Introduction
Alopecia areata (AA) has been classically associated
with several autoimmune disorders (1). Along with follicular mucinosis, AA
has also been historically documented in panniculitis-like T-cell
lymphoma, primary cutaneous follicle center lymphoma, cutaneous
T-cell lymphomas, including mycosis fungoides and Sézary syndrome,
as well as other non-Hodgkin’s lymphomas (NHLs) (2–5). AA,
however, as a paraneoplastic syndrome of Hodgkin’s lymphoma (HL),
remains limited to a few case studies, although its occurrence in
HL was anecdotally referenced in the early 1900s (6–9). The
etiology of AA as a paraneoplastic syndrome of HL remains unknown,
although it was considered to be a T-lymphocyte-mediated phenomenon
involving production of cytokines, initiation of the inflammatory
cascade and genetic predisposition (1,4,8,10).
Case report
A 46-year-old male with a history of hypertension
presented to the outpatient clinic with complaints of patchy hair
loss on his mustache and beard for 5 months. His review of systems
was otherwise unremarkable. The physical examination revealed at
least 8 prominent regions of patchy, non-tender, non-erythematous
hair loss, without scarring of the underlying skin, in the
patient’s beard and mustache area, with the largest lesion located
at mid-chin. The patient was treated for AA with a low-potency
topical corticosteroid and subsequent intralesional injection with
10 mg triamcinolone acetonide, with eventual improvement.
Approximately 8 months after the initial diagnosis of AA, the
patient presented to the emergency room with intermittent fevers,
night sweats, weight loss and abdominal pain. The assessment of the
vital signs revealed a temperature of 101.5°F and a heart rate of
122 bpm. On physical examination there was significant tachycardia,
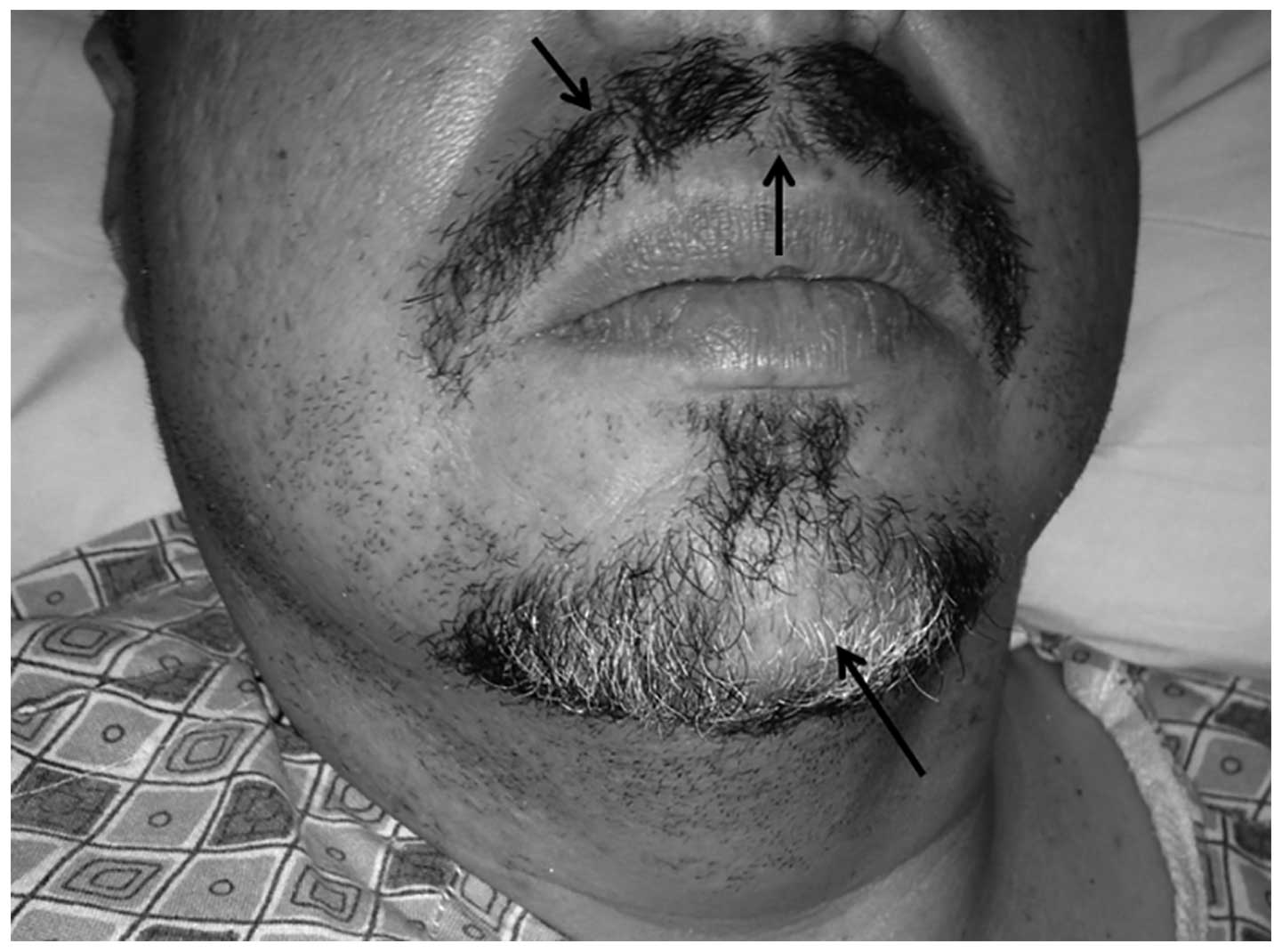
abdominal obesity and 3 regions of patchy hair loss in the mustache
and beard area, with the largest lesion (~3×4 cm) located at
mid-chin, with characteristics as previously described (Fig. 1).
The laboratory examinations revealed notable
pancytopenia, elevated erythrocyte sedimentation rate, normal
lactate dehydrogenase levels, normal thyroid function tests and a
negative antinuclear antibody (ANA) titer. The computed tomography
(CT) of the chest, abdomen and pelvis and magnetic resonance
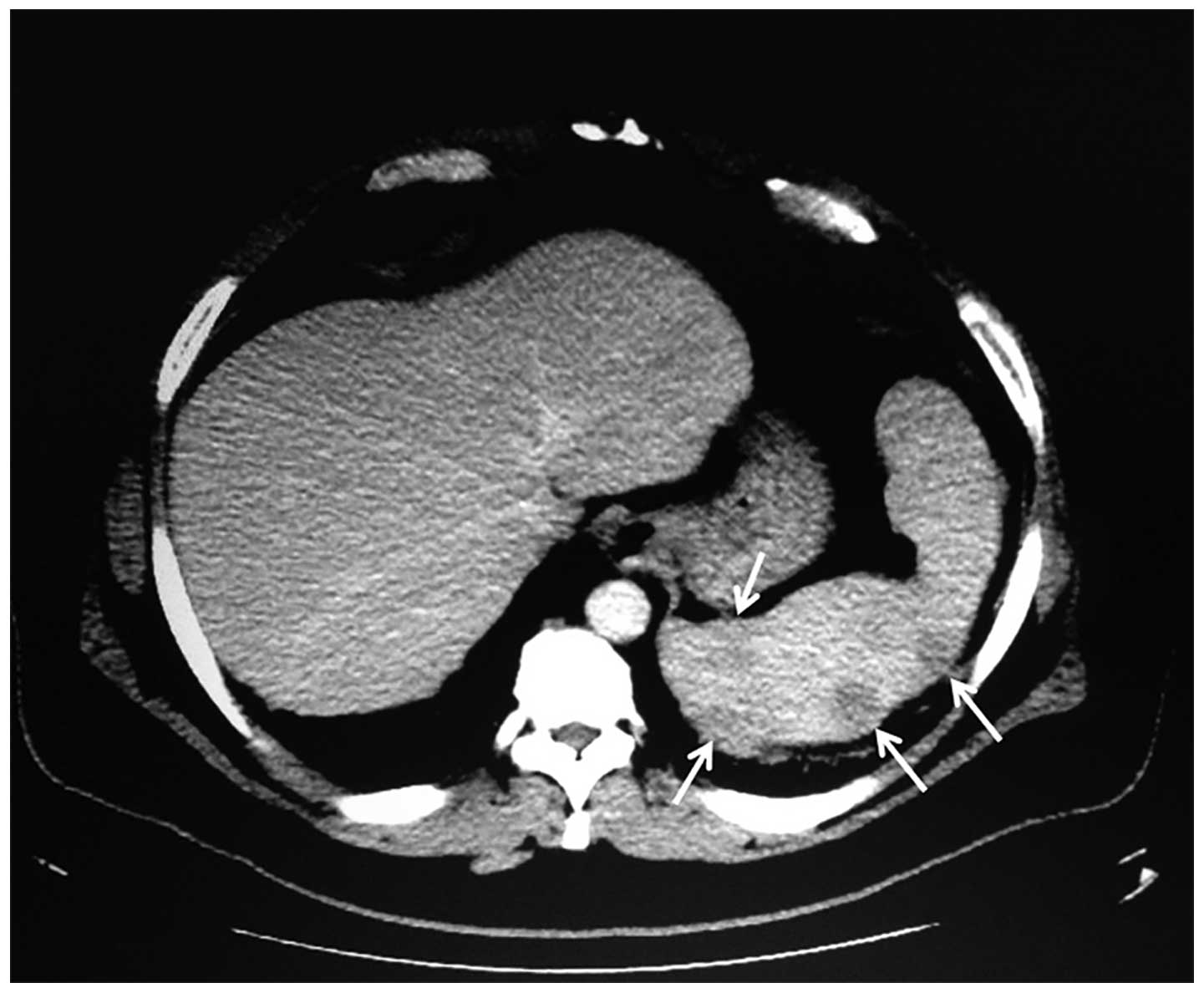
imaging of the abdomen and pelvis demonstrated splenomegaly with
multiple nodular hypodensities scattered throughout the spleen,
innumerable retroperitoneal and periportal lymphadenopathy and left
axillary lymphadenopathy, raising suspicion of lymphoma (Fig. 2). A laparoscopic splenectomy was
performed and the histopathological examination revealed the
presence of large atypical cells resembling Reed-Sternberg cells
admixed in a background of lymphocytes and fibrosis, with staining
characteristics consistent with those of classical HL of the
nodular sclerosis subtype (positive for CD15, CD30, fascin,
Epstein-Barr virus latent membrane protein-1 and CD20). The bone
marrow biopsy also revealed the presence of large atypical cells
with prominent nucleoli and staining characteristics similar to
those mentioned above. The patient subsequently underwent 6 cycles
of adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) for
stage IVB HL. At the time of this study, a surveillance positron
emission tomography-CT scan revealed no evidence of recurrence and
the patient’s AA had virtually resolved.
Discussion
AA is a benign condition characterized by sudden,
patchy, non-scarring hair loss, possibly due to
T-lymphocyte-mediated destruction of hair follicles via cytokine
production and initiation of the inflammatory cascade (1). AA has been associated with autoimmune
disorders, such as vitiligo and thyroid disease; however, the
association of AA with diabetes, lupus erythematosus, pernicious
anemia, myasthenia gravis, rheumatoid arthritis, polymyalgia
rheumatica, ulcerative colitis and lichen planus have also been
documented (1). With respect to
lymphomas, AA and follicular mucinosis or alopecia mucinosa, a
condition commonly characterized by erythematous plaques or
coalescing follicular papules with scarring and hair loss, have
been historically documented in cutaneous T-cell lymphomas,
including mycosis fungoides and Sézary syndrome (2–4).
A previous epidemiological study reported that, out
of 33,271 patients with NHL and 9,474 with multiple myeloma, 19 and
10 patients, respectively, had associated AA (5). The association between AA and HL, or
as then termed, Hodgkin’s disease, was anecdotally referenced in
the 1920s, or even earlier (6). It
was also reported that other dermatological conditions, including
pruritic, urticarial, hemorrhagic, exfoliative, pigmented and
bullous eruptions, were associated with HL (6). However, to the best of our knowledge,
the incidence of AA in association with HL is exceedingly rare. In
fact, out of 1,155 cases of HL, there were <5 cases of
associated AA at the time of a previously published review
(5) and our knowledge of AA as a
paraneoplastic phenomenon of HL is limited to a few case studies
(7–9).
The clinical presentations of AA in such cases were
characterized by patchy regions of hair loss to complete hair loss
on the scalp (alopecia totalis) (7–9). In
one case, the manifestations of AA preceded the majority of the
systemic manifestations of HL by several months (7), whereas the other cases presented with
concurrent manifestations of AA and HL (8,9). Two
of the cases occurred in young females (aged 17 and 30 years),
whereas one case occurred in a 43-year-old male (7–9).
Other clinical manifestations included generalized pruritus,
diffuse skin scaling, diffuse skin hyperpigmentation, fevers,
weight loss, anorexia, fatigue, hepatomegaly, back pain, scoliosis
and painless cervical, axillary and inguinal lymphadenopathy
(7–9). The thyroid function tests, serum
cortisol, serum adrenocorticotropic hormone, C3 and C4 levels and
ANA titers were notably negative (8,9). All
the reported cases had advanced disease (Ann Arbor stage IIIB or
IVB) and received chemotherapy with either ABVD, mustargen, oncovin
(vincristine), procarbazine and prednisone (MOPP) with ABV, or
oncovin, prednisone, procarbazine and doxorubicin (OPPA) with
cyclophosphamide, oncovin, prednisone and procarbazine (COPP)
(7–9). Following chemotherapy, the AA
resolved as early as after 1 cycle of ABVD to as late as 12 months
following completion of OPPA and COPP (7–9).
The etiology of AA in classical HL has not been
fully elucidated. It has been suggested that AA may be the result
of direct infiltration and destruction of hair follicles by the
disease or, more commonly, a paraneoplastic phenomenon (7). The mechanism underlying this
paraneoplastic process has been putatively attributed to impaired
cellular immune responses or the anergy seen in HL, which correlate
with the findings of reduced numbers of circulating T-lymphocytes
(in particular, CD8+ suppressor T-lymphocytes) in AA
(1,8). Alternatively, other studies suggested
that the induction of major histocompatibility complex class I and
II expression on hair follicles may lead to the activation of
CD8+ T-lymphocytes towards hair follicle melanocytes and
recruitment of CD4+ T-lymphocytes that further attack
the hair follicles, a process that may be perpetuated and amplified
by the clonal T-cell proliferation seen in lymphomas (4). There is also a genetic predisposition
contributing to such phenomena, given the numerous human leukocyte
antigen subtypes shared by AA and HL (1,10).
The resolution of AA observed with the remission of HL in our
patient and other case reports reaffirms the close association
between these two conditions. However, the phenomenon of AA as a
paraneoplastic manifestation of HL remains poorly characterized in
the literature. This case report serves to highlight the
significance of AA as a herald for possible underlying malignancies
and the need for greater awareness of AA and its implications on
the prognosis of HL.
References
|
1
|
Madani S and Shapiro J: Alopecia areata
update. J Am Acad Dermatol. 42:549–566. 2000. View Article : Google Scholar
|
|
2
|
Bonta MD, Tannous ZS, Demierre M, Gonzalez
E, Harris NL and Duncan LM: Rapidly progressing mycosis fungoides
presenting as follicular mucinosis. J Am Acad Dermatol. 43:635–640.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Török L, Gurbity TP, Kirschner Á and
Krenács L: Panniculitis-like T-cell lymphoma clinically manifested
as alopecia. Br J Dermatol. 147:785–788
|
|
4
|
Richmond HM, Lozano A, Jones D and Duvic
M: Primary cutaneous follicle center lymphoma associated with
alopecia areata. Clin Lymphoma Myeloma. 8:121–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson LA, Gadalla S, Morton LM, et al:
Population-based study of autoimmune conditions and the risk of
specific lymphoid malignancies. Int J Cancer. 125:398–405. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fox H: Lymphogranulomatosis of the skin in
Hodgkin’s disease. Arch Derm Syphilol. 2:578–593. 1920.
|
|
7
|
Garg S, Mishra S, Tondon R and Tripathi K:
Hodgkin’s lymphoma presenting as alopecia. Int J Trichology.
4:169–171. 2012.
|
|
8
|
Mlczoch L, Attarbaschi A, Dworzak M,
Gadner H and Mann G: Alopecia areata and multifocal bone
involvement in a young adult with Hodgkin’s disease. Leuk Lymphoma.
46:623–627. 2005.PubMed/NCBI
|
|
9
|
Chan PD, Berk MA, Kucuk O and Singh S:
Simultaneously occurring alopecia areata and Hodgkin’s lymphoma:
complete remission of both diseases with MOPP/ABV chemotherapy. Med
Pediatr Oncol. 20:345–348. 1992.PubMed/NCBI
|
|
10
|
Mani H and Jaffe ES: Hodgkin lymphoma: an
update on its biology with newer insights into classification. Clin
Lymphoma Myeloma. 9:206–216. 2009. View Article : Google Scholar : PubMed/NCBI
|