Introduction
The recommended dose of traditional cytotoxic
antitumor agents is generally determined at or near their
maximum-tolerated dose (1–3). However, in the case of epidermal
growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), the
recommended dose is determined at an optimal biological dose to
minimize the risk of adverse events (AEs) without compromising
efficacy (4–6). However, body size was not taken into
consideration in previous dose-finding studies. A significant
number of EGFR-mutated patients are female and, in their majority,
the body surface area (BSA) may be low (7–9).
Therefore, a dose reduction due to AEs or low BSA may be required
in daily clinical practice for such patients. However, there is
limited information on the association between low BSA and TKI dose
reduction and efficacy (10). In
addition, the efficacy of TKIs in patients undergoing a dose
reduction due to severe AEs has not been clearly determined. In the
present study, we evaluated the efficacy of TKIs in patients
undergoing a dose reduction due to AEs and in those with a low BSA
in clinical practice.
Patients and methods
Patients
A total of 282 consecutive patients with
pathologically confirmed non-small-cell lung cancer (NSCLC), who
were treated with TKIs at the University of Tsukuba Hospital, the
Tsukuba Medical Center Hospital, the Ryugasaki Saiseikai General
Hospital and the Mito Medical Center-Mito Kyodo General Hospital
between September, 2005 and May, 2013, were retrospectively
analyzed. The histopathological diagnoses were performed according
to the World Health Organization classification system (11) and the patients were staged
according to the Union for International Cancer Control tumor node
metastasis staging system (12).
The patient characteristics, efficacy and safety
were evaluated using patient data extracted from the database of
each participating institution.
Tumor response
Tumor responses were classified as complete
response, partial response, stable disease, progressive disease or
not evaluable, according to the Response Evaluation Criteria in
Solid Tumors, version 1.1 (13).
In this study, we defined ‘dose reduction’ of TKIs was as a
reduction of the starting dose, as well as a reduction during the
course of treatment. Patients undergoing discontinuation of TKIs
without any dose reduction were not included. The effect of BSA on
progression-free survival (PFS) and overall survival (OS) was
evaluated. The effect of TKIs, with or without dose reduction, was
also evaluated in the analysis of PFS and OS. The present
retrospective study conformed to the Ethical Guidelines for
Clinical Studies issued by the Ministry of Health, Labor and
Welfare of Japan.
Statistical analysis
Statistical significance between two groups was
determined using the Mann-Whitney U test and the Chi-square test.
The patient survival time was calculated from the day of TKI
initiation to death or last follow-up. The survival rate was
analyzed with the Kaplan-Meier method and comparisons were
performed using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
During the study period, a total of 282 patients
were treated with TKIs (213 with gefitinib and 69 with erlotinib).
The median age was 66 years (range, 21–90 years). The patient
characteristics are summarized in Table I. A total of 124 patients (44.0%)
were male, 128 (45.4%) had smoking history and 197 (69.9%) had good
performance status (0–1). The tumors were classified as 243 (86.2%)
adenocarcinomas, 35 (12.4%) squamous cell carcinomas and 4 (1.4%)
other types. Nineteen patients (6.7%) had a low BSA (<1.25
m2) and 112 (39.7%) had 1.25<BSA<1.5
m2. A dose reduction of TKIs for any reason was
performed in 53 (18.8%) of the 282 patients (12.2% receiving
gefitinib and 39.1% erlotinib). Of these, 21.4 and 31.6% with a BSA
of <1.5 and <1.25 m2, respectively, underwent a
dose reduction of TKIs.
 | Table ICharacteristics of 282 patients
treated with TKIs. |
Table I
Characteristics of 282 patients
treated with TKIs.
| Characteristics | No. (%) |
|---|
| Age, years (median,
range) | 66, 21–90 |
| Gender
(male/female) | 124 (44.0)/158
(56.0) |
| Smoking habit
(present/absent) | 128 (45.4)/154
(54.6) |
| Performance status
(0–1/2–4) | 197 (69.9)/85
(30.1) |
| Pathology |
| Adenocarcinoma | 243 (86.2) |
| Squamous cell
carcinoma | 35 (12.4) |
| Other | 4 (1.4) |
| EGFR mutation
type |
| Mutated | 96 (34.1) |
| Wild-type | 52 (18.4) |
| Unknown | 134 (47.5) |
| Body surface area
(m2) |
| <1.25 | 19 (6.7) |
| 1.25–1.50 | 112 (39.7) |
| >1.50 | 151 (53.6) |
Overall response rate (RR), disease
control rate (DCR), PFS and AEs
In the 282 patients, including 96 (34.1%) with
mutated-type EGFR, 52 (18.4%) with wild-type EGFR and 134 (47.5%)
with unknown EGFR status, the objective RR was 40.1%, the DCR was
62.1% and the PFS was 4.2 months. Of the 282 patients, 23 (8.2%)
developed AEs >grade 3. The most common AEs were severe skin
reaction and liver dysfunction.
AEs, BSA and dose reduction in patients
treated with gefitinib
Of the 213 patients receiving gefitinib, 16
exhibited AEs >grade 3. Among these, 6 (37.5%) underwent a dose
reduction. However, of the 197 patients who did not have AEs
>grade 3, 20 (10.2%) received a dose reduction. The difference
was statistically significant (P=0.006). The proportion of the
patients undergoing a dose reduction was not associated with BSA in
those with or without AEs >grade 3 (Table II).
 | Table IIAdverse events (AEs) >grade 3, body
surface area (BSA) and dose reduction in 213 patients treated with
gefitinib. |
Table II
Adverse events (AEs) >grade 3, body
surface area (BSA) and dose reduction in 213 patients treated with
gefitinib.
| Patients | No. | BSA
(m2) | Patients with dose
reduction no. (%) | Patients without dose
reduction no. (%) |
|---|
| With AEs | 16 | | 6 (37.5%) | 10 (62.5%) |
| | <1.25 | 0 | 0 |
| | 1.25–1.5 | 2 | 7 |
| | >1.5 | 4 | 3 |
| Without AEs | 197 | | 20 (10.2%) | 177 (89.2%) |
| | <1.25 | 3 | 11 |
| | 1.25–1.5 | 12 | 69 |
| | >1.5 | 5 | 97 |
AEs, BSA and dose reduction in patients
treated with erlotinib
Of the 69 patients receiving erlotinib therapy, 7
developed AEs >grade 3. Among these, 5 (71.4%) had a dose
reduction. Of the 62 patients who did not have AEs >grade 3, 22
(35.5%) received a dose reduction. The difference was not
statistically significant (P=0.162). The proportion of patients
undergoing a dose reduction was not associated with BSA in those
with or those without AEs >grade 3 (Table III).
 | Table IIIAdverse events (AEs) >grade 3, body
surface area (BSA) and dose reduction in 69 patients treated with
erlotinib. |
Table III
Adverse events (AEs) >grade 3, body
surface area (BSA) and dose reduction in 69 patients treated with
erlotinib.
| Patients | No. | BSA
(m2) | Patients with dose
reduction no. (%) | Patients without dose
reduction no. (%) |
|---|
| With AEs | 7 | | 5 (71.4%) | 2 (28.6%) |
| | <1.25 | 0 | 0 |
| | 1.25–1.5 | 1 | 0 |
| | >1.5 | 4 | 2 |
| Without AEs | 62 | | 22 (35.5%) | 40 (64.5%) |
| | <1.25 | 3 | 2 |
| | 1.25–1.5 | 7 | 14 |
| | >1.5 | 12 | 24 |
BSA and RR, DCR, PFS and OS in
EGFR-mutated patients treated with gefitinib
We compared RR, DCR and PFS between 45 patients with
BSA >1.5 m2 and 37 with BSA <1.5 m2.
The RR and DCR were 82.2 and 95.6% in the former patient group and
67.6 and 91.9% in the latter group, respectively. There was no
statistically significant difference between the two groups
(P=0.196 and 0.645, respectively). There was also no statistically
significant difference in the PFS between the two groups of
patients (BSA >1.5 m2, 12.1 months vs. <1.5
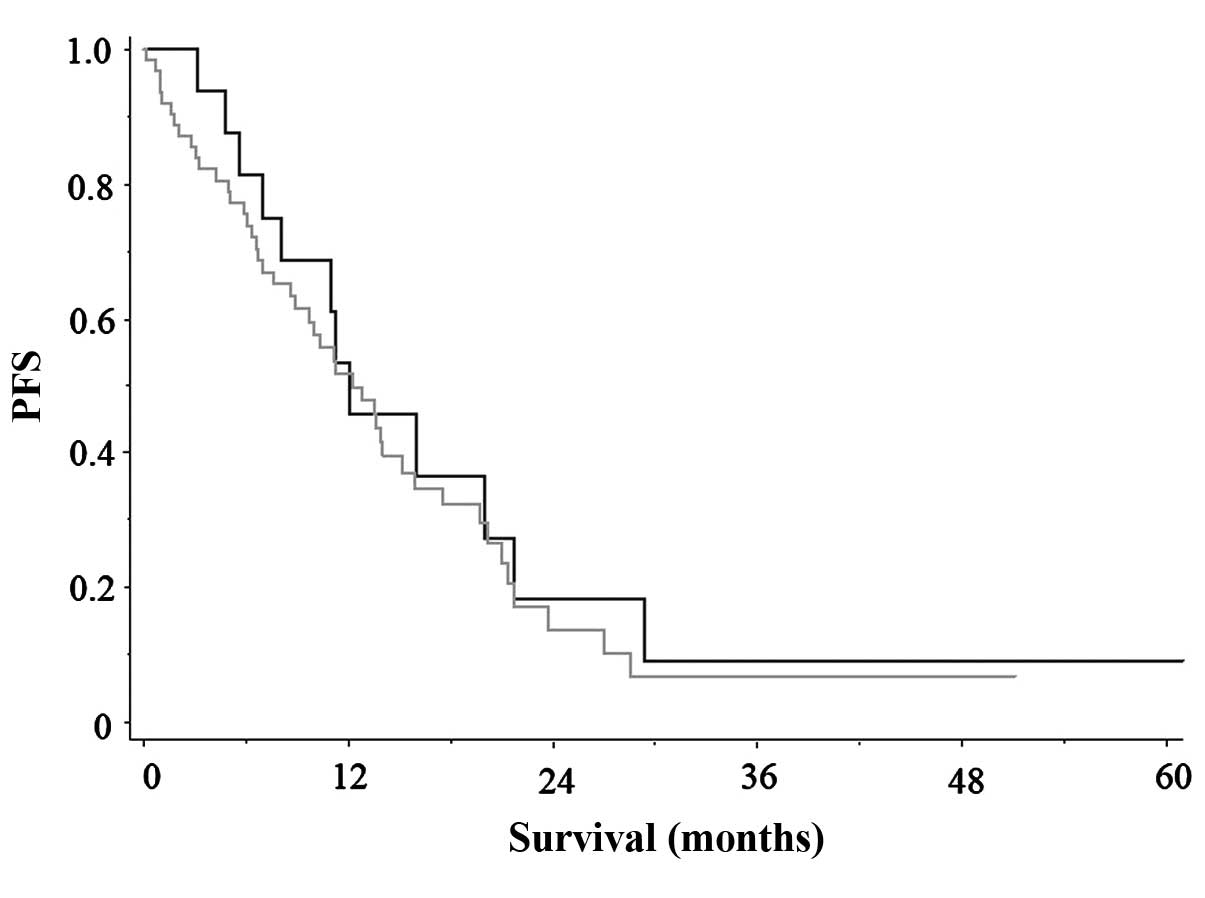
m2, 12.8 months; P=0.861) (Fig. 1). The OS appeared to be longer in
patients with BSA <1.5 m2 compared to that in
patients with >1.5 m2, although the difference was
not statistically significant (P=0.080).
Dose reduction and RR, DCR, PFS and OS in
EGFR-mutated patients treated with gefitinib
We next evaluated the effect of dose reduction of
gefitinib on RR, DCR, PFS and OS. There were 19 patients with and
63 without a dose reduction of gefitinib. The RR and DCR were 78.9
and 94.7% in the former group of patients and 74.6 and 93.7% in the
latter group, respectively. There was no statistically significant
difference between the two groups (P=0.699 and 0.862,
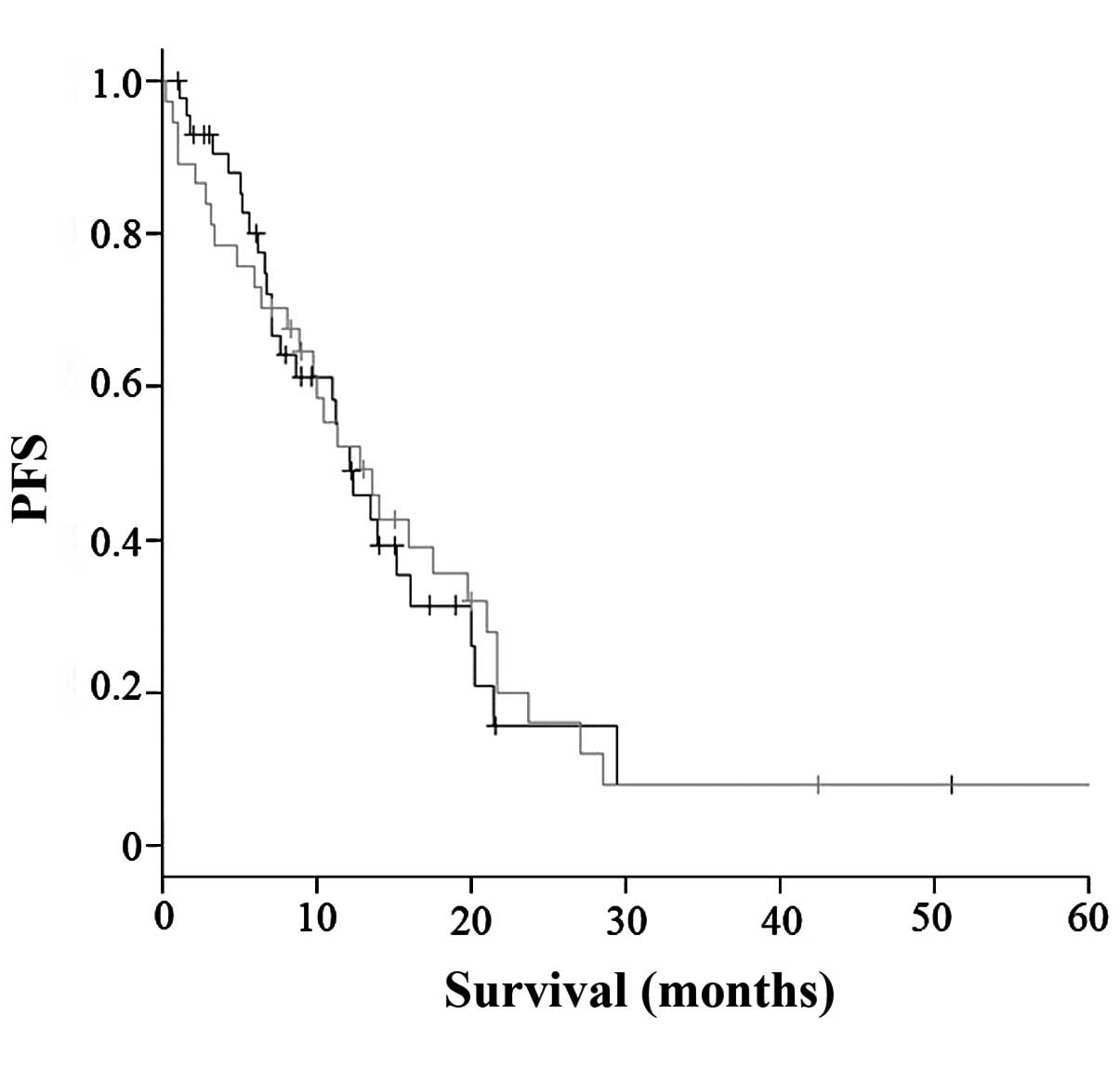
respectively). As shown in Fig. 2,
there was also no statistically significant difference in PFS
between patients with and those without dose reduction (12.1 vs.
12.3 months, respectively; P=0.522). There appeared to be a
difference in OS between patients with and those without dose
reduction, but the difference was not statistically significant
(P=0.154).
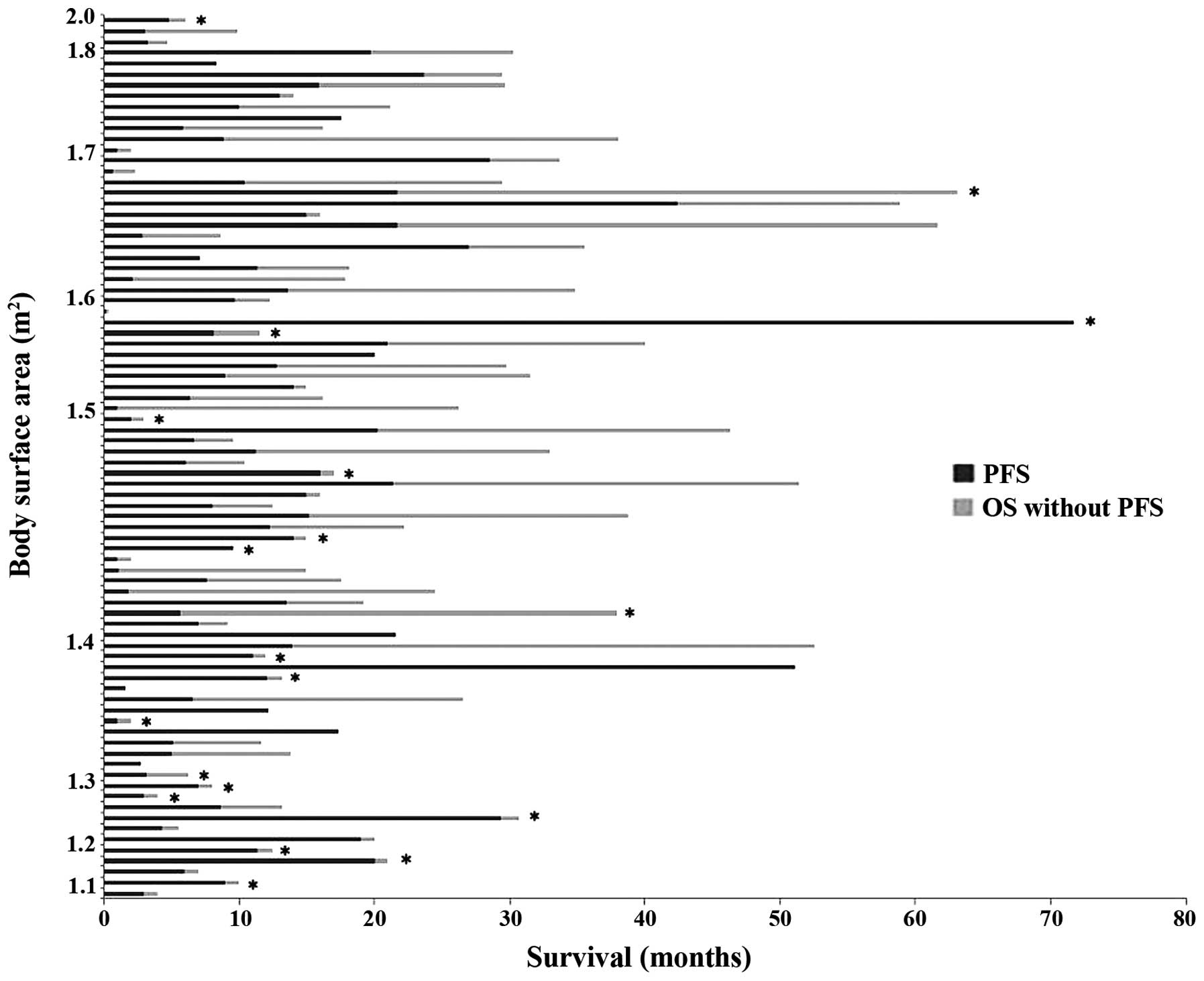
The association among BSA, dose reduction of
gefitinib, PFS and OS is shown in Fig.
3. Of the 24 patients with BSA <1.5 m2, 22
(91.7%) were female, 13 (54.2%) were aged ≥75 years, 10 (41.7%)
underwent a dose reduction and 19 (79.2%) received no further
cytotoxic antitumor chemotherapy. There were no statistical
differences among these variables.
BSA and RR, DCR, PFS and OS in patients
treated with erlotinib
In the 14 EGFR-mutated patients treated with
erlotinib, there was no statistically significant difference in RR,
DCR and PFS between those with and those without dose reduction
(RR: 87.5 vs. 50%, P=0.124; DCR: 100 vs. 89.3%, P=0.230; and PFS:
14.3 vs. 8.0 months, P=0.409).
In the 39 wild-type EGFR patients treated with
erlotinib, there was no statistically significant difference in PFS
between those with and those without dose reduction (P=0.794).
There was no apparent statistical difference in OS between patients
with a BSA of >1.5 and those with a BSA of <1.5 m2
(P=0.589). However, there was a statistically significant
difference in OS between those with and those without dose
reduction (P=0.172).
Discussion
Previous clinical trials reported that using a fixed
dose of EGFR-TKIs achieved a significant improvement in PFS with
acceptable AE profiles (14,15).
Either gefitinib or erlotinib are currently among the first-choice
treatments for advanced NSCLC patients with EGFR mutation (14,15).
However, the efficacy of TKIs in the clinical setting, particularly
in patients undergoing a dose reduction due to toxicity or low BSA,
remains unclear. There remained the question whether a strictly
fixed dose of TKIs, determined by dose-finding studies, would
exhibit the same efficacy in patients undergoing a dose reduction
as in those without a dose reduction. Therefore, we evaluated RR,
DCR, PFS and OS in patients with and those without dose reduction
of TKIs. In the present study, clinically relevant AEs >grade 3
were observed in 23 (8.2%) of the 282 patients, leading to a dose
reduction in 11 of the patients. Therefore, 42 patients underwent a
dose reduction due to reasons other than AEs. In addition, 46.4% of
the 282 patients had a BSA of <1.5 m2, demonstrating
that a considerable percentage of patients with low BSA received
TKI therapy, which may be associated with the fact that a number of
those patients were EGFR-mutated female patients. In fact, in
previous reports on treatment with TKIs, either in clinical trials
or clinical practice, there was a higher population of female
patients with NSCLC compared to that in the general population
(7–9,16).
Our study did not identify any disadvantage
regarding PFS from a dose reduction in patients receiving gefitinib
or erlotinib. However, as these findings were obtained from a
relatively small number of patients in a retrospective study, we
were unable to draw definitive conclusions on how dose reduction
would affect patients with AEs and those with low BSA. We recommend
that our results be meaningfully interpreted rather than be
overlooked as anecdotal findings. We may need to reconsider the
most appropriate dosage of TKIs for such patients, in order to
obtain maximum efficacy with an acceptable toxicity profile.
To the best of our knowledge, there has been only
one previous study evaluating the effect of BSA on the efficacy of
TKIs (10). That study
investigated the potential effect of BSA on the PFS and OS of
patients with advanced EGFR-mutated NSCLC who were treated with
gefitinib (10) and found that BSA
affected the outcome of gefitinib therapy, with a higher BSA being
associated with worse PFS (10).
The main focus of that study was treatment efficacy in patients
with a high BSA; by contrast, our interest was not only the effects
of dose reduction in patients with AEs as well as in patients with
a low BSA. In the previous study, the PFS of patients with low BSA
with treatment interruption or reduced dose was either equal to or
superior to that of all low-BSA patients (10), which was consistent with our
results. Ichihara et al (10) hypothesized that the patients who
required a dose reduction due to AEs may be those with relatively
high blood concentrations of the agent, due to factors such as drug
metabolism. This hypothesis requires confirmation in future
studies.
Another point investigated was the effect of BSA on
OS. Ichihara et al (10)
reported that OS was not associated with BSA in their study. In our
study, there was no apparent difference in OS between patients with
BSA <1.5 m2 and those with BSA >1.5 m2
receiving treatment with either gefitinib or erlotinib. The OS in
patients without dose reduction appeared to be longer compared to
that in patients with dose reduction, but the difference was not
statistically significant. As suggested by Ichihara et al
(10), a possible reason may be
the effect of preceding and/or subsequent therapies on OS. In their
study, as many as 70% of the patients received chemotherapy
following disease progression post-gefitinib monotherapy,
suggesting that post-progression therapy may have blunted any
difference in PFS between the high- and low-BSA subgroups (10). Another explanation for these
results may be the fact that further cytotoxic antitumor
chemotherapy was not indicated in the majority of the low-BSA
patients, as observed in our study. We were unable to identify a
statistically significant difference in dose reduction for patients
with low BSA.
The analysis of our data set and conclusions drawn
were limited by the small sample size, retrospective design and
heterogeneity in the EGFR mutations, TKIs used, clinicians’
experience with TKIs, reasons for dose reduction and exclusion of
patients with discontinuation of TKIs without any dose reduction.
Another major limitation is that pharmacokinetic data were not
included in this analysis. It remains unclear whether BSA
differences led to inter-patient pharmacokinetic variability,
resulting in the observed difference in PFS. A
pharmacokinetics-pharmacodynamics study is required to clarify this
issue. The blood concentration of cytotoxic agents is closely
associated with their efficacy (17–19).
Similarly, the blood concentration of TKIs also appears to be
associated with their efficacy (20,21).
Dose-reduction estimation studies for TKIs may be
crucial, particularly for patients with low BSA. Heterogeneity in
these factors should be taken into consideration, or exclude causes
of heterogeneity. Prospective studies investigating the incidence
of dose reduction in patients with AEs and those with low BSA may
be meaningful for common clinical practice. This approach may
further elucidate the clinical meaning of dose reduction of TKIs in
such patients.
References
|
1
|
Seymour L and Eisenhauer E: A review of
dose-limiting events in phase I trials: antimetabolites show
unpredictable relationships between dose and toxicity. Cancer
Chemother Pharmacol. 47:2–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoekstra R, Verweij J and Eskens FA:
Clinical trial design for target specific anticancer agents. Invest
New Drugs. 21:243–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zohar S, Lian Q, Levy V, Cheung K, Ivanova
A and Chevret S: Quality assessment of phase I dose-finding cancer
trials: proposal of a checklist. Clin Trials. 5:478–485. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolf M, Swaisland H and Averbuch S:
Development of the novel biologically targeted anticancer agent
gefitinib: determining the optimum dose for clinical efficacy. Clin
Cancer Res. 10:4607–4613. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takimoto CH: Maximum tolerated dose:
clinical endpoint for a bygone era? Target Oncol. 4:143–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto N, Horiike A, Fujisaka Y, et al:
Phase I dose-finding and pharmacokinetic study of the oral
epidermal growth factor receptor tyrosine kinase inhibitor
Ro50-8231 (erlotinib) in Japanese patients with solid tumors.
Cancer Chemother Pharmacol. 61:489–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boyer M, Horwood K, Pavlakis N, et al:
Efficacy of erlotinib in patients with advanced non-small-cell lung
cancer (NSCLC): analysis of the Australian subpopulation of the
TRUST study. Asia Pac J Clin Oncol. 8:248–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gahr S, Stoehr R, Geissinger E, et al:
EGFR mutational status in a large series of Caucasian European
NSCLC patients: data from daily practice. Br J Cancer.
109:1821–1828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaburagi T, Satoh H, Hayashihara K, et al:
Observational study on the efficacy and safety of erlotinib in
patients with non-small cell lung cancer. Oncol Lett. 5:435–439.
2013.PubMed/NCBI
|
|
10
|
Ichihara E, Hotta K, Takigawa N, et al:
Impact of physical size on gefitinib efficacy in patients with
non-small cell lung cancer harboring EGFR mutations. Lung Cancer.
81:435–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Travis WD, Brambilla E, Muller-Hermelink
HK and Harris CC: Pathology and Genetics: Tumours of the Lung,
Pleura, Thymus and Heart. IARC Press; Lyon: pp. 9–122. 2004
|
|
12
|
Kligerman S and Abbott G: A radiologic
review of the new TNM classification for lung cancer. AJR Am J
Roentgenol. 194:562–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sohaib A: RECIST rules. Cancer Imaging.
12:345–346. 2012. View Article : Google Scholar
|
|
14
|
Pennell NA: Integration of EGFR inhibitors
and conventional chemotherapy in the treatment of non-small-cell
lung cancer. Clin Lung Cancer. 12:350–359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roengvoraphoj M, Tsongalis GJ, Dragnev KH
and Rigas JR: Epidermal growth factor receptor tyrosine kinase
inhibitors as initial therapy for non-small cell lung cancer: focus
on epidermal growth factor receptor mutation testing and
mutation-positive patients. Cancer Treat Rev. 39:839–850. 2013.
View Article : Google Scholar
|
|
16
|
Hayashibara K, Satoh H, Shinohara Y, et
al: A population-based study of gefitinib in patients with
non-small cell lung cancer. Med Oncol. 26:222–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miles DW, Chan A, Dirix LY, et al: Phase
III study of bevacizumab plus docetaxel compared with placebo plus
docetaxel for the first-line treatment of human epidermal growth
factor receptor 2-negative metastatic breast cancer. J Clin Oncol.
28:3239–3247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wittenburg LA and Gustafson DL: Optimizing
preclinical study design in oncology research. Chem Biol Interact.
190:73–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujita KI and Sasaki Y: Optimization of
cancer chemotherapy on the basis of pharmacokinetics and
pharmacodynamics: from patients enrolled in ‘clinical trials’ to
those in the ‘real world’. Drug Metab Pharmacokinet. 29:20–28.
2014.
|
|
20
|
Fukudo M, Ikemi Y, Togashi Y, et al:
Population pharmacokinetics/pharmacodynamics of erlotinib and
pharmacogenomic analysis of plasma and cerebrospinal fluid drug
concentrations in Japanese patients with non-small cell lung
cancer. Clin Pharmacokinet. 52:593–609. 2013. View Article : Google Scholar
|
|
21
|
Ranson M and Wardell S: Gefitinib, a
novel, orally administered agent for the treatment of cancer. J
Clin Pharm Ther. 29:95–103. 2004. View Article : Google Scholar : PubMed/NCBI
|