Introduction
Gastric cancer is one of the most common malignant
tumors and the second leading cause of cancer-related mortality
(1). Elucidating the molecular
mechanism underlying the development of gastric cancer is crucial
in identifying gastric cancer-susceptible populations, screening
for tumor markers and in the application of gene therapy. Girdin is
an actin-binding protein that contains an actin-binding domain, a
membrane-binding domain and an Akt phosphorylation site, first
identified by a group of Japanese scholars in 2005 (2–4).
Girdin plays an essential role in promoting cell proliferation,
which is a critical factor in various physiological processes,
including embryonic development, inflammation, angiogenesis and
tumor development (5,6). The role of girdin in the development
of breast and colorectal cancer was recently confirmed (7–9);
however, the association between girdin and gastric cancer is yet
to be fully elucidated. This study aimed to determine girdin
expression in gastric cancer and para-cancer tissues using
immunohistochemical detection and to investigate the association
between girdin expression and the clinicopathological
characteristics of gastric cancer, as well as the function of
girdin in gastric cancer development and progression.
Materials and methods
Clinical data collection
In this study, we used 10×16 gastric cancer tissue
microarrays (FM-S4006-1; Outdo Biotechnology, Shanghai, China) with
a diameter of 1.5 mm and a thickness of 4 μm that were
prepared according to a standard method. The integrity of each chip
was >95%. A total of 105 gastric cancer patients (67 men and 38
women) with a median age of 60 years (range, 30–84 years) were
included in this study. According to the tumor differentiation
grading system recommended by the World Health Organization in
2000, 99 cases were determined as poorly-differentiated
adenocarcinomas and 6 cases were determined as well-differentiated
adenocarcinomas. According to the tumor-node-metastasis cancer
staging system recommended by the International Union Against
Cancer in 2002, 13, 17, 79 and 7 cases were determined as stage I,
II, III and IV cancer, respectively.
Experimental reagents
The rabbit anti-human girdin monoclonal antibody and
corresponding secondary donkey anti-rabbit antibody were purchased
from Santa Cruz Biotechnology, Inc. (sc-22218 and 711-065-152;
Santa Cruz, CA, USA). The antibodies were preserved at −20°C and
the working concentration of the primary antibody was 4
μg/ml. The streptavidin-peroxidase (S-P) immunohistochemical
staining hypersensitivity kit was purchased from Dako Japan Inc.
(Kyoto, Japan).
Experimental methods
S-P immunohistochemical staining was used to detect
the expression level of girdin. Known positive tissue sections and
phosphate-buffered saline (instead of primary antibody) were used
as positive and negative controls, respectively. The staining was
performed according to the instructions provided by the
manufacturer. The staining intensity of the cells was graded as
follows: 0, negative; 1, light yellow granules; 2, deep yellow
granules; and 3, brown granules points. Grading was performed as
follows: 0, positive cell ratio ≤5%; 1, 5% < positive cell ratio
≤ 25%; 2, 25% < positive cell ratio ≤ 50%; 3, 50% < positive
cell ratio ≤ 75%; and 4, 75% < positive cell ratio ≤ 100%
points.
The final point score for girdin expression was
determined through the multiplication of the points for staining
intensity by the points for positive cell ratio. The girdin
expression levels were graded as follows: −, points ≤ 4; +, 4 <
points ≤ 8; ++, 8 < points ≤ 12; and +++, points > 12. Grade
- was determined as girdin-negative, whereas +, ++ and +++ were
determined as girdin-positive.
Statistical analyses
Data analyses were performed using SPSS software,
version 13.0 (SPSS Inc., Chicago, IL, USA). The χ2 test
was used to evaluate the girdin expression differences between
gastric cancer and para-cancer tissues and the association between
girdin expression and other clinicopathological parameters,
including gender, age, clinical stage, histological grade, lymph
node metastasis, tumor size and depth of invasion.
Results
Quality control of tissue microarray
Following immunohistochemical staining, tissue
samples with defects or poor staining were excluded, leaving a
total of 177 samples, including 105 gastric cancer and 72 gastric
para-cancer tissue samples.
Assessment of girdin expression
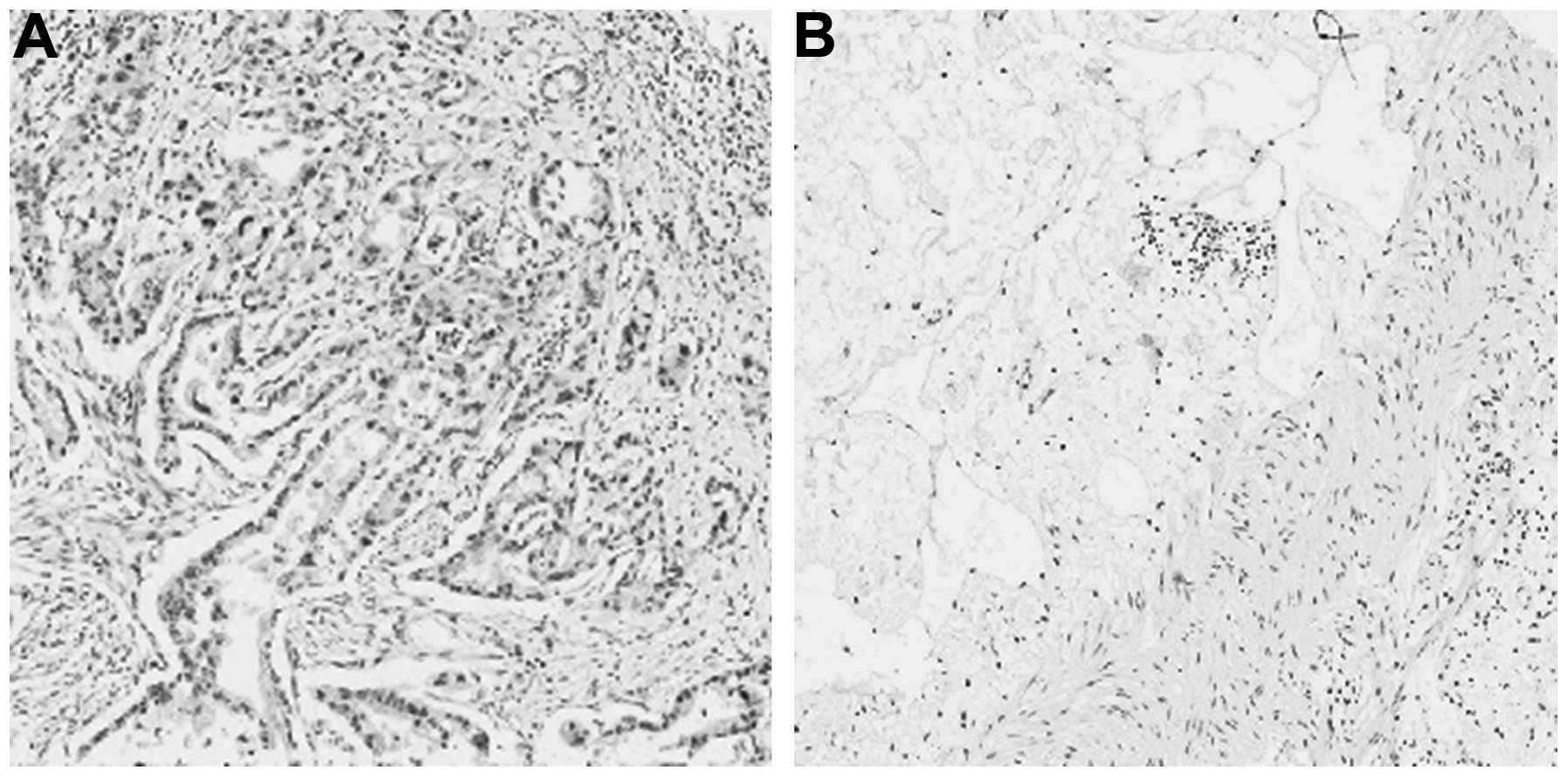
Of the 105 gastric cancer cases, 42 (44.4%)
exhibited positive girdin expression (mainly in the cytoplasm) and
72 (55.6%) exhibited negative girdin expression. Of the 72
para-cancer tissue samples, only 8 (11.1%) exhibited positive
girdin expression. The difference in girdin expression between
gastric cancer and para-cancer tissues was found to be
statistically significant (P<0.05). The results are shown in
Fig. 1 and Table I.
 | Table I.Girdin expression in gastric cancer
and para-cancer tissues. |
Table I.
Girdin expression in gastric cancer
and para-cancer tissues.
| Location | Cases | Girdin
expression | P-value |
|---|
|
|---|
| Positive | Negative | Expression ratio
(%) |
|---|
| Gastric cancer | 105 | 42 | 63 | 40.0 | <0.001 |
| Para-cancer
tissues | 72 | 8 | 64 | 11.1 | |
Association between girdin expression and
other clinicopathological parameters
There was no significant association between
girdin-positive expression and gender, age, tumor size, tumor
location, clinical stage and histological grade (P>0.05).
However, girdin expression exhibited a significant positive
correlation with tumor invasion depth and lymph node metastasis
(P<0.05). The results are presented in Table II.
 | Table II.Association between girdin expression
and other clinicopathological characteristics of gastric
cancer. |
Table II.
Association between girdin expression
and other clinicopathological characteristics of gastric
cancer.
| Clinicopathological
data | Cases | Girdin
expression | P-value |
|---|
|
|---|
| Positive | Negative | Expression ratio
(%) |
|---|
| Gender | | | | | 0.115 |
| Male | 67 | 23 | 44 | 34.3 | |
| Female | 38 | 19 | 19 | 50.0 | |
| Age (years) | | | | | 0.574 |
| ≤60 | 46 | 17 | 29 | 37.0 | |
| >60 | 59 | 25 | 34 | 42.4 | |
| Pathological
grading | | | | | 0.822 |
| Highly
differentiated (I, I–II) | 6 | 3 | 3 | 50.0 | |
| Moderately
differentiated (II, II–III) | 46 | 19 | 27 | 41.3 | |
| Poorly
differentiated (III, IV) | 53 | 20 | 33 | 37.7 | |
| Clinical staging | | | | | 1.000 |
| Stage I+II | 30 | 12 | 18 | 40.0 | |
| Stage III+IV | 75 | 30 | 45 | 40.0 | |
| Depth of
invasion | | | | | 0.048 |
| T1+T2 | 18 | 4 | 14 | 22.2 | |
| T3+T4 | 84 | 40 | 44 | 47.6 | |
| Lymph node
metastasis | | | | | 0.036 |
| No | 21 | 4 | 17 | 19.0 | |
| Yes | 84 | 37 | 47 | 44.0 | |
| Tumor size (cm) | | | | | 0.339 |
| <5 | 54 | 24 | 30 | 44.4 | |
| ≥5 | 51 | 18 | 33 | 35.3 | |
Discussion
Gastric cancer is one of the most pathogenic and
lethal malignancies in China. Epidemiological studies indicated
that the development of gastric cancer may be associated with
various factors, such as genetic, environmental and Helicobacter
pylori infection (10);
however, the molecular mechanism underlying gastric cancer
progression has not been fully elucidated. It was previously
suggested that the development and progression of gastric cancer is
a complicated process involving multiple genes, factors and stages
and that different tumor-associated genes or pathways participate
in the regulation of gastric cancer development (11). Girdin is an actin-binding protein
located at chromosome 2p16.1 and was first identified by a group of
Japanese scholars in 2005. This macromolecular protein contains
1,870 amino acid residues, has an approximate molecular mass of
220–250 kDa and interacts with Akt, Gαi/s, dynamin and guanosine
triphosphate hydrolase enzyme (GTPase) (2,3). It
was recently demonstrated that girdin is expressed by various tumor
cell lines and tissues and it may promote the formation of
malignant tumors (12). In a study
of 180 malignant tumor tissues, Jiang et al (13) detected high expression of girdin in
breast, colorectal, lung, cervical and thyroid cancer tissues. The
expression ratio of girdin varied between 10 and 50% among
different types of cancer, reaching 10–35% in invasive ductal
breast carcinoma tissues. Ghosh et al (14) demonstrated that girdin could only
be detected in colorectal cancer cells with high metastatic
ability, such as HCTll6 and DLDl cells and not in cells with low
metastatic ability (HT29p and Lsl74T). Kitamura et al
(5) suggested that endothelial
cell-derived tumors, such as hemangioma and angiosarcoma, exhibited
elevated girdin expression. Zhang et al (15) also found high levels of
Akt-mediated girdin phosphorylation in human malignant glioma
tissues.
Although several studies have been performed on
girdin expression in a variety of cancers, the number of studies on
girdin expression in gastric cancer tissues is currently limited.
Our study used S-P immunohistochemical staining to assess girdin
expression in gastric cancer and para-cancer tissues. The results
indicated that gastric cancer tissues expressed significantly
higher (P<0.05) levels of girdin compared to para-cancer tissues
and girdin expression was found to be positively correlated
(P<0.05) with the depth of tumor invasion and lymph node
metastasis, which are two significant prognostic indicators for
gastric cancer. We also demonstrated that girdin expression levels
in gastric cancer samples with different invasion depth were
statistically significantly different. The expression ratio of
girdin was 22.2% in gastric cancers with an invasion depth of T1
and T2 and it was significantly increased to 47.6% in cancers with
an invasion depth of T3 and T4. The expression ratio of girdin was
19.0% in gastric cancers without lymph node metastasis and was
significantly elevated to 44.0% in cancers with lymph node
metastasis. Thus, we hypothesized that increased girdin expression
may be an important event during gastric cancer progression.
In conclusion, girdin expression was found to be
positively associated with the depth of invasion and lymph node
metastasis. Therefore, girdin may be considered to be a novel
indicator in evaluating gastric cancer metastasis and prognosis, as
well as a candidate target in gastric cancer therapy. However,
elucidating the molecular mechanism underlying gastric cancer
induction by girdin requires further investigation.
Acknowledgements
This study was funded by grants from
the Natural Science Foundation of Jiangsu Province (no. BK2012481),
the Science and Technology Innovation Foundation of Nanjing Medical
University (no. 2011NJMU247) and the Technology Foundation for
Selected Overseas Chinese Scholars, Ministry of Personnel of
China.
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Enomoto A, Murakami H, Asai N, et al:
Akt/PKB regulates actin organization and cell motility via
Girdin/APE. Dev Cell. 9:389–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Enomoto A, Ping J and Takahashi M: Girdin,
a novel actin-binding protein, and its family of proteins possess
versatile functions in the Akt and Wnt signaling pathways. Ann NY
Acad Sci. 1086:169–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Weng L, Enomoto A, Ishida-Takagishi M,
Asai N and Takahashi M: Girding for migratory cues: roles of the
Akt substrate Girdin in cancer progression and angiogenesis. Cancer
Sci. 101:836–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kitamura T, Asai N, Enomoto A, et al:
Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate
Girdin. Nat Cell Biol. 10:329–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Enomoto A, Asai N, Namba T, et al: Roles
of disrupted-in-schizophrenia 1-interacting protein girdin in
postnatal development of the dentate gyrus. Neuron. 63:774–787.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Liu C, Zhang Y, Xu H, et al: Girdin
protein: a new potential distant metastasis predictor of breast
cancer. Med Oncol. 29:1554–1560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ling Y, Jiang P, Cui SP, et al: Clinical
implications for girdin protein expression in breast cancer. Cancer
Invest. 29:405–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Jun BY, Kim SW, Jung CK, et al: Expression
of girdin in human colorectal cancer and its association with tumor
progression. Dis Colon Rectum. 56:51–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Forte GI, Calà C, Scola L, et al: Role of
environmental and genetic factor interaction in age-related disease
development: the gastric cancerparadigm. Rejuvenation Res.
11:509–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Brivanlou AH and Darnell JE Jr: Signal
transduction and the control of gene expression. Science.
295:813–818. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ghosh P, Garcia-Marcos M and Farquhar MG:
GIV/Girdin is a rheostat that fine-tunes growth factor signals
during tumor progression. Cell Adh Migr. 5:237–248. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jiang P, Enomoto A, Jijiwa M, et al: An
actin-binding protein Girdin regulates the motility of breast
cancer cells. Cancer Res. 68:1310–1318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ghosh P, Garcia-Marcos M, Bornheimer SJ
and Farquhar MG: Activation of Galphai3 triggers cell migration via
regulation of GIV. J Cell Biol. 182:381–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhang B, Gu F, She C, et al: Reduction of
Akt2 inhibits migration and invasion of glioma cells. Int J Cancer.
125:585–595. 2009. View Article : Google Scholar : PubMed/NCBI
|