
|
Clinical practice guidelines for the
management of liver cirrhosis. Nankodo, Tokyo: pp. 116–149, issued
April 25, 2010.
|
|
3.
|
Guideline for blood product use and blood
transfusion therapy practice. ‘Yakusyokuhatsu Notification
0726002’. July 26–2007.
|
|
4.
|
Runyon BA: Management of adult patients
with ascites due to cirrhosis. Hepatology. 39:841–856. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sort P, Navasa M, Arroyo V, et al: Effect
of intravenous albumin on renal impairment and mortality in
patients with cirrhosis and spontaneous bacterial peritonitis. N
Engl J Med. 341:403–409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ortega R, Gines P, Uriz J, et al:
Terlipressin therapy with and without albumin for patients with
hepatorenal syndrome: results of a prospective, nonrandomized
study. Hepatology. 36:941–948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gentilini P, Casini-Raggi V, Di Fiore G,
et al: Albumin improves the response to diuretics in patients with
cirrhosis and ascites: results of a randomized, controlled trial. J
Hepatol. 30:639–645. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Nakamura T, Sata M, Suzuki K, et al:
Open-labeled randomized controlled trial to compare diuretic
therapy with recombinant human serum albumin and diuretic therapy
for therapeutic treatment of ascites in patients with advanced
liver cirrhosis: An exploratory trial. Hepatol Res. Apr
22–2013.(Epub ahead of print). View Article : Google Scholar
|
|
9.
|
Fogel MR, Sawhney VK, Neal EA, Miller RG,
Knauer CM and Gregory PB: Diuresis in the ascitic patient: a
randomized controlled trial of three regimens. J Clin
Gastroenterol. 3(Suppl 1): 73–80. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Brinch K, Moller S, Bendtsen F, Becker U
and Henriksen JH: Plasma volume expansion by albumin in cirrhosis.
Relation to blood volume distribution, arterial compliance and
severity of disease. J Hepatol. 39:24–31. 2003.PubMed/NCBI
|
|
11.
|
Wong PY, Carroll RE, Lipinski TL and
Capone RR: Studies on the renin-angiotensin-aldosterone system in
patients with cirrhosis and ascites: effect of saline and albumin
infusion. Gastroenterology. 77:1171–1176. 1979.PubMed/NCBI
|