Introduction
Borderline resectable (BR) pancreatic adenocarcinoma
is an advanced disease and conventional resection has been proven
to be inadequate for improving patient prognosis. The criteria of
the resectability status are defined by the National Comprehensive
Cancer Network guidelines as tumor infiltration into nearby major
vessels (1). A combination of
vascular resection (VR) is required to achieve no microscopic
residual tumor (R0) resection for BR pancreatic head carcinoma
(PhC). The principle underlying our surgical strategy for
resectable (R) PhC is total excision of the lymphatic basin of the
pancreatic head, which is termed meso-pancreatoduodenum (meso-pd).
For BR PhC, additional venous and/or arterial resection may be
required for R0 resection. In the present study, 78 patients with
PhC were evaluated, including 65 patients who underwent VR and were
consecutively treated at our institute between 2002 and 2012, in
order to clarify the benefit of the en bloc VR technique for R0
resection of BR PhC.
Patients and methods
Diagnostic procedures and staging
The PhCs were classified as follows: R; BR-V, BR
involving the superior mesenteric vein (SMV) or portal vein (PV);
BR-SMA, BR involving the superior mesenteric artery; and BR-HA, BR
involving the hepatic artery. The classification was performed on
the basis of the extent of the cancer nest, which was determined by
multi-detector row computed tomography (MDCT). The extent of nerve
plexus (PLX) invasion was determined by either the coarse reticular
pattern or the mass and strand pattern connected to the main lesion
of the carcinoma (2). Abutment or
near abutment of the SMV/PV, SMA or HA by the cancer nest was
considered an indication for en bloc resection of these
vessels.
The resected specimens were serially sliced into
5-mm stepwise sections along the axial plane. The tumor stage and
grade were classified according to the 7th edition of the
tumor-node-metastasis classification system of the International
Union against Cancer (UICC) (3).
Tumor-node-metastasis staging was performed in accordance with the
UICC/American Joint Commission on Cancer staging system (4), which corresponds to the
histopathological reporting of pancreatic cancer of the Royal
College of Pathologists (5).
Margin positivity was defined as tumor clearance of <1 mm.
This retrospective study was approved by the
appropriate Institutional Review Board, and informed consent was
obtained from each patient.
Surgical procedures
The basic and standard protocol for the treatment of
PhC was total meso-pd resection, en bloc resection of the
pancreatic head and the lymphatic basin. The lower dissection limit
of the mesentery was above the third duodenal portion and the
posterior dissection plane included the anterior renal fascia. The
PLX surrounding the SMA was not included in the meso-pd. VR was
optional, depending on the extent of tumor infiltration. All the
SMV/PV resections were performed using the sleeve resection
technique. The preferred reconstruction technique following
segmental resection was primary end-to-end anastomosis; however,
interpositioning of the autologous venous graft from the external
iliac vein was completed to provide a tension-free anastomosis,
when necessary. Following venous confluence resection, the splenic
vein stump was closed and the inferior mesenteric vein was
preserved, if possible. SMA resection was performed in 21 cases,
from its origin until the infiltration-free portion (6). In the first 17 cases, we performed
interpositioning of the autologous venous graft of the saphenous
vein for reconstruction with a tension-free, end-to-end
anastomosis. For the following 4 cases, we performed a direct
anastomosis of the aorta inferior to the origin of the inferior
mesenteric artery, using an autologous venous graft of the
saphenous vein, via side-to-end anastomosis for the proximal site
and end-to-side anastomosis for the distal site. Prior to SMV/PV or
SMA resection and reconstruction, occlusion of the SMA was repeated
3 times to induce ischemic preconditioning in the mesentery. HA
resection was performed in 7 cases. End-to-end reconstruction was
performed in 5 cases to restore the arterial blood supply to the
liver, whereas in the remaining 2 cases it was unnecessary. An
autologous venous graft of the saphenous vein was used for
reconstruction in 1 case. Vascular reconstruction following SMA or
HA resection was performed using a 2-step method. Arterial
reconstruction and reperfusion were performed, followed by SMV/PV
reconstruction. The specimen was mobilized prior to VR, resulting
in en bloc resection that included the involved vessel as the last
step of the surgical procedure.
In-hospital parameters
The following patient parameters were routinely
assessed, included in an online prospective database and analyzed:
Perioperative morbidity, particularly surgical complications
(occurrence of postpancreatectomy hemorrhage; thrombosis of the PV,
SMV, SMA or HA in patients undergoing VR; abdominal or liver
abscess formation and duodenal ulcer) and perioperative mortality,
defined as in-hospital mortality or death within the first month
following discharge from the hospital.
Follow-up
The routine postoperative evaluation included a
regularly scheduled physical examination, measurement of
carcinoembryonic antigen and carbohydrate antigen 19-9 levels and
imaging studies with MDCT every 3 months.
Statistical analysis
The associations between categorical variables were
assessed using the Fisher's exact test or the χ2 test.
The Kaplan-Meier method was used to estimate survival probability
at 24 and 60 months after surgery. The differences between patient
groups with respect to survival were assessed using log-rank tests.
P<0.05 was considered to indicate a statistically significant
difference. SPSS software for Windows®, version 13 (SPSS
Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Procedures and perioperative patient
characteristics
The patient characteristics, surgical procedures and
perioperative outcomes of the entire study cohort are summarized in
Table I. Of the 78 patients who
underwent pancreatoduodenectomy for PhC, 20 patients had R PhC, 28
had BR-V PhC, 21 had BR-SMA PhC and 9 had BR-HA PhC. Of the 20
patients with R PhC, 10 underwent SMV/PV resection. Of the 28
patients with BR-V PhC, 25 underwent SMV/PV resection and 3
underwent synchronous resection of the SMA. In the BR-SMA group,
all 21 patients underwent SMV/PV resection, with synchronous
resection of the SMA in 17 patients. In the BR-HA PhC group, all 9
patients underwent SMV/PV resection, with 7 patients undergoing
synchronous resection of the HA and 1 patient undergoing resection
of the SMA. Total pancreatectomy was performed in the remaining 2
BR-HA PhC patients who exhibited extensive involvement of the
splenic artery beyond the bifurcation of the common hepatic and
splenic arteries.
 | Table I.Characteristics of the study
population (n=78). |
Table I.
Characteristics of the study
population (n=78).
| Characteristics | Resectable n=20 | BR-V n=28 | BR-SMA n=21 | BR-HA n=9 | P-value |
|---|
| Gender (M/F) | (13/7) | (14/14) | (15/6) | (7/2) | 0.316 |
| Age, years
(range) | 66 (52–77) | 64 (44–78) | 60 (38–78) | 65 (53–79) | 0.295 |
| Operative time, min
(range) | 648 (422–811) | 750 (528–1,015) | 850 (690–1,045) | 829 (580–1,110) | <0.001 |
| PPPD/PD | 6/14 | 6/22 | 4/17 | 1/8a | <0.001 |
| Vascular
resection | | | | | |
| SMV/PV | 10 | 25 | 21 | 9 | |
| SMA | 0 | 3 | 17 | 1 | |
| HA | 0 | 0 | 0 | 7 | |
| Blood loss, ml
(range) | 662 (115–1,840) | 883 (210–3,510) | 2768 (250–8,880) | 2981
(1,170–5,640) | <0.001 |
| Surgical morbidity
(major) | 4 (20%) | 3 (11%) | 8 (38%) | 3 (33%) | 0.126 |
| Hemorrhage | 0 | 2 | 3 | 1 | |
| Pancreatic fistula
(grade B,C) | 3 | 2 | 2 | 0 | |
| PV thrombosis | 0 | 0 | 1 | 0 | |
| Arterial
thrombosis | 0 | 0 | 0 | 0 | |
| Abdominal
abscess | 0 | 2 | 2 | 1 | |
| Liver abscess | 0 | 0 | 0 | 1 | |
| Duodenal ulcer | 0 | 0 | 1 | 0 | |
| Perioperative
mortality | 0 | 1 | 2 | 2 | 0.120 |
Intraoperative parameters, morbidity and
mortality
The operative time was significantly longer in
patients with BR-V, BR-SMA and BR-HA PhC, compared to that in
patients with R PhC (P<0.001) and the intraoperative blood loss
was significantly greater for BR-SMA and BR-HA PhC compared to that
for R and BR-V PhC (P<0.001). Overall, 6 patients experienced
postoperative hemorrhage. In the BR-V PhC group, postoperative
hemorrhage occurred in 2 patients, 1 due to failure of the
anastomosis of the SMA and the other due to rupture of the ligated
stump of the right gastric artery. Both hemorrhages were induced by
abdominal abscess without pancreatic fistula, and the latter was
fatal. In the BR-SMA PhC group, postoperative hemorrhage occurred
in 3 patients, 1 due to rupture of a pseudo-aneurysm induced by a
pancreatic fistula, 1 due to rupture of an old aortic aneurysm
induced by an abdominal abscess and 1 due to failure of the SMA
anastomosis induced by an abdominal abscess. The resulting
hemorrhage in the former 2 patients was fatal. In the BR-HA PhC
group, postoperative hemorrhage occurred at the HA anastomosis site
in 1 patient with severe arterial sclerosis. Although hemostasis
was achieved, the patient succumbed to rapid recurrence of liver
and lung metastases. Overall, there were 5 cases (6.4%) of
perioperative mortality, with 4 deaths due to postoperative
hemorrhage and 1 due to suffocation by failure of expectoration,
without pneumonia or asthma.
Histopathology
The histopathological results of the patients are
summarized in Table II. All the
patients had histopathologically confirmed pancreatic ductal
adenocarcinoma. The microscopic R0 rates were 85% (17/20), 82%
(23/28), 71% (15/21) and 33% (3/9) in the R, BR-V, BR-SMA and BR-HA
PhC groups, respectively. Vascular infiltration was defined as
tumor clearance of <1 mm. The histopathological analysis of the
BR-SMA or BR-HA PhC groups revealed evidence of SMA or HA
infiltration in 20 (95%) and 9 (100%) patients, respectively
(Table II).
 | Table II.Histopathology. |
Table II.
Histopathology.
| Tumor characteristics
and resectability | R (n=20) | BR-V (n=28) | BR-SMA (n=21) | BR-HA (n=9) | P-value |
|---|
| T stage | | | | | <0.001 |
| T1 | 3 | 2 | 0 | 0 | |
| T2 | 0 | 0 | 0 | 0 | |
| T3 | 17 | 25 | 11 | 2 | |
| T4 | 0 | 1 | 10 | 7 | |
| N stage | | | | | 0.069 |
| N0 | 9 | 6 | 6 | 0 | |
| N1 | 11 | 22 | 15 | 9 | |
| Grade | | | | | 0.651 |
| G1 | 6 | 5 | 4 | 1 | |
| G2 | 12 | 18 | 16 | 6 | |
| G3 | 2 | 5 | 1 | 2 | |
| Resectability
status (%) | | | | | 0.062 |
| R0 | 17 (85) | 23 (82) | 15 (71) | 3 (33) | |
| R1 | 2 (10) | 3 (11) | 2 (10) | 2 (22) | |
| R2 | 1 (5) | 2 (7) | 4 (19) | 4 (45) | |
| Vascular
infiltrationa | | | | | |
| SMV/PV | 2 | 17 | 19 | 9 | |
| SMA | 0 | 0 | 20 | 1 | |
| HA | 0 | 1 | 0 | 9 | |
Survival
The median survival time (MST) and the 5-year
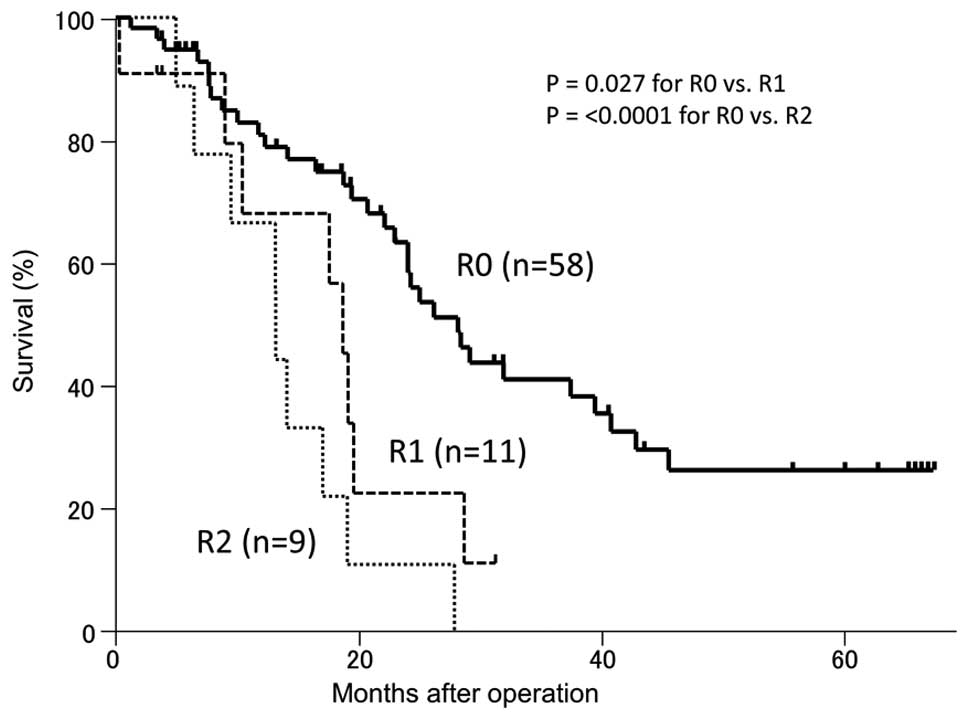
survival rate were 22 months and 26% for the R0 patients,
respectively (Fig. 1). No patients
with microscopic residual tumor (R1) or macroscopic residual tumor
(R2) remained alive at 3 years postoperatively. For the R0 cases,
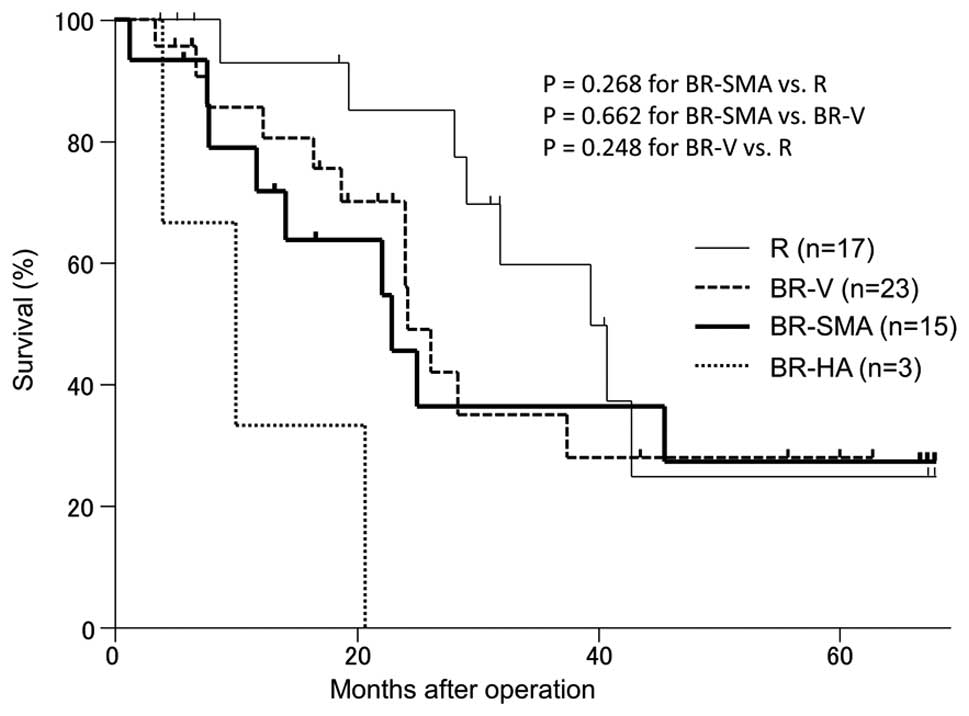
the MSTs and 5-year survival rates were 31 months and 25% for the R
PhC group, 22 months and 28% for the BR-V PhC group and 17 months
and 27% for the BR-SMA PhC group, respectively (Fig. 2), with no statistically significant
difference among these groups. Overall, 7 patients remained alive
at 5 years postoperatively (2 patients in the R PhC group, 2
patients in the BR-V PhC group and 3 patients in the BR-SMA PhC
group).
Discussion
Surgical resection is the only potentially curative
approach for the management of PhC. Our strategy for surgical
extirpation of PhC comprised total meso-pd resection, as a primary
lymphatic basin resection, and VR for R0 resection margins, when
necessary. In selected patients with arterial involvement, arterial
en bloc resection for PhC may result in an overall survival
comparable to that obtained with standard resection for R PhC and
improved compared to that obtained with palliative bypass for BR
PhC (7,8). In the present study, the prognoses of
the BR-V and BR-SMA PhC groups were comparable to that of the R PhC
group; however, the BR-HA PhC group had a significantly worse
prognosis. For BR-HA PhC, it was difficult to perform R0 resection
and hepatic recurrences developed within 1 year postoperatively in
6 of the 9 cases.
Achievement of an R0 resection margin status
following surgery is essential for the prolonged survival of
patients with PhC. Although the demarcation of the dissection line
for R0 resection using preoperative imaging is carefully performed,
local recurrence due to microscopically positive margins is common,
particularly at the SMA (4,9,10).
The involvement of the SMA in PhC is termed extrapancreatic PLX
invasion and is an indicator of poor prognosis (11–18).
The majority of PhCs are scirrhous and are characterized by a
fibrous stroma with scattered carcinoma cells. The normal PLX is
almost always composed of adipose tissue, with a low computed
tomography (CT) number, whereas PLX invasion is fibrous and imaged
by MDCT as a coarse reticular pattern or a mass and strand pattern
connecting to the main lesion of the carcinoma (2). The extent of the cancer nest is
assessed by the fibrous changes connected to the main tumor.
Histologically, these fibrous changes consist of desmoplastic
tissue with scattered carcinoma cells and have been described as
‘peritumoral inflammation’ or ‘mimicking tumor invasion’, according
to the low density of the carcinoma cells. To avoid an R1 resection
margin during curative surgery, the desmoplastic cancer nest should
be resected en bloc, with a macroscopic safety margin of 5 mm. The
extent of this safety margin remains controversial, but a
microscopic margin of >1 mm on histological examination is
recommended (19–25). As preoperative demarcation of the
dissection line is assessed by MDCT, which is a crucial decision
and must include an adequate safety margin macroscopically. At our
institution, VR was defined as abutment or near abutment of the
aforementioned vessels by the cancer nest. Therefore, careful
review of CT images is crucial in determining the extent of PLX
invasion. A window level and width of 40 and 350 HU, respectively,
are recommended.
The mesentery is a fan-shaped fold of the peritoneum
through which the blood vessels, lymph vessels and nerves of the
abdominal visceral organs pass. Therefore, the mesentery
corresponds to the initial field of infiltration of carcinoma
(26). Our ‘meso-pd’ concept
refers to the mesentery of the pancreatic head and the duodenum,
which is a firm and well-vascularized perineural lymphatic layer
located dorsal to the pancreas that reaches behind the mesenteric
vessels and has been described as the ‘mesopancreas’ (27). However, the term mesopancreas is
insufficient, as this mesentery is common to the pancreatic head
and the duodenum. Therefore, we considered the term ‘meso-pd’ to be
more descriptive of this mesentery. The meso-pd is fan-shaped and
its trunk is the inferior pancreatoduodenal artery, which is a
tributary of the SMA. The meso-pd is a counterpart of the mesocolon
and the mesentery, including the meso-pd, rotates between the 6th
and 12th week of the prenatal period. The envelope of fibrous
sheath or fascia enclosing the meso-pd is invisible (28), since the original fascia is fused
and lost during embryonal development. Therefore, a total meso-pd
resection was performed with respect to the PLX surrounding the SMA
and including the anterior renal fascia. The caudal border of the
meso-pd is the lower level of the third duodenal portion, where
tiny lymphatic emboli were observed (29).
We determined the manner of lymphatic extension and
PLX infiltration of the PhC depending on whether the tumor
originated from the embryonic dorsal or ventral pancreatic bud
(30,31). Tumors confined to the ventral
pancreas extend toward the SMA, whereas tumors confined to the
dorsal pancreas extend towards the common HA or hepatoduodenal
ligament. If the tumor infiltrates deeply into both areas, the
cancer is likely to extend in both directions. Therefore, the
meso-pd was considered to be the mesentery of the embryonic ventral
pancreas and total meso-pd resection would be essential for PhC
confined to the ventral pancreas. We developed an aggressive
surgical method termed ‘augmented regional pancreatoduodenectomy
(ARPD)’ in 2002 for the resection of the pancreatic head together
with the SMA and SMV/PV for cases of PhC (6). This procedure was performed in 21
patients: 3 with BR-V PhC, 17 with BR-SMA PhC and 1 with BR-HA PhC.
The 3 patients with BR-V and the patient with BR-HA were ‘nearly
BR-SMA cases’; therefore, ARPD was performed. ARPD has theoretical
advantages for en bloc and curative resection of carcinomas of the
ventral pancreas. By contrast, the mesentery corresponding to the
embryonic dorsal pancreas is currently unclear, although it is
associated with the HA. Survival following HA resection was poor in
our study and our procedure, which focuses on the meso-pd, was
shown to be insufficient for the treatment of carcinomas of the
dorsal pancreas.
Intraoperative blood loss during ARPD was higher in
patients with BR-SMA PhC compared to that in patients with R or
BR-V PhC; this difference was most likely due to the improvement in
the operative technique with increased experience, with an
estimated blood loss of 615±273 ml in the last 4 patients. All the
reported deaths occurred in patients who were operated on within
the first 3 years. Postoperative hemorrhage was fatal, particularly
when induced by a pancreatic fistula or intra-abdominal infection.
Failure of the arterial anastomosis occurred in 3 patients, with 1
patient successfully treated by arterial re-anastomosis. The
results of the present study indicate that the en bloc resection of
the meso-pd with major VR for R0 may be suitable for patients with
BR-V PhC and BR-SMA PhC, but not for those with BR-HA PhC.
References
|
1.
|
NCCN: Pancreatic adenocarcinoma
Version.1.2014. NCCN clinical practice guidelines in oncology,
2014. http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
Accessed January 3, 2013.
|
|
2.
|
Mochizuki K, Gabata T, Kozaka K, et al:
MDCT findings of extrapancreatic nerve plexus invasion by pancreas
head carcinoma: correlation with en bloc pathological specimens and
diagnostic accuracy. Eur Radiol. 20:1757–1767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sobin L, Gospodarowicz M and Wittekind C;
International Union against Cancer: TNM classification of malignant
tumours. 7th ed. West Sussex, UK: Wiley-Liss; 2009
|
|
4.
|
Edge SB BD, Compton CC, Fritz AG, Greene
FL and Trotti A; American Joint C ommittee on C ancer: AJCC cancer
staging manual. 7th ed. Springer-Verlag; New York, NY: 2010
|
|
5.
|
The Royal College of Pathologists:
Standards and Minimum Datasets for Reporting Cancers. Minimum
dataset for the histo-pathological reporting of pancreatic, ampulla
of Vater and bile duct carcinoma. The Royal College of
Pathologists; London, United Kingdom: 2002, http://www.mccn.nhs.uk/userfiles/documents/04i%20Pathology_pancreas_dataset280_2.pdf.
Accessed January 3, 2013.
|
|
6.
|
Miwa K, Ohta T, Shimizu K, et al:
Augmented regional pancreatoduodenectomy for pancreas head cancer;
combined resection of pancreas head and superior mesenteric artery
and vein. American College of Surgeons 90th Annual Clinical
Congress. 1902004.
|
|
7.
|
Bockhorn M, Burdelski C, Bogoevski D,
Sgourakis G, Yekebas EF and Izbicki JR: Arterial en bloc resection
for pancreatic carcinoma. Br J Surg. 98:86–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yekebas EF, Bogoevski D, Cataldegirmen G,
et al: En bloc vascular resection for locally advanced pancreatic
malignancies infiltrating major blood vessels: perioperative
outcome and long-term survival in 136 patients. Ann Surg.
247:300–309. 2008. View Article : Google Scholar
|
|
9.
|
Verbeke CS: Resection margins and R1 rates
in pancreatic cancer - are we there yet? Histopathology.
52:787–796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Campbell F, Foulis A and Verbeke CS:
Dataset for the histo-pathological reporting of carcinomas of the
pancreas, ampulla of Vater and common bile duct. The Royal College
of Pathologists; London, United Kingdom: 2010, https://www.rcpath.org/Resources/RCPath/Migrated%20Resources/Documents/D/datasethistopathologicalreportingcarcinomasmay10.pdf.
Accessed January 3, 2013.
|
|
11.
|
Kayahara M, Nagakawa T, Konishi I, Ueno K,
Ohta T and Miyazaki I: Clinicopathological study of pancreatic
carcinoma with particular reference to the invasion of the
extrapancreatic neural plexus. Int J Pancreatol. 10:105–111.
1991.PubMed/NCBI
|
|
12.
|
Kayahara M, Nagakawa T, Ueno K, Ohta T,
Tsukioka Y and Miyazaki I: Surgical strategy for carcinoma of the
pancreas head area based on clinicopathologic analysis of nodal
involvement and plexus invasion. Surgery. 117:616–623. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nagakawa T, Kayahara M, Ueno K, Ohta T,
Konishi I and Miyazaki I: Clinicopathological study on neural
invasion to the extrapancreatic nerve plexus in pancreatic cancer.
Hepatogastroenterology. 39:51–55. 1992.PubMed/NCBI
|
|
14.
|
Nagakawa T, Kayahara M, Ueno K, et al: A
clinicopathologic study on neural invasion in cancer of the
pancreatic head. Cancer. 69:930–935. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nagakawa T, Mori K, Nakano T, et al:
Perineural invasion of carcinoma of the pancreas and biliary tract.
Br J Surg. 80:619–621. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nakao A, Harada A, Nonami T, Kaneko T and
Takagi H: Clinical significance of carcinoma invasion of the
extrapancreatic nerve plexus in pancreatic cancer. Pancreas.
12:357–361. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Nakao A, Takeda S, Sakai M, et al:
Extended radical resection versus standard resection for pancreatic
cancer: the rationale for extended radical resection. Pancreas.
28:289–292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mitsunaga S, Hasebe T, Kinoshita T, et al:
Detail histologic analysis of nerve plexus invasion in invasive
ductal carcinoma of the pancreas and its prognostic impact. Am J
Surg Pathol. 31:1636–1644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Esposito I, Kleeff J, Bergmann F, et al:
Most pancreatic cancer resections are R1 resections. Ann Surg
Oncol. 15:1651–1660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Gaedcke J, Gunawan B, Grade M, et al: The
mesopancreas is the primary site for R1 resection in pancreatic
head cancer: relevance for clinical trials. Langenbecks Arch Surg.
395:451–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Campbell F, Smith RA, Whelan P, et al:
Classification of R1 resections for pancreatic cancer: the
prognostic relevance of tumour involvement within 1 mm of a
resection margin. Histopathology. 55:277–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Westgaard A, Tafjord S, Farstad IN, et al:
Resectable adenocarcinomas in the pancreatic head: the
retroperitoneal resection margin is an independent prognostic
factor. BMC Cancer. 8:52008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Konstantinidis IT, Warshaw AL, Allen JN,
et al: Pancreatic ductal adenocarcinoma: is there a survival
difference for R1 resections versus locally advanced unresectable
tumors? What is a ‘true’ R0 resection? Ann Surg. 257:731–736.
2013.PubMed/NCBI
|
|
24.
|
Frampton AE, Gall TM, Krell J, Ahmad R and
Jiao LR: Is there a ‘margin’ for error in pancreatic cancer
surgery? Future Oncol. 9:31–34. 2013.PubMed/NCBI
|
|
25.
|
Gnerlich JL, Luka SR, Deshpande AD, et al:
Microscopic margins and patterns of treatment failure in resected
pancreatic adenocarcinoma. Arch Surg. 147:753–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Cancrini A Jr, Mongardini M, Bellotti C,
et al: Mesorectal resection in oncologic surgery. G Chir. 10:51–54.
1989.(In Italian).
|
|
27.
|
Gockel I, Domeyer M, Wolloscheck T,
Konerding MA and Junginger T: Resection of the mesopancreas (RMP):
a new surgical classification of a known anatomical space. World J
Surg Oncol. 5:442007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Agrawal MK, Thakur DS, Somashekar U,
Chandrakar SK and Sharma D: Mesopancreas: myth or reality? JOP.
11:230–233. 2010.PubMed/NCBI
|
|
29.
|
Noto M, Miwa K, Kitagawa H, et al:
Pancreas head carcinoma: frequency of invasion to soft tissue
adherent to the superior mesenteric artery. Am J Surg Pathol.
29:1056–1061. 2005.PubMed/NCBI
|
|
30.
|
Kitagawa H, Ohta T, Makino I, et al:
Carcinomas of the ventral and dorsal pancreas exhibit different
patterns of lymphatic spread. Front Biosci. 13:2728–2735. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Makino I, Kitagawa H, Ohta T, et al: Nerve
plexus invasion in pancreatic cancer: spread patterns on
histopathologic and embryological analyses. Pancreas. 37:358–365.
2008. View Article : Google Scholar : PubMed/NCBI
|