Introduction
A number of randomized trials with a prolonged
follow-up reported that breast-conserving surgery (BCS) and
modified radical mastectomy (MRM) have comparable overall survival
(OS) rates (1,2). The standard treatment for operable
breast cancer is currently BCS followed by postoperative radiation
therapy (XRT) (1). Several
previous randomized trials demonstrated that adjuvant chemotherapy
improves disease-free survival (DFS) and OS in axillary
node-positive (N+) high-risk breast cancer patients (3). In the adjuvant setting, it has been
observed an advantage of anthracycline-based over
non-anthracycline-based chemotherapeutic regimens for N+ high-risk
breast cancer (4). However, XRT
administered concomitantly with anthracycline-based chemotherapy
may be associated with excessive cardiac toxicity (1,5). It
was previously suggested that the local recurrence rate may be
higher when the initiation of XRT is delayed (6). Although the administration of
adjuvant chemotherapy prior to XRT has become common practice for
patients with early-stage breast cancer who undergo conservative
surgery, the optimal integration of chemotherapy and XRT remains
controversial. To avoid delaying the administration of XRT
following administration of adriamycin and docetaxel, we sought to
deliver cyclophosphamide, methotrexate and 5-fluorouracil (CMF)
chemotherapy concomitantly with XRT. In high-risk breast cancer,
the concurrent administration of both treatments may be feasible by
selectively using drugs that do not have cumulative toxic effects
with XRT. Such a scheme may have the advantage of exerting
synergistic effects on tumor response and shortening the delay in
initiating XRT, with obvious survival benefits. A randomized phase
III trial of patients with N+ breast cancer demonstrated that the
5-year locoregional relapse-free survival decreased by 39% in the
concurrent chemotherapy-XRT arm compared to the sequential arm
(7). In addition, the mortality
risk associated with XRT is minor compared to its survival benefit.
In the latest Oxford Overview, XRT following mastectomy and
axillary node dissection provided an 11.4% absolute gain in the
10-year recurrence-free survival and a 9.4% gain in the 15-year
breast cancer mortality in patients with 1–3 positive nodes
(8). Based on those findings, a
phase II trial was designed in order to investigate sequential
adriamycin, docetaxel and CMF concurrent with XRT in patients who
undergo surgical resection. The schedule was personalized for
patients with the highest risk of relapse, such as women with >5
positive axillary nodes and women with triple-negative disease
(9). Those patients received a
dose-dense chemotherapeutic regimen including carboplatin,
ifosfamide and etoposide, which was previously shown to be
efficient at both the standard (10) and high doses (11) in breast cancer patients previously
treated with anthracyclines.
Patients and methods
Eligibility
This multicenter, phase II, single-arm study
included premenopausal and postmenopausal breast cancer patients,
aged 24–70 years, who were treated with MRM or BCS and axillary
node dissection. All the subjects had high-risk, invasive pT2-pT3a
breast cancer with >1 positive axillary lymph nodes and no
distant metastasis. Continuing ovarian function was biochemically
confirmed by the following laboratory data: follicle-stimulating
hormone <10 mU/ml; luteinizing hormone (LH) <0.8 mU/ml;
17β-estradiol (E2), 20–693 pg/ml; and progesterone, 0.15–28 ng/ml.
Other eligibility criteria included an Eastern Cooperative Oncology
Group performance status of 0–1 and adequate baseline bone marrow
function (absolute neutrophil count >1,500/ml, platelet count
>100,000 cells/ml), hepatic function [serum bilirubin <2.0
mg/dl, transaminase (aspartate aminotransferase, alanine
aminotransferase) levels <5x the upper limit of institutional
norm] and renal function (creatinine <1.4 mg/dl). Patients with
histotypes characterized by a more indolent behavior (papillary,
medullary or mucinous), metastases, or malignancies other than
curatively treated skin and cervical cancer were excluded. This
phase II study was performed in accordance with the Declaration of
Helsinki and the European Union Guidelines on Good Clinical
Practice and was approved by the Ethics Committees of the
participating institutions. Written informed consent was obtained
from each patient. Immediately after surgery and throughout the 5
years covered by the study, the patients received psychological
support to improve treatment compliance.
Surgical and medical treatment
The patients underwent a surgical procedure
consisting of either MRM or BCS. Patients with N+ disease were
treated as follows: starting 2 weeks after surgery, premenopausal
patients received an LH-releasing hormone (LH-RH) analogue (11.25
mg) by deep intramuscular injection every 12 weeks for 5 years
(12) and chemotherapy was
initiated 2 weeks after the LH-RH analogue. The initiation of
cytotoxic therapy was delayed for 3 weeks after surgery in
postmenopausal patients and consisted of 4 courses of adriamycin
(50 mg/m2) and docetaxel (75 mg/m2) every 3
weeks. Concurrently with XRT, 6 courses of CMF (600
mg/m2 cyclophosphamide, 600 mg/m2
5-fluorouracil and 60 mg/m2 methotrexate) were
administered every 3 weeks. XRT was administered to all the
patients at a total dose of 5,000 cGy (200 cGy/fraction, 5
fractions/week) to the chest wall following mastectomy or to the
residual breast tissue following BCS. Irradiation (5,000 cGy in 200
cGy/fraction, 5 fractions/week) of the axillary (II and III levels)
and supraclavicular lymph nodes was performed in patients with
>4 histologically confirmed axillary lymph node metastases. A
boost of 1,000 cGy was delivered to the tumor bed. One month after
completion of the chemoradiation therapy, patients with >5
histologically confirmed axillary lymph node metastases (n=30) and
patients with triple-negative disease (n=38) received 2 courses of
dose-dense chemotherapy with ifosfamide (5 g/m2),
carboplatin (area under the concentration-time curve=7) and
etoposide (300 mg/m2), supported by 300 μg/day of
glycosylated recombinant granulocyte colony-stimulating factor,
without peripheral blood progenitor cell support (11,13).
Estrogen receptor-positive (ER+) patients received an aromatase
inhibitor for 5 years.
Statistical analysis
The number of patients required for the study was
calculated according to Simon’s two-stage design (14). The first stage required that ≥80 of
128 patients exhibited an improvement from 60% (P0) to 70% (P1) in
the expected 5-year DFS of N+ breast cancer (P1), with a 5%
one-sided type I error (α) and with a 90% statistical power (1-β).
In the second stage, a total of 200 assessable patients could be
added, if ≥130 patients exhibited an improvement in DFS. All the
analyses were performed on an intent-to-treat basis. DFS was
defined as the time from the initiation of adjuvant chemotherapy
until objective disease progression or death. The OS was calculated
from the initiation of adjuvant chemotherapy until death from any
cause. The DFS and OS were calculated using the Kaplan-Meier life
table method (15). The survival
curves for patient subgroups were compared using the log-rank test
(16). Data analyses were
performed on May 31, 2013. An adjusted P<0.05 was considered to
indicate a statistically significant difference. All the tests were
two-sided unless otherwise specified. All the statistical analyses
were performed using the SPSS® statistical analysis
software, version 10.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 200 women were recruited between March,
1998 and August, 2010. The median age was 55 years (range, 24–70
years) and 52% (n=103) of the patients were premenopausal. Stage II
and III breast cancer was diagnosed in 63 and 37% of patients,
respectively. In total, 76% of patients were treated with BCS,
whereas 24% underwent MRM. The median number of recovered positive
axillary lymph nodes was 4.4 (range, 2–37). The majority (74%) of
the patients were ER+ and 26% were estrogen receptor-negative
(ER−). The median Ki-67 index was 30% (range, 10–100%). Of the 41
patients with human epidermal growth factor receptor 2
(HER2)-positive tumors, 28 were treated with trastuzumab after
2006; the remaining 13 patients did not receive trastuzumab, as
their treatment occurred prior to its approval as adjuvant therapy.
The mean baseline characteristics of the patients are summarized in
Table I. All the patients were
included in the study according to the intent-to-treat
principle.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | No. | Percentage |
|---|
| No. of patients | 200 | 100.0 |
| Age, years | | |
| Median | 55 | |
| Range | 24–70 | |
| ECOG performance
status | | |
| 0 | 180 | 90.0 |
| 1 | 20 | 10.0 |
| Grading | | |
| G1 and
mucinous | 16 | 8.0 |
| G2 | 106 | 53.0 |
| G3 | 78 | 39.0 |
| Hormone receptor
status | | |
| ER+ | 148 | 74.0 |
| ER− | 52 | 26.0 |
| HER status
(FISH) | | |
| Positive | 41 | 20.5 |
| Negative | 159 | 79.5 |
| Ki-67 index, % | | |
| Median | 30 | |
| Range | 10–100 | |
| Clinical stage | | |
| IIA | 94 | 47.0 |
| IIB | 32 | 16.0 |
| IIIA | 30 | 15.0 |
| IIIB | 14 | 7.0 |
| IIIC | 30 | 15.0 |
| Type of primary
surgery | | |
| Mastectomy | 47 | 23.5 |
| Quadrantectomy | 153 | 76.5 |
| Menstrual status | | |
| Premenopausal | 103 | 51.5 |
| Postmenopausal | 97 | 48.5 |
Treatment efficacy
After a median follow-up of 73 months (range, 38–180
months), all the patients were evaluable for toxicity and efficacy.
The median DFS and OS had not yet been reached. In total, 42 (21%)
subjects experienced disease progression, corresponding to a
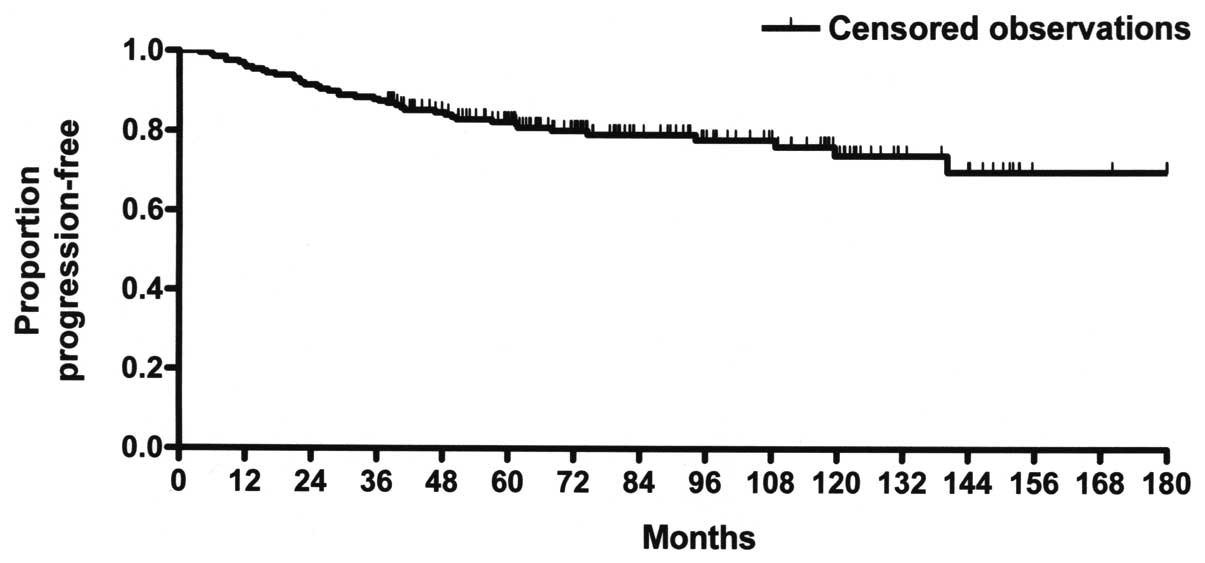
10-year DFS rate of 73.8% (Fig.
1). The corresponding 5- and 15-year DFS rates were 82.3 and
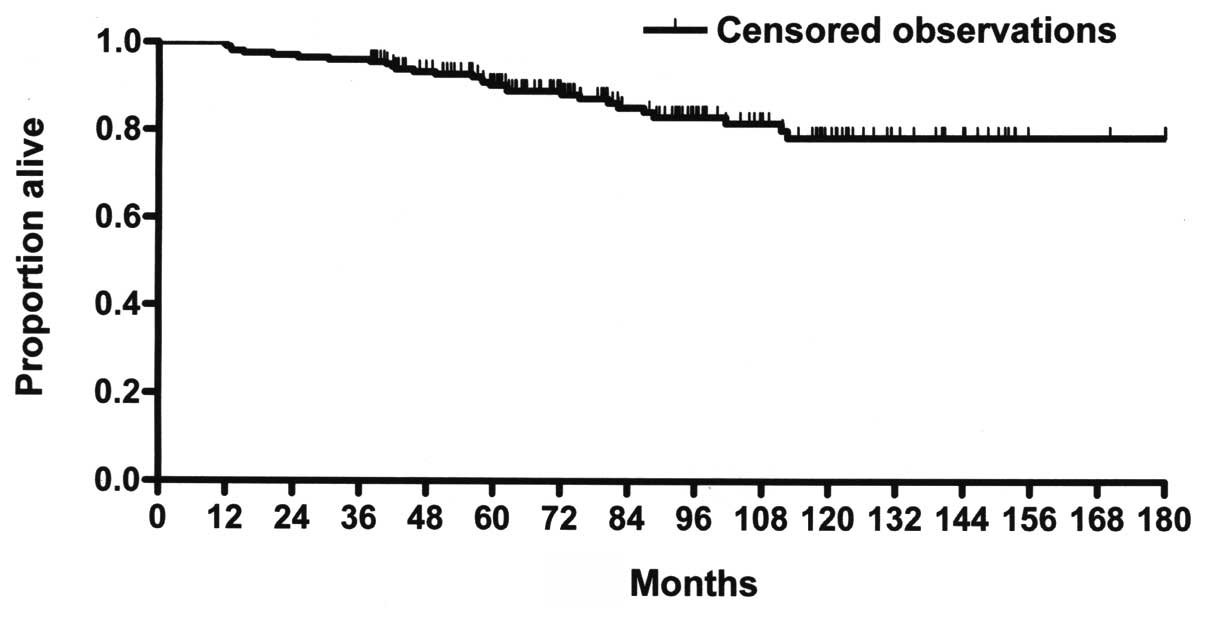
69.5%, respectively. There were a total of 29 deaths (14.5%),
corresponding to a 10-year OS rate of 77.3% (Fig. 2). The parallel 5- and 15-year OS
rates were 92.2 and 77.3%, respectively. There were no significant
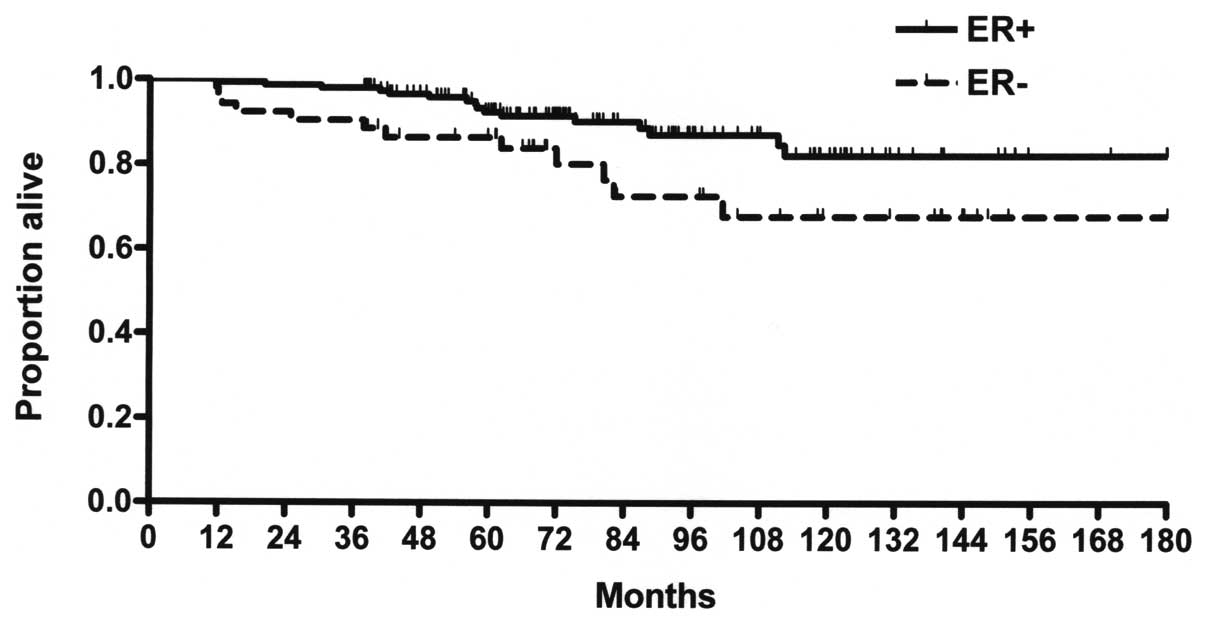
differences between the DFS of ER+ (80.7) and ER− (62.8%) patients
(P=0.088); however, the difference in OS between ER+ (80.7) and ER−
(67.6%) patients was significant (P<0.005; Fig. 3). The 5-year DFS rate was 88.3% in
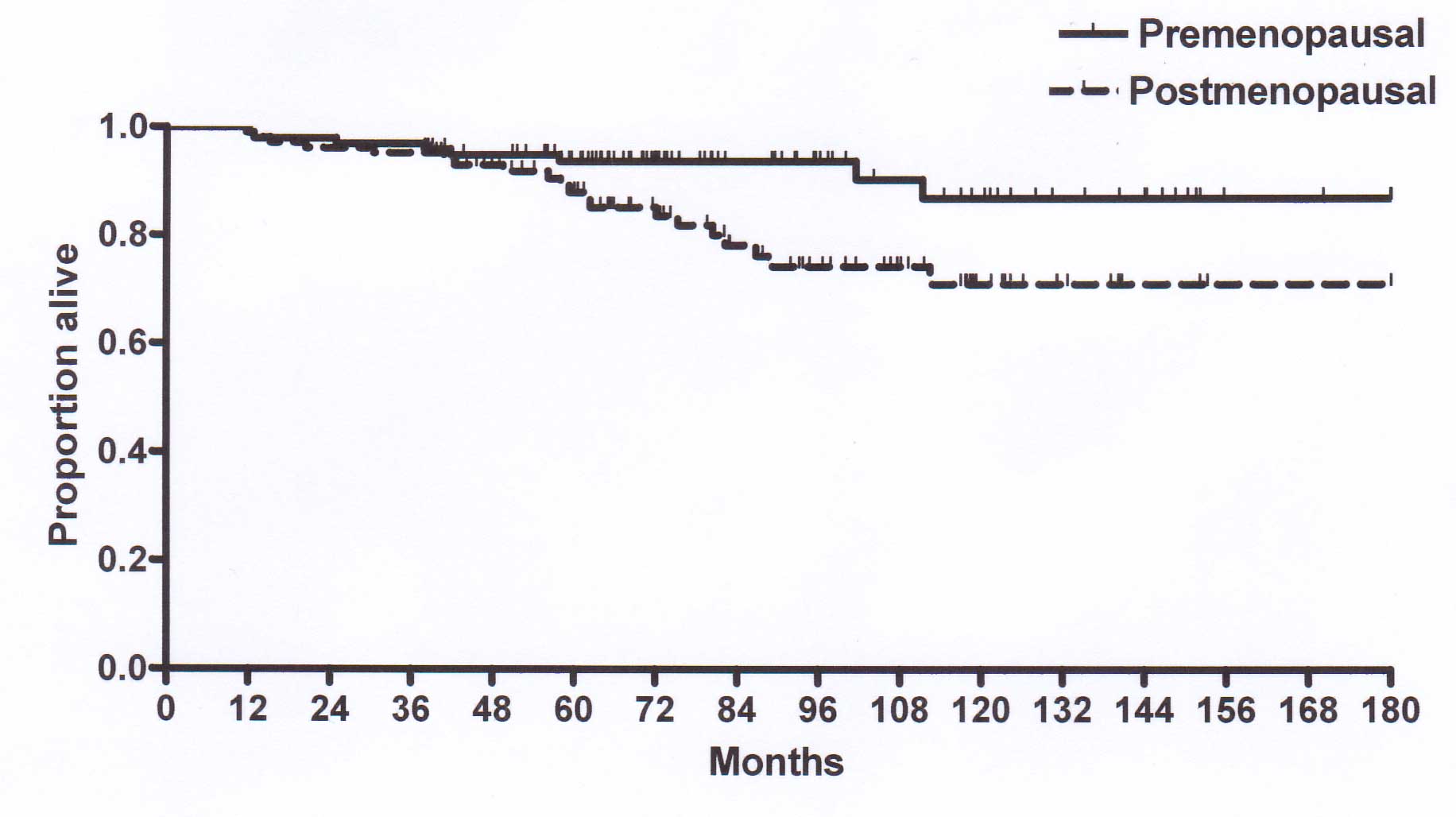
premenopausal and 75% in postmenopausal patients and the
corresponding 15-year DFS rate was 80.8% in premenopausal and 54.5%
in postmenopausal patients (P<0.05). The 15-year OS rate was
also improved in premenopausal (86.7) compared to that in
postmenopausal patients (70.8%, P<0.05; Fig. 4). There were significant
differences in the DFS (P<0.0001) and the OS (P<0.0001)
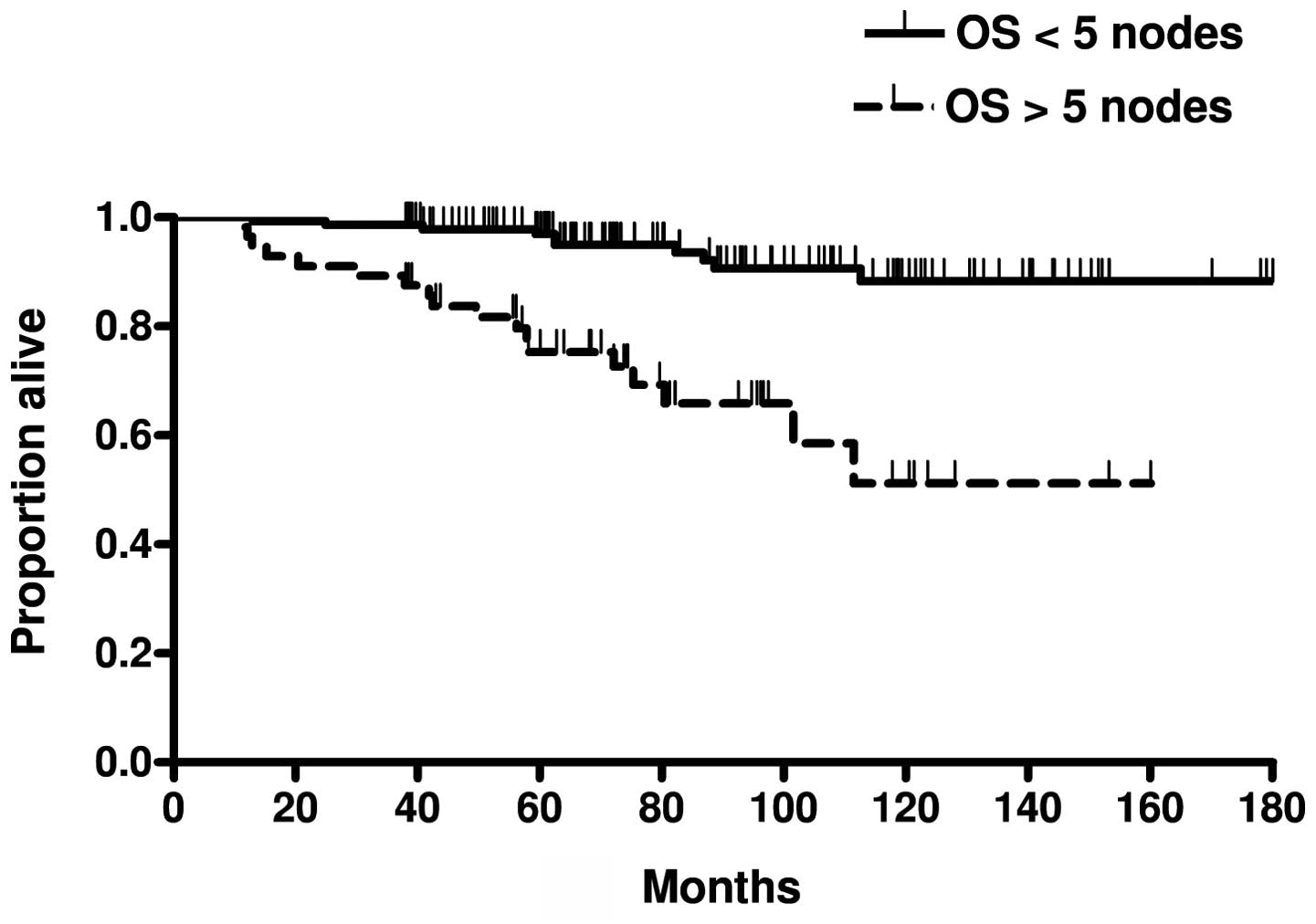
(Fig. 5) between patients with
>5 and those with <5 histologically-confirmed axillary lymph
node metastases. A total of 6 patients suffered from recurrence (3
ER+ and 3 ER− patients) after a median time of 31.3 months. Tumor
recurrence occurred locoregionally in 3 cases, in the brain and
lung in 2 cases and in osseous tissue in 1 case. A total of 3
patients with recurrence had >10 positive axillary nodes and the
remaining 3 were HER2-positive and were not treated with
trastuzumab.
Toxicity
The adverse events are summarized in Table II. Toxicity grading was performed
according to the National Cancer Institute-Common Terminology
Criteria for Adverse Events, version 3.0. The delivered dose
intensities were as follows: 95% for docetaxel and adriamycin, 98%
for CMF and 98% for dose-dense chemotherapy. In total, 90% of the
patients treated with LH-RH analogues experienced hot flashes, mood
modification and vaginal dryness. The bone mineral density was
assessed at baseline and annually thereafter. The median T-score
was −1.3 (range, −2 to 1.5) at baseline and −2.0 (range, −4 to
−1.2) in the fifth year of LH-RH analogue treatment. Arthralgias
and muscle weakness were common but did not interfere with daily
activities. Upon anthracycline chemotherapy, grade 3–4
hematological toxicity was observed in 64 patients (32%), whereas
thrombocytopenia and anemia were each observed in 18% of the
patients. Alopecia was universal. Only 6 patients (3%) required
discontinuation of concomitant XRT and CMF for 1 week due to
leukopenia, whereas gastrointestinal toxicity and mucositis were
observed in 13 and 8% of the patients, respectively. In dose-dense
chemotherapy, nausea and vomiting were observed in 6 cases;
however, they were mild and manageable with ondansetron and
dexamethasone. Grade 4 leukopenia and thrombocytopenia were
observed in all 68 patients undergoing dose-dense chemotherapy. The
absolute neutrophil count was <5×103/ml for a median
of 4.5 days (range, 3–5 days) and the platelet count was
<50×103/ml for a median of 1 day (range, 0–3 days).
No patients required platelet transfusion. There was no reported
cardiac toxicity or significant reduction of the left ventricular
ejection fraction. Anemia was infrequent due to the use of
erythropoietin and occurred in only 4 patients (18%). A total of 3
patients developed a fever >38°C for a median duration of 3 days
(range, 0–6 days). Grade 2 mucositis and grade 3 diarrhea occurred
in 4 and 3 patients, respectively. One patient had a documented
infection, with a positive blood culture for Staphylococcus
epidermidis. A total of 2 patients suffered from bone pain,
with a median duration of 2 days. There were no reported
treatment-related deaths.
 | Table II.Treatment toxicities. |
Table II.
Treatment toxicities.
| Adverse events | Type of treatment
|
|---|
| LH-RH analogue (n=96)
n (%) | Anthracycline-taxanes
(n=200) n (%) | CMF+XRT (n=200) n
(%) | DD-CT (n=68) n
(%) |
|---|
| Hematological | | | | |
| Leukopenia | 0 (0) | 64 (32) | 6 (3) | 68 (100) |
|
Thrombocytopenia | 0 (0) | 18 (9) | 0 (0) | 68 (100) |
| Anemia | 0 (0) | 18 (9) | 0 (0) | 12 (18) |
| Gastrointestinal | | | | |
|
Nausea-vomiting | 0 (0) | 38 (19) | 26 (13) | 18 (27) |
| Diarrhea | 0 (0) | 22 (11) | 6 (3) | 9 (13) |
| Mucositis | 0 (0) | 22 (11) | 16 (8) | 0 (0) |
| Infection | 0 (0) | 4 (2) | 0 (0) | 9 (13) |
| Neurotoxicity
(grade 2) | 0 (0) | 30 (15) | 0 (0) | 0 (0) |
| Alopecia | 0 (0) | 200 (100) | 0 (0) | 68 (100) |
| Hot flashes | 86 (90) | 0 (0) | 0 (0) | 0 (0) |
Discussion
Advances in medical oncology, including several
approaches to adjuvant treatment, have improved the prognosis of
breast cancer patients. In particular, the integration of surgery
with different treatments, such as chemotherapy and XRT, has
decreased local and systemic recurrence.
The role of concurrent chemotherapy and XRT has been
well established in the treatment of several types of tumors,
including cancer of the esophagus, stomach, pancreas, head and neck
and lungs; however, its role in the treatment of breast cancer
remains unclear and the optimal sequence of chemotherapy and XRT
has not yet been determined. Toledano et al (7) reported a significant reduction of 39%
in the risk of locoregional recurrence in N+ breast cancer with the
administration of concurrent XRT and chemotherapy, with respect to
their sequential administration. Another important issue is the
delay in initiating XRT following chemotherapy. It was reported
that delaying the initiation of intact breast irradiation for
patients with N+ breast cancer increases the risk of local
recurrence (17,18).
A recently published Cochrane Database Review
indicated that different methods of sequencing chemotherapy and XRT
do not appear to exert a major effect on recurrence or survival in
women with breast cancer, provided that XRT is initiated within 7
months of surgery (19). However,
∼10 months are likely to elapse between surgery and XRT if
anthracycline-taxane-based therapy followed by 6 courses of CMF is
administered.
In high-risk breast cancer with the involvement of
≥4 axillary nodes, intense dose-dense chemotherapy may be superior
to conventional chemotherapy (20). A previous study reported that,
after a median follow-up of 62 months, the 5-year event-free
survival rates were 62% in the conventional and 70% in the
dose-dense chemotherapy arms, representing a 28% reduction in the
relative risk of relapse (P<0.001) (20). This benefit was independent of the
menopausal, hormone receptor or HER2 status. In addition, the
5-year OS rates were 77 vs. 82%, representing a 24% reduction in
the relative mortality risk (P=0.0285).
In the present study, the adjuvant therapy was very
successful in patients with 1–5 positive axillary lymph nodes. As
regards high-risk patients with >5 positive axillary nodes who
were treated with two platinum-based dose-dense regimens, a
significant difference was observed in the DFS and OS between
patients with >5 and those with <5 positive axillary nodes.
These results indicate that researchers should seek new methods of
improving the results of adjuvant therapy in this group of patients
with high-risk breast cancer.
In our non-randomized multicenter phase II study,
the patients were accrued consecutively for 12 years, with no
selection bias. Only N+ patients were accrued. In contrast to other
studies, our protocol planned the administration of LH-RH analogues
concurrently with chemotherapy and XRT in premenopausal patients
(12). Particularly good results
were obtained in the 103 premenopausal patients. Prior to
chemotherapy initiation, these patients were treated with an LH-RH
analogue, which was continued for 5 years (12). With this approach, we used the two
most significant modalities in the treatment of premenopausal
patients with breast cancer, total estrogen blockade and
chemotherapy (21). High blood
estrogen levels are a relevant risk factor for the development of
breast cancer. The Nurses’ Health Study II demonstrated that the
highest follicular total and free E2 levels are associated with a
significantly increased risk of breast cancer (22).
In conclusion, we demonstrated that XRT administered
concomitantly with CMF chemotherapy is a feasible therapeutic
option, is not associated with excessive toxicity and allows XRT to
be initiated within 7 months of surgery (17).
References
|
1.
|
Veronesi U, Cascinelli N, Mariani L, Greco
M, Saccozzi R, Luini A, Aguilar M and Marubini E: Twenty-year
follow-up of a randomized study comparing breast-conserving surgery
with radical mastectomy for early breast cancer. N Engl J Med.
347:1227–1232. 2002.PubMed/NCBI
|
|
2.
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241.
2002.PubMed/NCBI
|
|
3.
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomised trials. Lancet. 365:1687–1717.
2005.PubMed/NCBI
|
|
4.
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG); Peto R, Davies C, Godwin J, et al:
Comparisons between different polychemotherapy regimens for early
breast cancer: meta-analyses of long-term outcome among 100,000
women in 123 randomised trials. Lancet. 379:432–444. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Darby SC, Ewertz M, McGale P, et al: Risk
of ischemic heart disease in women after radiotherapy for breast
cancer. N Engl J Med. 368:987–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Recht A, Come SE, Gelman RS, et al:
Integration of conservative surgery, radiotherapy, and chemotherapy
for the treatment of early-stage, node-positive breast cancer:
sequencing, timing, and outcome. J Clin Oncol. 9:1662–1667.
1991.PubMed/NCBI
|
|
7.
|
Toledano A, Azria D, Garaud P, Fourquet A,
Serin D, Bosset JF, Miny-Buffet J, Favre A, Le Floch O and Calais
G: Phase III trial of concurrent or sequential adjuvant
chemoradiotherapy after conservative surgery for early-stage breast
cancer: final results of the ARCOSEIN trial. J Clin Oncol.
25:405–410. 2007. View Article : Google Scholar
|
|
8.
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG); Darby S, McGale P, Correa C, et al:
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 378:1707–1716. 2011.
|
|
9.
|
Rodenhuis S, Bontenbal M, Beex LV, et al:
High-dose chemotherapy with hematopoietic stem-cell rescue for
high-risk breast cancer. N Engl J Med. 349:7–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Recchia F, Nuzzo A, Lalli A, De Filippis S
and Torchio P: Activity of standard-dose carboplatin,
cyclophosphamide, and etoposide in patients with metastatic breast
cancer with previous exposure to anthracyclines. Am J Clin Oncol.
20:166–168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Recchia F, De Fillipis S, Piccinini M and
Rea S: High-dose carboplatin, cyclophosphamide, etoposide with
hematological growth factors, without stem cell support in patients
with advanced cancer. Anticancer Res. 23:4141–4147. 2003.PubMed/NCBI
|
|
12.
|
Recchia F, Saggio G, Amiconi G, Di Blasio
A, Cesta A, Candeloro G and Rea S: Gonadotropin-releasing hormone
analogues added to adjuvant chemotherapy protect ovarian function
and improve clinical outcomes in young women with early breast
carcinoma. Cancer. 106:514–523. 2006. View Article : Google Scholar
|
|
13.
|
Recchia F, Candeloro G, Desideri G,
Necozione S, Recchia CO, Cirulli V and Rea S: Triple-negative
breast cancer: multipronged approach, single-arm pilot phase II
study. Cancer Med. 1:89–95. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Simon R: Confidence intervals for
reporting results of clinical trials. Ann Intern Med. 105:429–435.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kaplan EL and Meyer P: Nonparametric
estimation from incomplete observation. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
16.
|
Cox DR: Regression models and Life-Tables.
J R Stat Soc. 34:187–220. 1972.
|
|
17.
|
Hartsell WF, Recine DC, Griem KL and
Murthy AK: Delaying the initiation of intact breast irradiation for
patients with lymph node positive breast cancer increases the risk
of local recurrence. Cancer. 76:2497–2503. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Buchholz TA, Austin-Seymour MM, Moe RE,
Ellis GK, Livingston RB, Pelton JG and Griffin TW: Effect of delay
in radiation in the combined modality treatment of breast cancer.
Int J Radiat Oncol Biol Phys. 26:23–35. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hickey BE, Francis DP and Lehman M:
Sequencing of chemotherapy and radiotherapy for early breast
cancer. Cochrane Database Syst Rev. 4: View Article : Google Scholar
|
|
20.
|
Moebus V, Jackisch C, Lueck HJ, et al:
Intense dose-dense sequential chemotherapy with epirubicin,
paclitaxel, and cyclophosphamide compared with conventionally
scheduled chemotherapy in high-risk primary breast cancer: mature
results of an AGO phase III study. J Clin Oncol. 28:2874–2880.
2010. View Article : Google Scholar
|
|
21.
|
Recchia F, Candeloro G, Necozione S,
Desideri G, Cesta A, Recchia L and Rea S: Vascular endothelial
growth factor expression and T-regulatory cells in premenopausal
breast cancer. Oncol Lett. 5:1117–1122. 2013.PubMed/NCBI
|
|
22.
|
Eliassen AH, Missmer SA, Tworoger SS,
Spiegelman D, Barbieri RL, Dowsett M and Hankinson SE: Endogenous
steroid hormone concentrations and risk of breast cancer among
premenopausal women. J Natl Cancer Inst. 98:1406–1415. 2006.
View Article : Google Scholar : PubMed/NCBI
|