Introduction
Approximately one-third of patients with
non-small-cell lung cancer (NSCLC) have locally advanced disease at
the time of diagnosis. The prognosis of locally advanced NSCLC is
poor and the 5-year survival rates of patients with clinical stage
IIIA and IIIB disease are 18 and 8%, respectively (1). A meta-analysis of randomized trials
demonstrated that platinum-based chemoradiotherapy confers a
survival advantage over radio-therapy alone in patients with
locally advanced NSCLC (2).
Another meta-analysis of randomized trials demonstrated that
concurrent chemoradiotherapy improves survival compared to
sequential chemoradiotherapy in patients with locally advanced
NSCLC (3). Therefore, concurrent
chemoradiotherapy has been a standard treatment for patients with
locally advanced NSCLC. However, a standard chemoradiotherapy
regimen, including radiation dose and schedule as well as selection
and dosage of chemotherapeutic agents, has not been determined thus
far. Therefore, more investigations are required to develop more
effective and less toxic chemoradiotherapeutic regimens for locally
advanced NSCLC.
The combination chemotherapy of cisplatin and
vinorelbine with concurrent thoracic radiotherapy has been
demonstrated to be an effective and tolerable regimen (4). However, the major limitation of this
regimen is cisplatin toxicity and the inconvenience of extensive
hydration requirements. Carboplatin causes less renal, neurological
and gastrointestinal toxicity and is more convenient to administer
compared to cisplatin (5). In
patients with locally advanced NSCLC, overall survival rates do not
differ significantly between carboplatin- and cisplatin-based
chemoradiotherapy (6), although
previous meta-analyses demonstrated that combination chemotherapy
with cisplatin plus a new agent provides a survival advantage over
carboplatin plus a new agent in patients with metastatic advanced
NSCLC (7,8). Therefore, carboplatin-based
chemoradiotherapy may be a viable option in patients with locally
advanced NSCLC.
Preclinical studies demonstrated that both
carboplatin and vinorelbine act as radiation enhancers. Carboplatin
enhances the cytotoxic effects of radiation against tumor cells
in vitro as well as in vivo (9) and vinorelbine enhances the antitumor
effects of radiation in vitro in a cell cycle-dependent
manner, with optimal effects when the cells are in the G2/M phase
(10). Moreover, previous studies
reported that the combination chemotherapy of carboplatin and
vinorelbine without thoracic radiotherapy achieves promising
outcomes in patients with advanced NSCLC (11,12).
However, to date, few trials have evaluated the effectiveness and
safety of the combination chemotherapy of carboplatin and
vinorelbine with concurrent thoracic radiotherapy in patients with
locally advanced NSCLC (13,14).
In a previous phase I study, Hoffman et al
(13) recommended that
carboplatin, with a target area under the plasma concentration
versus time curve of (AUC) of 3 mg/ml/min using the Calvert’s
formula and vinorelbine, at a dose of 15 mg/m2, be
administered on days 1 and 8 every 3 weeks with concurrent thoracic
radiotherapy in patients with locally advanced NSCLC. However, this
recommended dose of vinorelbine was significantly lower compared to
doses in trials without concurrent thoracic radiotherapy in
patients with metastatic advanced NSCLC. Furthermore, findings of
the phase I/II study by Masters et al (15) suggested that a combination of
carboplatin at a target AUC of 2.5 mg/ml/min and vinorelbine at a
dose of 25 mg/m2 be administered on days 1 and 8 every 3
weeks without concurrent thoracic radiotherapy in patients with
metastatic advanced NSCLC. The vinorelbine dose was reduced from 25
to 20 mg/m2 due to concurrent thoracic radiotherapy
being added to the chemotherapy. The aim of this phase II study was
to assess the antitumor activity and safety of a divided schedule
of carboplatin and vinorelbine combined with concurrent thoracic
radiotherapy in patients with locally advanced NSCLC.
Patients and methods
Eligibility criteria
Patients with histologically or cytologically proven
unresectable stage IIIA or IIIB NSCLC who had not previously
received chemotherapy or radiotherapy were eligible for this study.
Other eligibility criteria included: i) age 20–75 years; ii)
Eastern Cooperative Oncology Group performance status of 0–2; iii)
a tumor within an estimated irradiation field no larger than half
the hemithorax; iv) a measurable lesion; v) life expectancy of ≥3
months; and vi) adequate bone marrow function (white blood cell
count of ≥4000/μl, neutrophil count of ≥2000/μl,
platelet count of ≥100,000/μl and hemoglobin level of ≥9.0
g/dl), adequate renal (serum creatinine levels <1.5 mg/dl and
creatinine clearance rate of ≥50 ml/min) and hepatic function
(total serum bilirubin level within the upper limit of the normal
range, levels of aspartate and alanine aminotransferase ≤twice the
upper limits of the normal ranges) and arterial oxygen pressure of
≥60 mmHg. Patients were excluded in case of malignant pleural or
pericardial effusion, active infections, severe heart disease,
interstitial pneumonia, or an active second malignancy. The study
protocol was approved by the Institutional Review Board of Showa
University School of Medicine and the patients provided written
informed consent.
Treatment schedule
The treatment regimen consisted of carboplatin and
vinorelbine with concurrent thoracic radio-therapy. Both
carboplatin and vinorelbine were administered on days 1 and 8.
These agents were administered every 3 weeks for a maximum of 4
courses. Vinorelbine at a dose of 20 mg/m2 was diluted
in 20 ml of normal saline and administered as an intravenous
infusion over 6 min. Carboplatin with a target AUC of 2.5 mg/ml/min
was diluted in 500 ml of normal saline and administered over 60
min. The carboplatin dose was calculated using the Calvert’s
formula.
Chemotherapy was discontinued in case of ≥grade 3
non-hematological toxicity, except for nausea/vomiting, anorexia,
constipation, diarrhea, esophagitis, alopecia and fatigue; serum
creatinine levels >2.0 mg/dl; a treatment outcome of progressive
disease at any time; or an interval of ≥2 weeks after the scheduled
initiation of the next course, until the criteria mentioned below
were satisfied. Carboplatin and vinorelbine were not administered
on day 8 of treatment if the neutrophil count was
<1,000/μl or if the platelet count was
<75,000/μl. Full doses of carboplatin and vinorelbine
were then administered on day 15 of the treatment. The next course
of treatment was initiated after the neutrophil count had increased
to 1,500/μl; the platelet count had increased to
100,000/μl; the creatinine level had decreased to ≤1.5
mg/dl; and the non-hematological toxicity, except for anorexia,
constipation, alopecia and fatigue, had decreased to ≤grade 2. The
doses of carboplatin and vinorelbine were reduced by a target AUC
of 0.5 mg/ml/min and 5 mg/m2, respectively, if the
patient had grade 4 thrombocytopenia. The dose of vinorelbine was
reduced by 5 mg/m2 if the patient had grade 4
neutropenia lasting ≥3 days or grade 3 or 4 neutropenia associated
with a temperature of >38˚C. Prophylactic antiemetic treatment
with 5-hydroxytryptamine receptor type 3 antagonists and
dexamethasone was routinely administered prior to carboplatin in
the patients. If the neutropenia had decreased to grade 4 during
chemotherapy, granulocyte colony-stimulating factor (G-CSF) was
administered until the neutrophil counts recovered.
Thoracic radiotherapy
Thoracic radiotherapy consisted of standard chest
irradiation in single daily fractions of 2 Gy for 6 weeks, up to a
total dose of ∼60 Gy. The planned initial radiation field was not
to exceed 50% of one lung. The initial dose (up to 40 Gy) was
administered to the original volume that had been determined with
the size and locations of the primary tumor and the draining
lymphatic vessels and included a 2 cm margin around the
pretreatment primary tumor and the ipsilateral hilum. The entire
width of the mediastinum was included, with a 2 cm margin around
the radiographically visible area of involvement. The inferior
margin extended 3 cm below the carina or 2 cm below the
radiographically visible tumor mass. Subsequently, an additional 20
Gy dose was administered to the boost volume, including the entire
primary tumor and clinically involved regional hilar and
mediastinal lymph nodes, as determined by computed tomography (CT).
The original volume was treated with an anterior-posterior
parallel-opposed pair of portals and the boost volume was treated
with the same pair or with a pair of oblique fields, if the
cumulative radiation dose to the spinal cord exceeded 40 Gy. The
percentage of lung volume receiving >20 Gy (i.e., V20) of
radiotherapy was not mandatory in the planning of thoracic
radiotherapy.
Thoracic radiotherapy was discontinued for grade 3–4
radiation pneumonitis. Thoracic radiotherapy was suspended for
grade 3–4 esophagitis, temperature of >38˚C or active infection,
during administration of G-CSF, or for a platelet concentration of
<20,000/μl and was resumed when these toxicities had
decreased to ≤grade 2.
Evaluation
Pretreatment evaluation included a baseline history
and physical examination, complete blood count with differential,
routine chemistry profiles, chest radiography, CT of the chest and
abdomen, magnetic resonance or CT imaging of the brain and
radionucleotide bone scan. Mediastinoscopy and positron-emission
tomography (PET) scans were not considered mandatory for this
trial.
Tumor response was classified according to the
Response Evaluation Criteria in Solid Tumors version 1.0. Acute
toxicities were assessed and graded according to the National
Cancer Institute Common Toxicity Criteria version 3.0 and late
toxicity associated with thoracic radiotherapy, occurring >90
days after the initiation of radiotherapy, was graded according to
Radiation Therapy Oncology Group late-toxicity criteria. The
patients who received at least 1 cycle of chemotherapy were
assessable for response, toxicity and survival.
Statistical analysis
Progression-free survival (PFS) was measured from
the initiation of this treatment to the identifiable time of the
first progression or death from any cause. Survival time was
measured from the initiation of the present treatment until death
or last follow-up. The Kaplan-Meier method was used to calculate
survival curves. Survival differences between subgroups were
compared by means of the log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
The present trial was designed as a phase II study,
with response rate as the main endpoint. According to Simon’s
minimax design, our study, with a sample size of 28, had 90% power
to accept the hypothesis that the true response rate was >75%
and had a 5% significance to reject the hypothesis that the true
response rate was <50%.
Results
Patient characteristics
Between March, 2006 and February, 2010, 28 patients
were enrolled (Table I). Thirteen
patients had stage IIIA and 15 had stage IIIB disease. Mutations in
the epidermal growth factor receptor (EGFR) gene were evaluated in
12 patients. Of these, 3 patients with adenocarcinoma exhibited
activating mutations in the EGFR gene. Response, survival and
toxicity were assessable in the patients. A total of 102 courses of
chemotherapy were administered. The median number of courses given
per patient was 4 (range, 1–4).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Values |
|---|
| Total number of
patients | 28 |
| Gender
(male/female) | 23/5 |
| Age, years
(range) | 67 (47–75) |
| Performance status
(0/1/2) | 5/20/3 |
| Stage
(IIIA/IIIB) | 13/15 |
| Pathology | |
|
Adenocarcinoma | 14 |
| Squamous cell
carcinoma | 11 |
| Other | 3 |
Treatment response and survival
Out of the 28 patients, 3 (10.7%) achieved a
complete response, 21 (75.0%) achieved a partial response, 3
(10.7%) had stable disease and 1 (3.6%) had progressive disease,
reaching an overall response rate of 85.7% [95% confidence interval
(CI), 67.3–96.0%] and a disease control rate of 96.4% (95% CI,
81.7–99.9%).
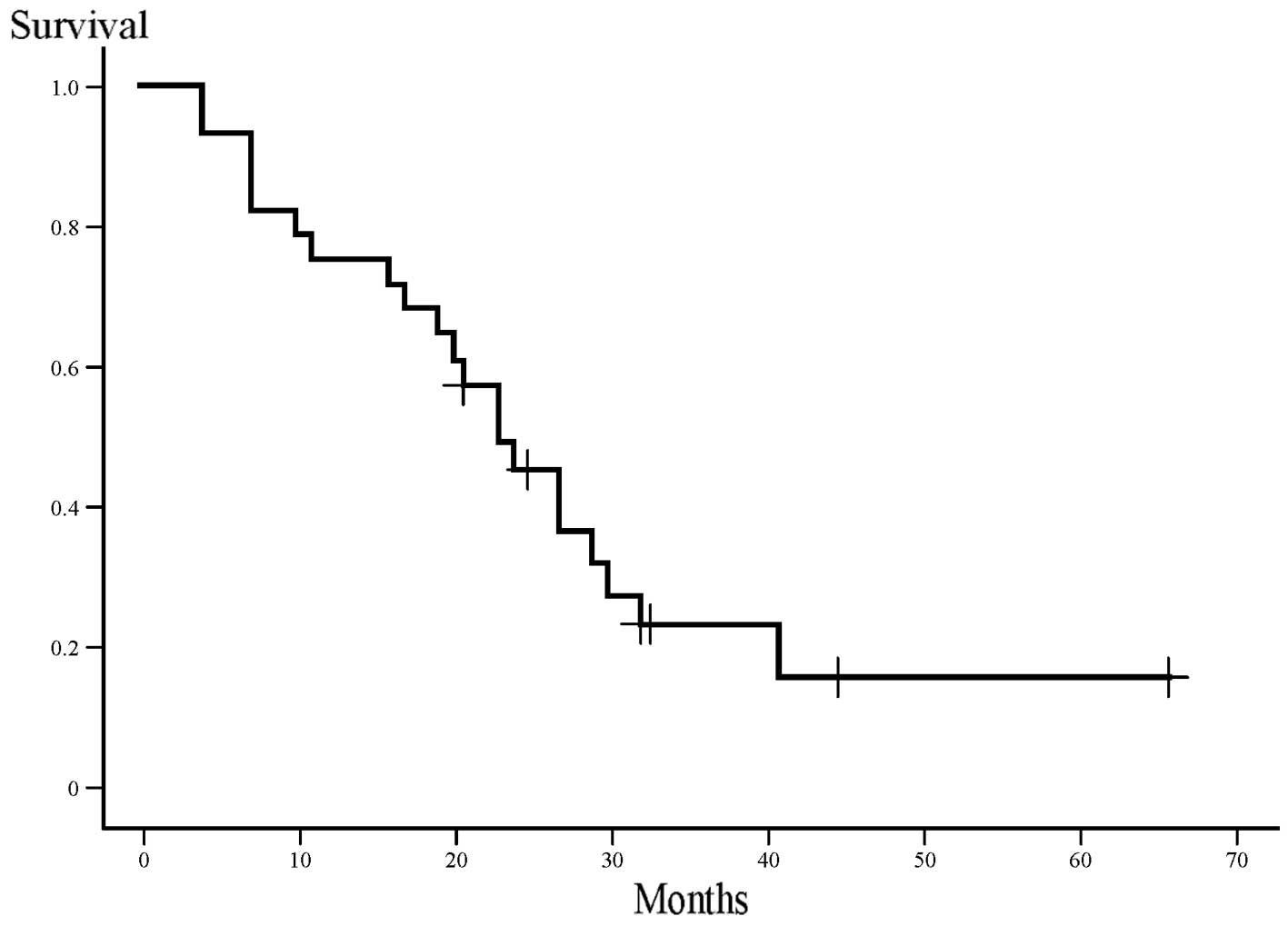
Survival was analyzed when the median follow-up time
of the patients was 22 months. At the time of the analysis, 6
patients (21%) were alive and no patients had been lost to
follow-up. One patient succumbed to bacterial sepsis during
second-line chemotherapy for disease recurrence. The median
survival time (MST) was 23 months (range, 4–66 months) and the
2-year survival rate was 43% (Fig.
1). The median PFS time was 8 months (range, 2–36 months;
Fig. 2).
Recurrence pattern and subsequent
therapy
At the time of the analysis, 24 (85.7%) out of the
28 patients exhibited disease recurrence. Of these, 8 (33.3%) had
local recurrence, 11 (45.8%) had distant recurrence and 5 (20.8%)
had local as well as distant recurrence. The most common site of
distant metastasis was the brain, followed by intrathoracic
sites.
Of these 24 patients, 18 (75%) received second-line
chemotherapy; 1 patient (4%) had a complete response to
chemoradiotherapy followed by recurrence at the primary site and
surgery; and the remaining 5 patients (21%) received only best
supportive care. Salvage chemotherapy was performed as follows: 7
patients received cytotoxic chemotherapy alone, 9 received both
cytotoxic chemotherapy, and an EGFR tyrosine kinase inhibitor and 2
received an EGFR tyrosine kinase inhibitor alone.
Toxicity
The most commonly occurring toxicity was
myelosuppression (Table II). Grade
3–4 hematological toxicities were frequently encountered:
neutropenia, thrombocytopenia and anemia occurred in 100, 14 and
46% of the patients. Treatment with G-CSF was required during 48%
of the courses (49 out of the 102 courses; median duration of
administration, 3 days; range, 1–7 days). Three patients received
erythrocyte transfusions. No patients received platelet
transfusion.
 | Table II.Treatment toxicity. |
Table II.
Treatment toxicity.
| Toxicity | National Cancer
Institute common terminology criteria grade
|
|---|
| 1 | 2 | 3 | 4 | 3/4 (%) |
|---|
| Neutropenia | 0 | 0 | 7 | 21 | 100 |
| Thrombocytopenia | 9 | 6 | 4 | 0 | 14 |
| Anemia | 5 | 10 | 10 | 3 | 46 |
| Nausea | 6 | 2 | 0 | 0 | 0 |
| Vomiting | 3 | 1 | 0 | 0 | 0 |
| Diarrhea | 3 | 0 | 1 | 0 | 4 |
| Infection | 0 | 7 | 9 | 1 | 36 |
| Esophagitis | 5 | 13 | 1 | 0 | 4 |
| Radiation
pneumonitis | 11 | 5 | 2 | 0 | 7 |
| Elevation of serum
creatinine | 0 | 0 | 0 | 0 | 0 |
| Elevation of
aminotransferases | 15 | 8 | 0 | 0 | 0 |
| Abnormality of
sodium balance | 21 | 0 | 4 | 0 | 14 |
| Abnormality of
potassium balance | 13 | 1 | 0 | 0 | 0 |
The majority of the non-hematological toxicities
were mild to moderate and transient. A total of 18 patients (64%)
presented with grade 1 or 2 radiation esophagitis and 1 patient
(4%) developed grade 3 radiation esophagitis, which resolved
completely without residual dilation. No patients developed grade 4
radiation esophagitis. Grade 2 and 3 radiation pneumonitis
requiring treatment with corticosteroids developed in 18 and 7% of
the patients (5 and 2 out of the 28 patients), respectively.
Corticosteroid therapy led to resolution of symptoms and
radiographic abnormalities of the patients. Grade 3 and 4 infection
occurred in 32 and 4% of the patients (9 and 1 out of the 28
patients), respectively. Eight patients had neutropenic fever which
resolved quickly with antibiotic therapy and 1 patient had herpes
zoster infection and recovered with antiviral therapy. One patient
had bacterial pneumonia and required endotracheal intubation and
ventilatory support. Although extubation was possible after the
patient had received antibiotic treatment, steroids and oxygen, his
condition gradually deteriorated and he expired 4 months after
completion of chemoradiotherapy, without any recorded disease
recurrence.
Dose intensity
The doses of carboplatin were not reduced. The doses
of vinorelbine were reduced due to toxicity in 13 patients (46%)
(neutropenic fever in 9 and grade 4 neutropenia lasting ≥3 days in
4 patients). During the 102 courses of chemotherapy, 6 (6%) doses
of vinorelbine were cancelled on day 8, usually due to neutropenia.
Out of the 102 courses of chemotherapy, 25 (25%) were delayed,
usually due to prolonged neutropenia. The actual delivered mean
individual doses of vinorelbine and carboplatin were 16.8
mg/m2 (84% of planned dose) and 2.35 mg/ml/min (94% of
planned dose), respectively.
The patients were able to complete the radiotherapy
according to the dose and schedule modification of the protocol.
Radiotherapy was suspended (median, 4 days; range, 2–16 days) in 14
(50%) patients due to grade 4 neutropenia (11 patients) and
neutropenic fever or infection (3 patients).
Discussion
Platinum-based third-generation chemotherapeutic
agents, such as vinorelbine, gemcitabine and paclitaxel should not
be used at their full doses in concurrent chemoradiotherapy due to
the high incidence of associated toxicity. Therefore, these agents
have been used at reduced doses in previous clinical studies of
concurrent chemoradiotherapy (4,6,16).
Furthermore, in previous phase III studies, platinum-based
third-generation agents failed to demonstrate a survival benefit
over platinum-based second-generation agents in concurrent
chemoradiotherapy in patients with locally advanced NSCLC (6,17).
In addition, consolidation chemotherapy with docetaxel following
concurrent chemoradiotherapy did not prolong survival; however, it
increased the rates of toxicities, including pneumonitis (18). Moreover, gefitinib maintenance
therapy following concurrent chemoradiotherapy and docetaxel
consolidation showed lower survival rates compared to lack thereof
(19). Furthermore, concurrent
chemoradiotherapy in combination with bevacizumab was associated
with a high incidence of tracheoesophageal fistulae formation
(20). Thus far, a standard
chemoradiotherapy regimen has not been determined, although
concurrent chemoradiotherapy has been a standard treatment in
patients with locally advanced NSCLC.
In previous phase III trials of concurrent
chemoradiotherapy with new agents in patients with locally advanced
NSCLC, the response rate range was 56–84%, the median PFS range was
8–13.4 months and the MST range was 16.5–26.8 months (6,16,17,21).
In the present study, the overall response rate was 85.7%, the
median PFS time was 8 months and the MST was 23 months. Therefore,
our data compare favorably with those of previously published
trials in patients with locally advanced NSCLC.
The principal disadvantage of concurrent
chemoradiotherapy is increased normal-tissue toxicity, particularly
hematological, esophageal and pulmonary. In the present study, the
most frequent toxicity was myelosuppression, particularly
neutropenia. Grade 3–4 hematological toxicities included
neutropenia, thrombocytopenia and anemia in 100, 14 and 46% of the
patients, respectively. This high rate of myelosuppression may be
associated with the use of carboplatin, which is strongly
myelosuppressive, rather than cisplatin. However, the episodes of
myelosuppression were manageable. Additionally, these toxicity
rates compare favorably to those reported by previously published
trials on patients with locally advanced NSCLC, in which the rates
of grade 3–4 neutropenia, thrombocytopenia and anemia were 23–99%,
2–53% and 3–16%, respectively (6,16,17,21).
Severe esophagitis has been reported in many trials.
The rate of grade 3–4 esophagitis in recent trials has ranged from
3–18% (6,16,17,21).
In the present study, only 1 patient (4%) developed grade 3
radiation esophagitis and no patients developed grade 4
esophagitis. In addition, severe radiation pneumonitis has been
reported in several trials. The rates of grade 3–4 radiation
pneumonitis in previous trials have been 1–10% (6,16,17,21).
In our study, although grade 3 radiation pneumonitis developed in
7% of patients, corticosteroid therapy led to satisfactory
resolution of symptoms and radiographic abnormalities of the
patients. In the future, with the routine employment of modern
radiotherapy technologies, such as 4-dimensional CT and
respiration-gated radiotherapy, the rates of radiation-induced
esophagitis and pneumonitis are expected to decrease, as improved
imaging and radiotherapy delivery techniques may enable significant
reductions in toxicity (22).
An important limitation in our study was the
compromised accuracy of mediastinal lymph node staging, since
mediastinoscopy and FDG PET were not mandatory in the staging
work-up. In the present study, metastatic lymph nodes were defined
as mediastinal lymph nodes >10 mm along the short axis on CT.
However, for the diagnosis of metastatic mediastinal lymph nodes,
CT (sensitivity, 50–71%; specificity, 66–89%) is inferior to FDG
PET (sensitivity, 67–91%; specificity, 82–96%) and mediastinoscopy
(sensitivity, 80%; specificity, 100%) (23–25).
In conclusion, concurrent chemoradiotherapy with a
divided schedule of carboplatin and vinorelbine is well-tolerated
and effective in patients with locally advanced NSCLC. Therefore,
this treatment is an acceptable option for patients with locally
advanced NSCLC, particularly for patients who are not eligible for
cisplatin-based chemoradiotherapy. Investigations are required for
the design of more active regimens, including molecular-targeted
therapies and modern radiotherapy technologies.
References
|
1.
|
Goldstraw P, Crowley J, Chansky K, et al:
International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions. The
IASCL Lung Cancer Staging Project: proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007.
|
|
2.
|
Auperin A, Le Pechoux C, Pignon JP, et al:
Concomitant radio-chemotherapy based on platin compounds in
patients with locally advanced non-small cell lung cancer (NSCLC):
a meta-analysis of individual data from 1764 patients. Ann Oncol.
17:473–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Auperin A, Le Pechoux C, Rolland E, et al:
Meta-analysis of concomitant versus sequential radiochemotherapy in
locally advanced non-small-cell lung cancer. J Clin Oncol.
28:2181–2190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Vokes EE, Herndon JE II and Crawford J:
Randomized phase II study of cisplatin with gemcitabine or
paclitaxel or vinorelbine as induction chemotherapy followed by
concomitant chemoradiotherapy for stage IIIB non-small-cell lung
cancer: cancer and leukemia group B study 9431. J Clin Oncol.
20:4191–4198. 2002. View Article : Google Scholar
|
|
5.
|
Go RS and Adjei AA: Review of the
comparative pharmacology and clinical activity of cisplatin and
carboplatin. J Clin Oncol. 17:409–422. 1999.PubMed/NCBI
|
|
6.
|
Yamamoto N, Nakagawa K, Nishimura Y, et
al: Phase III study comparing second-and third-generation regimens
with concurrent thoracic radiotherapy in patients with unresectable
stage III non-small-cell lung cancer: West Japan Thoracic Oncology
Group WJTOG0105. J Clin Oncol. 28:3739–3745. 2010. View Article : Google Scholar
|
|
7.
|
Hotta K, Matsuo K, Ueoka H, Kiura K,
Tabata M and Tanimoto M: Meta-analysis of randomized clinical
trials comparing cisplatin to carboplatin in patients with advanced
non-small-cell lung cancer. J Clin Oncol. 22:3852–3859. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ardizzoni A, Boni L, Tiseo M, et al:
Cisplatin-versus carboplatin-based chemotherapy in first-line
treatment of advanced non-small-cell lung cancer: an individual
patient data meta-analysis. J Natl Cancer Inst. 99:847–857. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Douple EB, Richmond RC, O’Hara JA and
Coughlin CT: Carboplatin as a potentiator of radiation therapy.
Cancer Treat Rev. 12:111–124. 1985. View Article : Google Scholar
|
|
10.
|
Edelstein MP, Wolfe LA III and Duch DS:
Potentiation of radiation therapy by vinorelbine (Navelbine) in
non-small cell lung cancer. Semin Oncol. 23:41–47. 1996.PubMed/NCBI
|
|
11.
|
Riedel RF, Andrews C, Garst J, et al: A
phase II trial of carboplatin/vinorelbine with pegfilgrastim
support for the treatment of patients with advanced non-small cell
lung cancer. J Thorac Oncol. 2:520–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bretti S, Manzin E, Celano A, Ritorto G,
Loddo C and Berruti A: Low-dose carboplatin (AUC4.5) combined with
vinorelbine in the treatment of advanced non-small cell lung
cancer: A single institution phase II study. Oncol Rep. 8:381–385.
2001.PubMed/NCBI
|
|
13.
|
Hoffman PC, Cohen EE, Masters GA, et al:
Carboplatin plus vinorelbine with concomitant radiation therapy in
advanced non-small cell lung cancer: a phase I study. Lung Cancer.
38:65–71. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Edelman MJ, Suntharalingam M, Burrows W,
et al: Phase I/II trial of hyperfractionated radiation and
chemotherapy followed by surgery in stage III lung cancer. Ann
Thorac Surg. 86:903–911. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Masters GA, Hahn EA, Shevrin DH and Kies
MS: Phase I/II trial of vinorelbine and divided-dose carboplatin in
advanced non-small cell lung cancer. Lung Cancer. 39:221–226. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zatloukal P, Petruzelka L, Zemanova M, et
al: Concurrent versus sequential chemoradiotherapy with cisplatin
and vinorelbine in locally advanced non-small cell lung cancer: a
randomized study. Lung Cancer. 46:87–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Segawa Y, Kiura K, Takigawa N, et al:
Phase III trial comparing docetaxel and cisplatin combination
chemotherapy with mitomycin, vindesin, and cisplatin combination
chemotherapy with concurrent thoracic radiotherapy in locally
advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol.
28:3299–3306. 2010. View Article : Google Scholar
|
|
18.
|
Hanna N, Neubauer M, Yiannoutsos C, et al:
Phase III study of cisplatin, etoposide, and concurrent chest
radiation with or without consolidation docetaxel in patients with
inoperable stage III non-small-cell lung cancer: the Hoosier
Oncology Group and U.S. Oncology. J Clin Oncol. 26:5755–5760. 2008.
View Article : Google Scholar
|
|
19.
|
Kelly K, Chansky K, Gaspar LE, et al:
Phase III trial of maintenance gefitinib or placebo after
concurrent chemoradiotherapy and docetaxel con4=8solidation in
inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin
Oncol. 26:2450–2456. 2008.
|
|
20.
|
Spigel DR, Hainsworth JD, Yardley DA, et
al: Tracheoesophageal fistula formation in patients with lung
cancer treated with chemoradiation and bevacizumab. J Clin Oncol.
28:43–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Furuse K, Fukuoka M, Kawahara M, et al:
Phase III study of concurrent versus sequential thoracic
radiotherapy in combination with mitomycin, vindesin, and cisplatin
in unresectable stage III non-small-cell lung cancer. J Clin Oncol.
17:2692–2699. 1999.
|
|
22.
|
Haasbeek CJ, Slotman BJ and Senan S:
Radiotherapy for lung cancer: clinical impact of recent technical
advances. Lung Cancer. 64:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Gould MK, Kuschner WG, Rydzak CE, et al:
Test performance of positron emission tomography and computed
tomography for mediastinal staging in patients with non-small-cell
lung cancer. Ann Intern Med. 139:879–892. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Birim O, Kappetein AP, Stijnen T and
Bogers AJ: Meta-analysis of positron emission tomographic and
computed tomographic imaging in detecting mediastinal lymph node
metastases in non-small cell lung cancer. Ann Thorac Surg.
79:375–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Detterbeck FC, Jantz MA, Wallace M,
Vansteenkiste J and Silvestri GA: American College of Chest
Physicians. Invasive mediastinal staging of lung cancer: AACP
evidence-based clinical practice guidelines (2nd Edition). Chest.
132:S202–S220. 2007. View Article : Google Scholar : PubMed/NCBI
|