Introduction
Lung cancer is one of the most common and lethal
types of cancer, accounting for 17% of the total new cancer cases
and approximately one-fourth of the total cancer-related deaths
worldwide (1). Non-small-cell lung
cancer (NSCLC) includes adenocarcinoma, squamous cell carcinoma and
large-cell carcinoma and is responsible for ~85% of newly diagnosed
lung cancer cases (2).
Approximately 80% of lung cancer cases have already advanced at the
time of diagnosis, thus eliminating surgery as a treatment option.
Multidisciplinary treatment is currently the main therapeutic
approach for advanced NSCLC. The main method to quickly and
effectively control tumor growth is a combination of local
treatment and systemic therapy (3). However, the clinical outcomes for
radiotherapy combined with chemotherapy, the main therapeutic
regimen for advanced NSCLC under the traditional multidisciplinary
approach, have been disappointing, with a 5-year survival rate of
<20% (4–6).
With the increase of the application of targeted
therapy for lung cancer, the combination of targeted therapy and
radiotherapy has also become a popular therapeutic modality.
Theoretically, due to its low toxicity, the combination regimen may
be an effective approach, particularly suitable for elderly
patients and those with additional underlying diseases (7,8).
However, certain specifications that have been
traditionally used for chemotherapy may not be applicable to
targeted therapy. Unless disease progresses significantly during
the course of targeted therapy, the general recommendation is to
continue medication without interruption, as treatment termination
is likely to result in rapid disease progression (9). Nishie et al (9) reported a statistically significant
difference in the median survival of NSCLC patients who continued
epidermal growth factor receptor tyrosine kinase inhibitors
(EGFR-TKIs) and those who discontinued such therapy after disease
progression, indicating that the management strategy of targeted
therapy should be differentiated from that of radiotherapy or
chemotherapy.
Stereotactic body radiation therapy (SBRT) delivers
high doses of radiation to the involved target field and diminishes
the radiation fields by reducing the effects of tumor motion for
accuracy and precision, thus contributing to a reduction in the
volume of irradiation of normal tissues and an increase in the dose
delivered to the target field (10). SBRT was recently used for lung
cancer in patients for whom surgery is not a suitable option, with
an efficacy comparable to that of surgery for early lung cancer
(11). Rowe et al (12) expanded SBRT to central lung cancer
and reported that the approach was safe and effective, with mostly
tolerable early complications. However, late complications were not
addressed in that study. In this study, we report the development
of severe obstructive atelectasis as a late complication in two
patients with central lung cancer who received SBRT. The
obstructive atelectasis interrupted the evaluation of the efficacy
of the subsequent gefitinib treatment for NSCLC. Informed consent
was obtained from the two patients prior to the study. This study
was approved by the Ethics Committee of Daping Hospital
[(2012)NO.10].
Case reports
Case 1
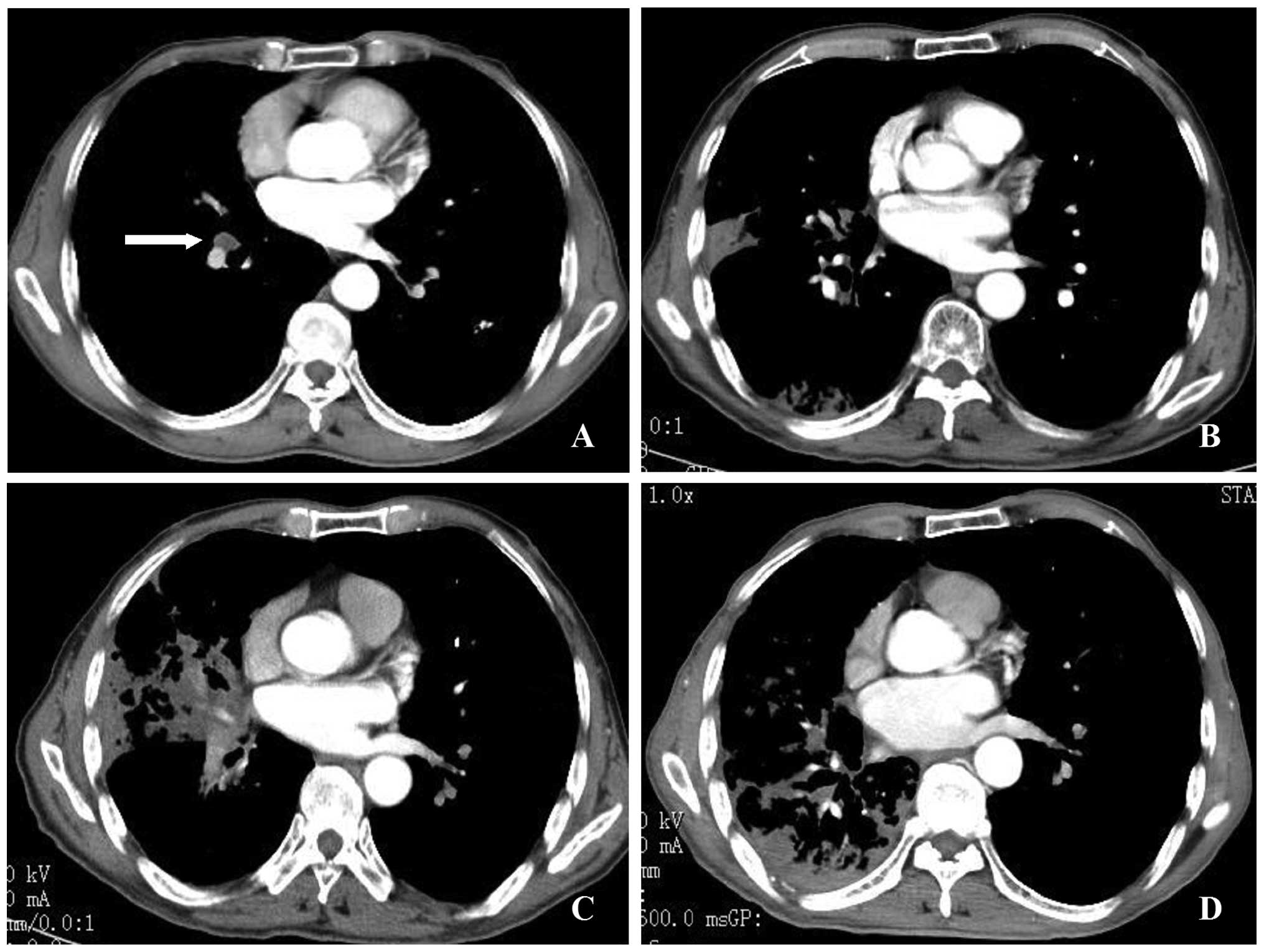
An 83-year-old man was diagnosed with stage T2N1M1
adenocarcinoma of the right lung (Fig.
1A). Palliative γ-ray SBRT was administered to the lesions in
the right lower lung, right hilum and left lower lung at doses and
fractions as previously described (13). Briefly, computed tomography
(CT)-guided simulation was available for delineation of the gross
tumor volume (GTV). The planning target volume (PTV) was generated
with an additional 0.5 cm to the GTV in the axial plane and 1.0 cm
in the longitudinal plane. The γ-SBRT plan included a 50% isodose
line covering >95% of the PTV and a 70% isodose line covering
>90% of the GTV. A total dose of 40 Gy was prescribed
encompassing the PTV in 10 fractions (5 fractions per week) at 4 Gy
per fraction and the corresponding dose of 56 Gy was prescribed
encompassing the GTV (5.6 Gy/fraction).
Considering the patient’s age, pemetrexed
monotherapy was administered 4 times intermittently after
radiotherapy. A review conducted at 6 months indicated mild
radioactive inflammation of the right lung on CT scan (Fig. 1B). Oral gefitinib was initiated at
9 months post-radiotherapy. Two weeks later, the cough of the
patient was exacerbated. A second CT scan revealed obstructive
atelectasis in the lateral segment of the right middle lobe
(Fig. 1C). The atelectasis was
attributed to disease progression and gefitinib was terminated.
Given the patient’s age, no further treatment options
(chemotherapy, radiotherapy, or surgery) were contemplated. A CT
scan at 14 months post-radiotherapy revealed that the obstructive
atelectasis was mitigated in the lateral segment of the right
middle lobe (Fig. 1D). However,
the obstructive atelectasis in the posterior segment of the right
upper lobe, the dorsal segment and the basal segment was
aggravated. Metastasis to the contralateral lung was identified 6
months later, indicating disease progression.
Case 2
A 77-year-old man was diagnosed with stage T2N3M0
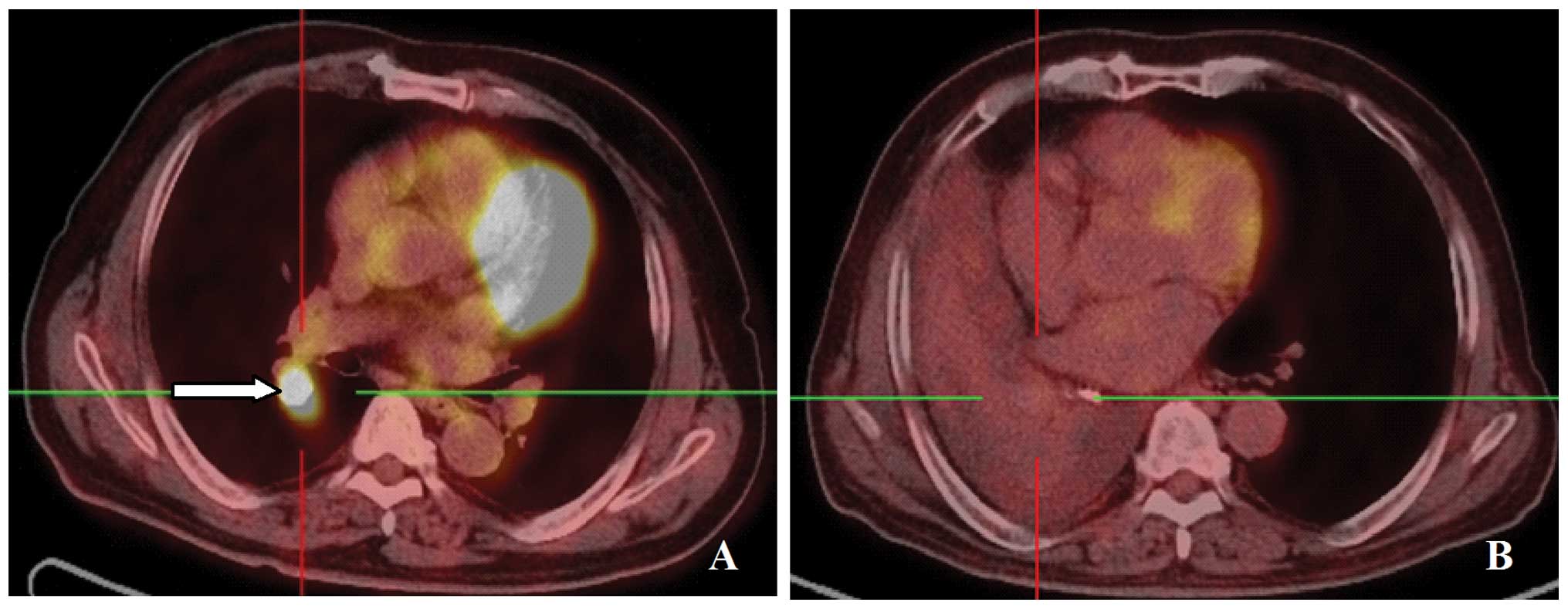
adenocarcinoma of the right lung. Positron emission tomography
(PET)-CT examination revealed nodules in the right lower lobe,
enlargement of the lymph nodes at the right hilum and above the
left supraclavicular fossa, with increased standardized uptake
values (SUV) of fluorodeoxyglucose (Fig. 2A). Palliative γ-ray SBRT was
administered to the right hilum, right lower lobe and lymph nodes
on the left supraclavicular fossa with a similar dose schedule to
that of Case 1. Following radiotherapy, the patient received
bevacizumab, pemetrexed, and nedaplatin. At the 1-month review, the
CT scan revealed multiple small nodules in the right middle lobe
and disease progression was considered. The patient declined
further chemotherapy. Second-line oral gefitinib was initiated,
with a decrease in the number of nodules in the right middle lobe 1
month after gefitinib treatment initiation. Partial regression was
achieved. Gefitinib was continued for a further 7 months, when the
patient experienced worsened post-exertional shortness of breath. A
CT scan review revealed obstructive atelectasis in the right lung
(Fig. 2B). Progressive disease was
contemplated by an outpatient respiratory physician, who
recommended terminating gefitinib. However, the PET-CT revealed a
mass in the right lower lobe and enlargement of the lymph nodes in
the right hilum and left supraclavicular fossa. No increase in SUV
was observed in either area, indicating that the activity of the
lesions was inhibited. Thus we recommended continuing gefitinib
treatment.
Discussion
As interstitial pneumonia is one of the most severe
side effects of EGFR-TKIs and radiotherapy, EGFR-TKIs and
concurrent radiotherapy have been used with caution in clinical
practice. Wang et al (13)
reported only a low incidence rate (4%) of third-degree radiation
pneumonitis in NSCLC patients receiving EGFR-TKI treatment plus
radiotherapy. Okamoto et al (6), however, reported radiation
pneumonitis in 2 of 7 stage III unresectable NSCLC patients. Onal
et al (14) also reported
on a patient who received erlotinib after radiotherapy, which
induced radiation pneumonitis. Due to the aforementioned reasons,
combination therapy was not prescribed for our two patients.
Rather, additional EGFR-TKI treatment was administered sequentially
after the completion of radiotherapy.
The main advantage of SBRT is its short radiation
time and less damage to the surrounding tissues, resulting in a
decreased risk of radiation pneumonitis in lung cancer. Wang et
al (15) reported that in 14
patients with advanced NSCLC, SBRT combined with gefitinib resulted
in a disease remission rate of up to 57.1%. The median time of
disease remission was 8 months, while the 1-year survival rate was
69.6%, suggesting that this regimen may improve local control and
disease remission rates with few side effects. Compared to 3D-RT or
intensity-modulated radiotherapy, SBRT in combination with
EGFR-TKIs may be a more suitable treatment option for elderly
patients or those with diseases that are a contraindication to
radiotherapy, such as chronic obstructive pulmonary disease
(16).
However, our two patients with central lung lesions
developed severe obstructive atelectasis while on gefitinib after
undergoing SBRT, which interrupted our evaluation of gefitinib
efficacy. Our analysis indicated that the occurrence of obstructive
atelectasis mainly resulted from SBRT for lesions located in the
hilum of the lung, which caused the late complication of
radioactive bronchial fibrosis, in turn leading to bronchial
stenosis. However, such severe obstructive atelectasis cannot
exclude the synergistic role of gefitinib. Due to the misjudgement
of obstructive atelectasis as a result of disease progression in
Case 1, gefitinib was wrongly terminated after 2 weeks, depriving
the patient of the opportunity to receive effective treatment. Case
2 was slightly more complicated. The patient did not undergo any
gene mutation detection tests. The clinical trial from IPASS
(17), suggested that the majority
of the patients developed drug resistance within 6–8 months of
taking the medication. This patient developed atelectasis exactly 7
months after gefitinib initiation. A PET-CT examination confirmed
that there was no progression of the original lesions. Hence, we
did not adopt the suggestion by outpatient physicians to
discontinue the medication. In fact, Takeda et al (18) reported that, for patients with
NSCLC after SBRT, the SUV from PET-CT are of higher diagnostic
value regarding local recurrence compared to those from CT.
Our experience with patients who have recently
undergone radiotherapy suggests that, if disease progression occurs
at the site of radiotherapy during the course of EGFR-TKI
treatment, the decision to discontinue the medication should be
based on PET-CT rather than CT alone. Furthermore, radioactive
atelectasis differs from radiation pneumonitis. If the patient is
able to tolerate the condition without severe breathing
difficulties, the recommendation is to continue medication.
However, our empirical findings must be substantiated and confirmed
through further clinical trials.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81272599).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Moran C: Importance of molecular features
of non-small cell lung cancer for choice of treatment. Am J Pathol.
178:1940–1948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salama JK and Vokes EE: New radiotherapy
and chemoradiotherapy approaches for non-small-cell lung cancer. J
Clin Oncol. 31:1029–1038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pfister DG, Johnson DH, Azzoli CG, et al;
American Society of Clinical Oncology. American Society of Clinical
Oncology treatment of unresectable non-small-cell lung cancer
guideline: update 2003. J Clin Oncol. 22:330–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furuse K, Fukuoka M, Kawahara M, et al:
Phase III study of concurrent versus sequential thoracic
radiotherapy in combination with mitomycin, vindesine, and
cisplatin in unresectable stage III non-small-cell lung cancer. J
Clin Oncol. 17:2692–2699. 1999.PubMed/NCBI
|
|
6
|
Okamoto I: Overview of chemoradiation
clinical trials for locally advanced non-small cell lung cancer in
Japan. Int J Clin Oncol. 13:112–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang CC, Chi KH, Kao SJ, et al: Upfront
gefitinib/erlotinib treatment followed by concomitant radiotherapy
for advanced lung cancer: a mono-institutional experience. Lung
Cancer. 73:189–194. 2011. View Article : Google Scholar
|
|
8
|
Sato Y, Ebara T, Sunaga N, Takahashi T and
Nakano T: Interaction of radiation and gefitinib on a human lung
cancer cell line with mutant EGFR gene in vitro. Anticancer Res.
32:4877–4881. 2012.PubMed/NCBI
|
|
9
|
Nishie K, Kawaguchi T, Tamiya A, et al:
Epidermal growth factor receptor tyrosine kinase inhibitors beyond
progressive disease: a retrospective analysis for Japanese patients
with activating EGFR mutations. J Thorac Oncol. 7:1722–1727. 2012.
View Article : Google Scholar
|
|
10
|
Graham JD, Nahum AE and Brada M: A
comparison of techniques for stereotactic radiotherapy by linear
accelerator based on 3-dimensional dose distributions. Radiother
Oncol. 22:29–35. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Senan S: Surgery versus stereotactic
radiotherapy for patients with early-stage non-small cell lung
cancer: More data from observational studies and growing clinical
equipoise. Cancer. 119:2668–2270. 2013. View Article : Google Scholar
|
|
12
|
Rowe BP, Boffa DJ, Wilson LD, Kim AW,
Detterbeck FC and Decker RH: Stereotactic body radiotherapy for
central lung tumors. J Thorac Oncol. 7:1394–1399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Xia TY, Wang YJ, et al:
Prospective study of epidermal growth factor receptor tyrosine
kinase inhibitors concurrent with individualized radiotherapy for
patients with locally advanced or metastatic non-small-cell lung
cancer. Int J Radiat Oncol Biol Phys. 81:e59–e65. 2011. View Article : Google Scholar
|
|
14
|
Onal C, Abali H, Koc Z and Kara S:
Radiation recall pneumonitis caused by erlotinib after palliative
definitive radiotherapy. Onkologie. 35:191–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Zhu XX, Wu XH, et al: Gefitinib
combined with stereotactic radiosurgery in previously treated
patients with advanced non-small cell lung cancer. Am J Clin Oncol.
Dec 1–2012.(Epub ahead of print).
|
|
16
|
Yoshitake T, Nakamura K, Shioyama Y, et
al: Stereotactic body radiation therapy for stage I non-small cell
lung cancer patients with chronic respiratory insufficiency
requiring domiciliary oxygen therapy. Anticancer Res. 32:4041–4044.
2012.
|
|
17
|
Fukuoka M, Wu YL, Thongprasert S, et al:
Biomarker analyses and final overall survival results from a phase
III, randomized, open-label, first-line study of gefitinib versus
carboplatin/paclitaxel in clinically selected patients with
advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol.
29:2866–2874. 2011. View Article : Google Scholar
|
|
18
|
Takeda A, Kunieda E, Fujii H, et al:
Evaluation for local failure by 18F-FDG PET/CT in comparison with
CT findings after stereotactic body radiotherapy (SBRT) for
localized non-small-cell lung cancer. Lung Cancer. 79:248–253.
2013. View Article : Google Scholar : PubMed/NCBI
|