Introduction
Gastric cancer is the second leading cause of
cancer-related mortality worldwide, accounting for ~1 in 10 of all
deaths from cancer (1). The
outcome of gastric cancer is generally poor, with a 5-year relative
survival of <30% in most countries (2). Although radical surgery remains the
cornerstone of treatment for gastric cancer, surgery alone appears
to have reached its limits in terms of local control and survival.
The achievement of locoregional control remains difficult in the
presence of advanced disease (3).
The majority of patients with advanced gastric cancer receive
palliative chemotherapy, which is associated with a median survival
of 11–12 months (4). In addition
to standard cytotoxic regimens, targeted therapies, using small
molecules or antibodies designed to disrupt the activity of
specific oncogenic signaling pathways, have recently emerged as a
promising treatment strategy. A number of receptor tyrosine kinases
(RTKs) have been associated with tumor progression and patient
outcomes in various types of cancer. RTK inhibitors, such as human
epidermal growth factor receptor (HER), have been evaluated and
some have been used to treat gastrointestinal cancers. In a recent
ToGA trial (5), trastuzumab, a
monoclonal antibody against the p185HER2 protein, improved the
overall survival of patients with HER2-positive tumors when
combined with chemotherapy. However, only 7–17% of gastric cancer
patients have HER2-positive tumors and are considered as suitable
candidates for anti-HER2 therapy (6,7).
Further investigations are required to increase the number of
patients with gastric cancer for whom targeted treatments may be a
viable clinical option.
The fibroblast growth factor receptor (FGFR) family
(FGFR1-4) belongs to the receptor tyrosine kinase superfamily.
FGFRs regulate fundamental developmental pathways by interacting
with fibroblast growth factors (FGFs) and thereby control a wide
range of events, extending from mesoderm patterning in the early
embryo to the development of multiple organ systems (8,9). FGF
signaling participates in several biological functions in the adult
organism, including regulation of angiogenesis and wound repair.
FGFRs are expressed on a number of different cell types and
regulate key cell activities, such as proliferation, survival,
migration and differentiation, which renders FGF signaling
susceptible to subversion by cancer cells (10).
FGFR2 amplifications have been reported in 10% of
gastric cancers, the majority of which are of the diffuse type
(11). FGFR2 amplification may
correlate with poor outcomes in patients with diffuse-type gastric
cancer (12). Moreover, the
presence of FGFR2 gene amplification in gastric cancer is
associated with sensitivity to inhibition of FGFR signaling by
tyrosine kinase inhibitors and monoclonal antibodies in preclinical
models (13,14). Thus, FGFR2 has attracted
considerable attention as a novel therapeutic candidate for the
development of targeted anticancer agents (15).
In contrast to FGFR2, the roles of FGFR1, FGFR3 and
FGFR4 have not been fully elucidated. Overexpression of these FGFRs
in gastric cancer was reported by a few small studies (16–19).
In this study, we aimed to investigate the correlations of FGFR1-4
immunohistochemical expression with clinicopathological
characteristics and outcomes in gastric cancer.
Patients and methods
Patients
Our study group comprised 222 patients with primary
gastric adenocarcinoma who underwent surgical resection between
January, 2003 and December, 2007 in the Department of
Esophagogastric Surgery, Tokyo Medical and Dental University. Each
tumor was classified according to the tumor-node-metastasis staging
system recommended by the International Union Against Cancer. Of
the 222 patients, 168 were men and 54 were women. The mean age of
the patients was 64.6 years (range, 21–92 years). All the patients
were evaluated for recurrent disease by tumor marker analysis or
diagnostic imaging (computed tomography, ultrasonography, magnetic
resonance imaging and endoscopy) every 3–6 months. Patients with
distant metastasis or recurrent disease received chemotherapy with
S-1 alone or combined chemotherapy. A total of 20 patients (9%)
received adjuvant chemotherapy with S-1 following radical
resection. All the patients were followed up until July, 2012. The
median follow-up was 60 months (range, 3–111 months). A total of 77
patients (35%) succumbed to gastric cancer, 66 (30%) had recurrent
disease and 11 (5%) died from other causes.
Immunostaining of the FGFR family
Immunohistochemical analysis was performed with the
use of secondary antibodies conjugated to a peroxidase-labeled
polymer [Histofine Simple Stain MAX PO (Multi); Nichirei Co.,
Tokyo, Japan]. Polyclonal rabbit antibodies against FGFR1, FGFR2,
FGFR3 and FGFR4 were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). All the available hematoxylin and
eosin-stained slides of the surgical specimens were reviewed. For
each case, representative formalin-fixed, paraffin-embedded tissue
blocks were selected for immunohistochemical studies and sliced
into 4-μm sections. Following deparaffinization and rehydration,
antigen retrieval was performed at 98°C for 30 min, using a pH 6.0,
10 mmol/l sodium citrate buffer (Mitsubishi Chemical Medience
Corporation, Tokyo, Japan) in a microwave processor (MI-77;
Azumaya, Tokyo, Japan). Endogenous peroxidase was blocked with 3%
hydrogen peroxide in methanol. Subsequently, non-specific binding
was blocked by treating the slides with 10% normal goat serum for
10 min at room temperature. The slides were incubated with the
primary antibodies, including anti-FGFR1 (dilution, 1:100),
anti-FGFR2 (dilution, 1:300), anti-FGFR3 (dilution, 1:500) and
anti-FGFR4 (dilution, 1:100) in 1% bovine serum
albumin/phosphate-buffered saline overnight at 4°C. The sections
were then incubated with Simple Stain Max PO (Multi) for 30 min at
room temperature. The chromogen substrate was 3,3′-diaminobenzidine
tetrahydrochloride solution (Histofine Simple Stain DAB solution;
Nichirei Co.). Subsequently, the sections were counterstained with
Mayer’s hematoxylin (Wako, Tokyo, Japan). Negative controls were
treated similarly, except for the antibodies being replaced by
normal rabbit IgG (Santa Cruz Biotechnology, Inc.).
Interpretation of immunostaining
results
The staining intensity was scored into four grades
as follows: 0, no staining; 1, weakly positive; 2, moderately
positive; and 3, strongly positive. The staining extent (positive
frequency) was also scored into four grades according to the
percentage of stained tumor cells as follows: 0, complete absence
of staining; 1, ≤20%; 2, >20 to ≤50%; and 3, >50% stained
cells. Composite scores were derived by addition of the intensity
score and the staining extent score. For the statistical analysis,
composite scores of ≥4 were defined as high expression and scores
of <4 as low expression. Two investigators (Hideaki Murase and
Yoko Takagi), who were blinded to the patients’ outcomes separately
counted the stained cancer cells. Any disagreements between the two
investigators were resolved by reassessment and consensus.
Statistical analysis
The statistical analysis was performed using IBM
SPSS Statistics 20 software (IBM, Inc., Armonk, NY, USA). The
χ2 test was used to investigate the possible
associations between the expression of each FGFR receptor and
clinicopathological variables. The χ2 test was also used
to assess the correlations between FGFR expressions. The
Mann-Whitney U test was used to analyze the associations between
FGFR expression and patient age. Kaplan-Meier curves were plotted
to assess the effects of FGFR expression on disease-specific
survival (DSS) and different DSS curves were compared using the
log-rank test. Multivariate proportional Cox models were used to
assess the prognostic significance of FGFR and of factors
associated with DSS. P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemical analysis of the FGFR
family
Expression of FGFR1, FGFR2, FGFR3 and FGFR4 was
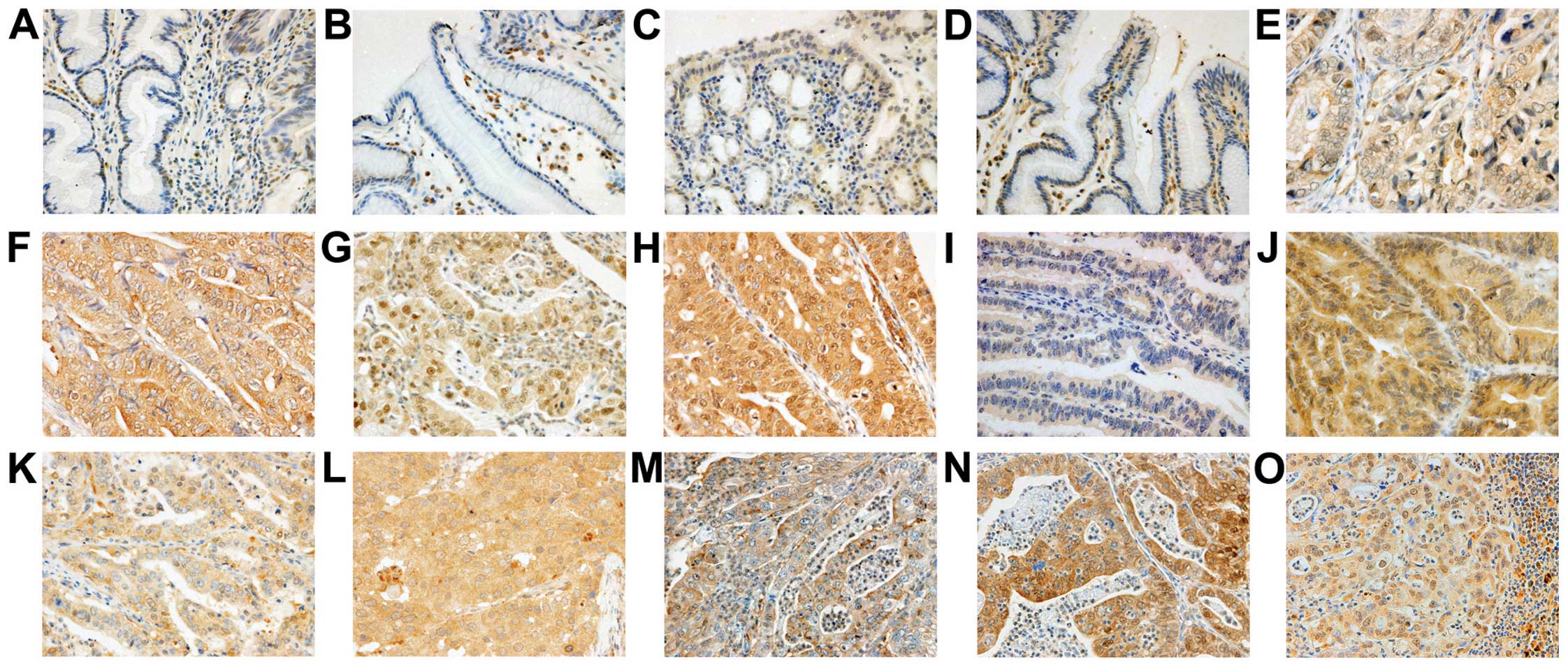
mainly observed in the cytoplasm of cancer cells (Fig. 1) and fibroblasts in cancer tissue.
Weak expression was observed in certain regions of the normal
epithelium in proximity to the cancer cells. Among the 222 tumors
investigated, the number of tumors exhibiting high FGFR expression
were 66 (30%) for FGFR1, 114 (51%) for FGFR2, 142 (64%) for FGFR3
and 175 (79%) for FGFR4. High expression of FGFR1, FGFR2, FGFR3 or
FGFR4 was significantly correlated with high expression of each of
the other three proteins (Table
I).
 | Table ICorrelations among the expressions of
FGFR1, FGFR2, FGFR3 and FGFR4. |
Table I
Correlations among the expressions of
FGFR1, FGFR2, FGFR3 and FGFR4.
| Expression
level | FGFR2 | FGFR3 | FGFR4 |
|---|
|
|
|
|---|
| Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| FGFR1 |
| Low | 94 | 62 | <0.001 | 64 | 92 | 0.022 | 43 | 113 | <0.001 |
| High | 14 | 52 | | 16 | 50 | | 4 | 62 | |
| FGFR2 |
| Low | | | | 49 | 59 | 0.005 | 39 | 69 | <0.001 |
| High | | | | 31 | 83 | | 8 | 106 | |
| FGFR3 |
| Low | | | | | | | 26 | 54 | 0.003 |
| High | | | | | | | 21 | 121 | |
Association with clinicopathological
variables
The clinicopathological variables are summarized in
Table II. A high expression of
FGFR1, FGFR2 and FGFR4 was significantly associated with the depth
of tumor invasion (T3–T4 vs. T1–T2: P<0.001, P=0.011 and
P<0.001, respectively), lymph node metastasis (P=0.002, P=0.011
and P<0.001, respectively), and tumor stage (III–IV vs. I–II:
P=0.001, P=0.012 and P<0.001, respectively). Distant metastasis
or recurrence was found in a significantly higher proportion of
patients with high expression of FGFR1, FGFR2 and FGFR4 compared to
those with low expression of these proteins (P<0.001, P=0.004
and P<0.001, respectively). As the high expression of FGFR1,
FGFR2 and FGFR4 was significantly associated with lymph node
metastasis, we immunohistochemically evaluated the expression of
these proteins in lymph node metastases from 88 patients and
compared it to their expression in the primary tumor. A high
expression of FGFR1, FGFR2 and FGFR4 was found in 61 (69%), 44
(50%), and 67 (76%) patients, respectively. However, only FGFR4
exhibited a significant association between its expression in the
primary tumor and that in metastatic lymph nodes (P=0.017)
(Table III).
 | Table IICorrelations of the expressions of
FGFR1, FGFR2, FGFR3 and FGFR4 with clinicopathological factors. |
Table II
Correlations of the expressions of
FGFR1, FGFR2, FGFR3 and FGFR4 with clinicopathological factors.
| Clinicopathological
factors | n | FGFR1 | FGFR2 | FGFR3 | FGFR4 |
|---|
|
|
|
|
|---|
| Low (156) | High (66) | P-value | Low (108) | High (114) | P-value | Low (80) | High (142) | P-value | Low (47) | High (175) | P-value |
|---|
| Age (years) | | | | 0.36 | | | 0.036 | | | 0.38 | | | 0.006 |
| <70 | 142 | 103 | 39 | | 77 | 65 | | 48 | 94 | | 38 | 104 | |
| ≥70 | 80 | 53 | 27 | | 31 | 49 | | 32 | 48 | | 9 | 71 | |
| Gender | | | | 1.00 | | | 1.00 | | | 1.00 | | | 0.70 |
| Female | 54 | 38 | 16 | | 26 | 28 | | 19 | 35 | | 10 | 44 | |
| Male | 168 | 118 | 50 | | 82 | 86 | | 61 | 107 | | 37 | 131 | |
| Main location | | | | 0.20 | | | 0.029 | | | 0.39 | | | 0.84 |
| Middle or
lower | 177 | 128 | 49 | | 93 | 84 | | 61 | 116 | | 37 | 140 | |
| Upper | 45 | 28 | 17 | | 15 | 30 | | 19 | 26 | | 10 | 35 | |
| WHO pathological
type | | | | 0.47 | | | 0.023 | | | 0.002 | | | 0.74 |
|
Differentiated | 106 | 77 | 29 | | 43 | 63 | | 27 | 79 | | 21 | 85 | |
|
Undifferentiated | 116 | 79 | 37 | | 65 | 51 | | 53 | 63 | | 26 | 90 | |
| Depth of
invasion | | | | <0.001 | | | 0.011 | | | 0.58 | | | <0.001 |
| T1/2 | 118 | 96 | 22 | | 67 | 51 | | 45 | 73 | | 41 | 77 | |
| T3/4 | 104 | 60 | 44 | | 41 | 63 | | 35 | 69 | | 6 | 98 | |
| Lymphatic
invasion | | | | 0.001 | | | 0.020 | | | 0.45 | | | <0.001 |
| Negative | 69 | 59 | 10 | | 42 | 27 | | 22 | 47 | | 26 | 43 | |
| Positive | 153 | 97 | 56 | | 66 | 87 | | 58 | 95 | | 21 | 132 | |
| Venous
invasion | | | | 0.019 | | | 0.004 | | | 0.66 | | | <0.001 |
| Negative | 73 | 59 | 14 | | 46 | 27 | | 28 | 45 | | 30 | 43 | |
| Positive | 149 | 97 | 52 | | 62 | 87 | | 52 | 97 | | 17 | 132 | |
| LN metastasis | | | | 0.002 | | | 0.011 | | | 0.41 | | | <0.001 |
| Negative (N0) | 114 | 91 | 23 | | 65 | 49 | | 38 | 76 | | 36 | 78 | |
| Positive
(N1/2/3) | 108 | 65 | 43 | | 43 | 65 | | 42 | 66 | | 11 | 97 | |
| Stage | | | | 0.001 | | | 0.012 | | | 0.47 | | | <0.001 |
| I/II | 141 | 110 | 31 | | 78 | 63 | | 48 | 93 | | 42 | 99 | |
| III/IV | 81 | 46 | 35 | | 30 | 51 | | 32 | 49 | | 5 | 76 | |
| Distant metastasis
or recurrence | | | | <0.001 | | | 0.004 | | | 0.29 | | | <0.001 |
| Negative | 152 | 119 | 33 | | 84 | 68 | | 51 | 101 | | 43 | 109 | |
| Positive | 70 | 37 | 33 | | 24 | 46 | | 29 | 41 | | 4 | 66 | |
 | Table IIICorrelations of FGFR1, FGFR2, FGFR3
and FGFR4 expression between primary tumors and metastatic lymph
nodes. |
Table III
Correlations of FGFR1, FGFR2, FGFR3
and FGFR4 expression between primary tumors and metastatic lymph
nodes.
| Expression
level | Metastatic lymph
nodes |
|---|
|
|---|
| Low | High | P-value |
|---|
| Primary tumor |
| FGFR1 |
| Low | 17 | 38 | 0.95 |
| High | 10 | 23 | |
| FGFR2 |
| Low | 21 | 14 | 0.13 |
| High | 23 | 30 | |
| FGFR4 |
| Low | 5 | 3 | 0.017 |
| High | 16 | 64 | |
Association with DSS
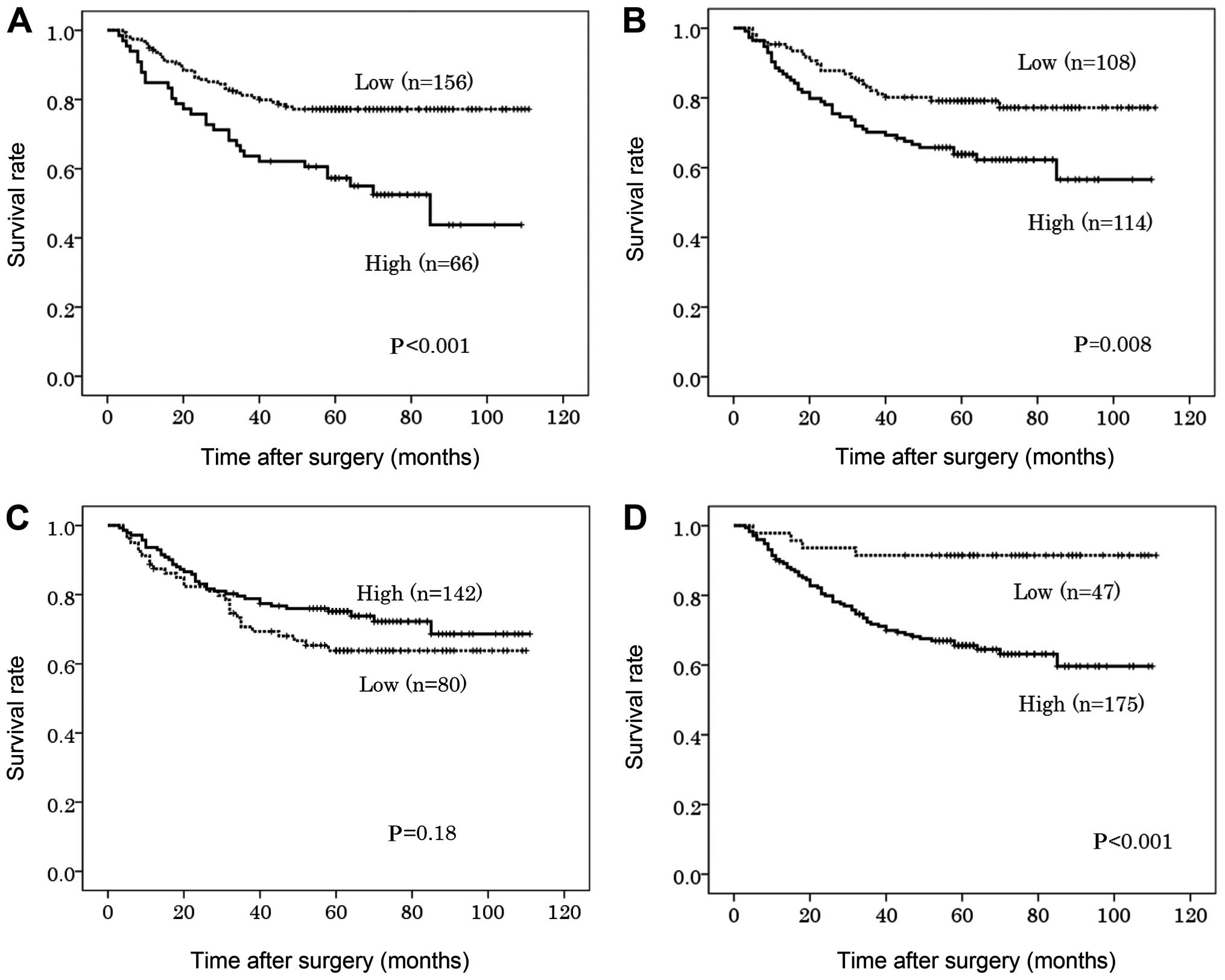
High expression of FGFR1, FGFR2 and FGFR4 in the
primary tumor was significantly associated with poorer DSS on the
univariate analysis (P<0.001, P=0.008 and P<0.001,
respectively) (Fig. 2). The 5-year
DSS in patients with high expression of FGFR1, FGFR2 and FGFR4 was
57, 63 and 66%, respectively, compared to 77, 79 and 91%,
respectively, in patients with low expression of these proteins
(Table IV). On the multivariate
analysis, the depth of tumor invasion and lymph node involvement
were independent prognostic factors [hazard ratio (HR)=6.80, 95%
confidence interval (CI): 2.63–15.6, P<0.001; and HR=4.48, 95%
CI: 1.90–10.5, P=0.001, respectively], unlike FGFR1, FGFR2 and
FGFR4 (HR=1.20, 95% CI: 0.73–2.00, P=0.47; HR=1.32, 95% CI:
0.77–2.24, P=0.31; and HR=0.96, 95% CI: 0.32–2.90, P=0.95,
respectively).
 | Table IVPrognostic factors in univariate and
multivariate Cox proportional-hazards regression models for DSS in
the study group as a whole. |
Table IV
Prognostic factors in univariate and
multivariate Cox proportional-hazards regression models for DSS in
the study group as a whole.
| Prognostic
factors | Univariate
(log-rank) | Multivariate |
|---|
|
|
|---|
| 5-year DSS (%) | P-value | HR | 95% CI | P-value |
|---|
| Age (years) |
| <70 | 72 | | | | |
| ≥70 | 69 | 0.37 | | | |
| Gender |
| Female | 72 | | | | |
| Male | 71 | 0.81 | | | |
| Main location |
| Middle or
lower | 74 | | | | |
| Upper | 62 | 0.15 | | | |
| WHO pathological
type |
|
Differentiated | 80 | | 1 | | |
|
Undifferentiated | 62 | 0.004 | 1.42 | 0.84–2.40 | 0.18 |
| Depth of
invasion |
| T1/2 | 97 | | 1 | | |
| T3/4 | 54 | <0.001 | 6.80 | 2.63–15.6 | <0.001 |
| LN metastasis |
| Negative | 95 | | 1 | | |
| Positive | 46 | <0.001 | 4.48 | 1.90–10.5 | 0.001 |
| FGFR1 |
| Low | 77 | | 1 | | |
| High | 57 | <0.001 | 1.20 | 0.73–2.00 | 0.47 |
| FGFR2 |
| Low | 79 | | 1 | | |
| High | 64 | 0.008 | 1.32 | 0.77–2.24 | 0.31 |
| FGFR3 |
| Low | 64 | | | | |
| High | 75 | 0.18 | | | |
| FGFR4 |
| Low | 91 | | 1 | | |
| High | 66 | <0.001 | 0.96 | 0.32–2.90 | 0.95 |
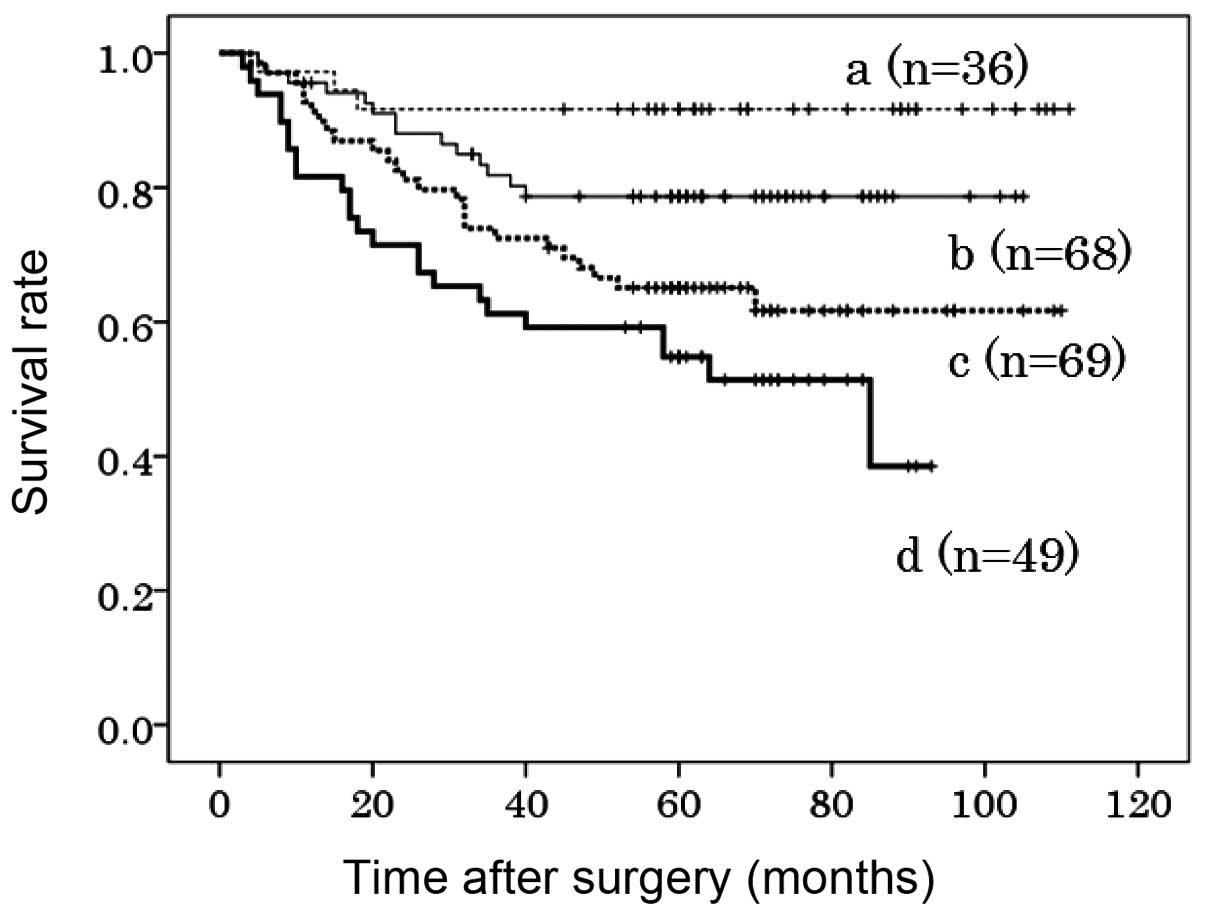
Co-overexpression of FGFR1, FGFR2 and
FGFR4
The co-overexpression of FGFR1, FGFR2 and FGFR4 in
the primary tumors was found to be significantly associated with a
poorer DSS compared to the expression of none or only one of these
proteins (P<0.001 and P=0.001). The 5-year DSS was 55, 65, 78
and 92% in patients exhibiting high expression of all three, two,
one and none of these FGFRs, respectively (Fig. 3). Although tumor stage was the most
significant prognostic factor (HR=22.3, 95% CI: 10.1–49.6,
P<0.001), the co-overexpression of these three FGFRs was also
identified as an independent prognostic factor (HR=1.71, 95% CI:
1.02–2.85, P=0.041) (Table V).
 | Table VPrognostic factors in multivariate
Cox proportional-hazards regression models for disease-specific
survival of patients with co-overexpression of FGFR1, FGFR2 and
FGFR4. |
Table V
Prognostic factors in multivariate
Cox proportional-hazards regression models for disease-specific
survival of patients with co-overexpression of FGFR1, FGFR2 and
FGFR4.
| Prognostic
factors | Multivariate |
|---|
|
|---|
| HR | 95% CI | P-value |
|---|
| WHO pathological
type | | | |
|
Differentiated | 1 | | |
|
Undifferentiated | 1.29 | 0.77–2.19 | 0.33 |
| Stage | | | |
| Stage I/II | 1 | | |
| Stage III/IV | 22.3 | 10.1–49.6 | <0.001 |
| Co-overexpression
of FGFR1, 2 and 4 | | | |
| Others | 1 | | |
| All high | 1.71 | 1.02–2.85 | 0.041 |
Discussion
Our results suggested that high expression of FGFR1,
FGFR2 or FGFR4 may be crucial in tumor progression, metastasis and
outcomes in gastric cancer patients. Moreover, the
co-overexpression of FGFR1, FGFR2 and FGFR4 was found to be an
independent prognostic factor in gastric cancer.
FGFR-dependent signaling occurs through two main
pathways: via the intracellular receptor substrates FGFR substrate
2 (FRS2) and phospholipase Cg (PLCg), ultimately upregulating the
Ras-dependent mitogen-activated protein kinase, and the
Ras-independent phosphoinositide 3-kinase-Akt signaling pathways
(15). Other pathways may also be
activated by FGFRs, including STAT-dependent signaling (20). Although all four FGFRs generally
signal through a similar network of pathways, a number of
qualitative and quantitative differences have been identified and
FGFR-specific differences in signaling pathways associated with
genetic alterations of each FGFR have been confirmed in different
types of cancer (21–24).
FGFR1 amplification was previously identified in
breast (25), ovarian (26), bladder (27) and lung cancer (28). In gastric cancer, a previous study
reported the presence of FGFR1 amplifications in 12 (50%) of the 24
cases and FGFR1 protein was overexpressed in 37 (61%) of the 61
specimens on immunohistochemical analysis using a monoclonal
antibody that differed from the one used in the present study.
However, that study reported no significant correlation between
FGFR1 expression and clinicopathological characteristics (17). To the best of our knowledge, the
present study is the first to demonstrate that the high expression
of FGFR1 is associated with poor survival in gastric cancer. The
overexpression of FGFR1 was also found to be correlated with liver
metastasis in colorectal cancer (29), whereas the amplification and
overexpression of FGFR1 may contribute to poor outcomes in
luminal-type breast cancer by driving anchorage-independent
proliferation and resistance to endocrine therapy (25). The co-overexpression of FGF1 and
FGFR1 has also been associated with poor outcomes in esophageal
squamous cell carcinoma (30);
however, we did not assess FGF expression in this study.
The FGFR4 Gly388Arg polymorphism has attracted
considerable attention since the discovery of this germline
polymorphism by Bange et al (31). In the human FGFR4 gene, a
single-nucleotide polymorphism (SNP) from G to A at codon 388 at
exon 9 changes the amino acid sequence of FGFR4 from glycine to
arginine (Gly388 to Arg388). FGFR4 Gly388Arg was found to be
associated with poor outcomes in breast (32), ovarian (33), lung (34) and gastric cancer (18). FGFR4 amplification was also found
in pancreatic (35), renal cell
(36) and gastric cancer (19). Ye et al (19) reported that the high expression of
FGFR4 is associated with lymph node metastasis and a trend toward
worse survival. Our findings are consistent with the findings of
that study.
FGFR2 amplification was previously reported in
gastric (37) and breast cancer
(38), whereas FGFR2 missense
mutations have been identified in gastric (39), lung (40), ovarian (41) and endometrial cancer (42), as well as in melanoma (43). FGFR2 genetic amplification or
mutation leads to abnormal activation of the FGFR2 signaling
pathway and contributes to carcinogenesis and tumor progression in
gastric cancer. Overexpression of FGFR2 protein was detected on
immunohistochemical staining in 20 of 38 diffuse-type gastric
cancers, but in none of 11 intestinal-type lesions (44). FGFR2 amplifications were found in
10% of gastric cancers, the majority of which were of the
undifferentiated type (11).
Furthermore, FGFR2 amplification may correlate with poor outcomes
in undifferentiated gastric cancer (12). In the present study, a high
expression of FGFR2 was observed in undifferentiated as well as in
differentiated-type gastric cancer. Although high expression of
FGFR2 was not identified as an independent prognostic factor, it
was significantly associated with poorer survival. Our findings are
consistent with the results of previous studies.
FGFR3 amplification has been rarely reported in
cancer (45,46). FGFR3 mutations have been identified
in several types of cancer, including cervical cancer (47), multiple myeloma, prostate cancer
(48) and spermatocytic seminomas
(49). Bladder cancer exhibits the
most clearly established association with FGFR3 mutations, which
are strongly associated with low-grade non-invasive disease
(50). Overexpression of FGFR3 was
reportedly associated with low-stage bladder cancers on
immunohistochemical analysis (51). However, overexpression of FGFR3 has
also been associated with poor differentiation and high nuclear
grade in hepatocellular carcinoma (52). The overexpression of FGFR3 in
invasive breast cancer was not significantly associated with
specific clinicopathological characteristics, although it was
suggested to be a candidate marker for a poor prognosis (53). In this study, the expression of
FGFR3 was not significantly associated with clinicopathological
findings or survival. Shin et al (16) investigated the expression of the
FGFRs in gastric cancer tissues and cell lines on northern blot
analysis, ribonuclease protection assay and immunohistochemical
analysis and reported that the mRNAs of FGFR1, FGFR2 and FGFR4 were
upregulated in cancer tissues, whereas FGFR3 mRNA was not. These
FGFR mRNAs were coexpressed in various combinations of two or three
in the same tissue. The immunohistochemical analysis confirmed
specific staining of multiple FGFRs, excluding FGFR3, in cancer
specimens. In the present study, a high expression of FGFR3 was
detected in addition to that of the other three FGFRs. The
discrepancies among studies may be attributed to the differences in
disease stage or the techniques used for immunohistochemical
analysis.
We also evaluated the expression of FGFR1, FGFR2 and
FGFR4 in metastatic lymph nodes. To the best of our knowledge,
FGFRs in metastatic sites of gastric cancer had not been previously
investigated. The expression of FGFR1 and FGFR2 differed between
the primary tumors and lymph node metastases and were significantly
correlated with the expression of only FGFR4. The differences in
FGFR1 or FGFR2 expression among different tumor sites may represent
a challenge regarding chemotherapy against these molecular
targets.
FGFR-targeted therapeutics using small-molecule
compounds that inhibit binding of FGF to FGFR is an active topic in
the field of clinical oncology (54). Ki23057, a FGFR2 inhibitor, was
reported to enhance the chemosensitivity of drug-resistant gastric
cancer cells (55). Inhibition of
FGFR2 signaling by AZD4547, a selective inhibitor of FGFR1, FGFR2
and FGFR3, was shown to significantly inhibit tumor growth in a
dose-dependent manner in FGFR2-amplified xenografts (56). AZD4547 is currently being compared
to paclitaxel as second-line treatment for patients with gastric
cancer whose tumors exhibit FGFR2 gene amplification (NCT01457846,
SHINE). Monoclonal antibodies that selectively recognize FGF or
FGFR represent additional options for FGFR-targeted cancer therapy.
Anti-FGFR2 monoclonal antibodies inhibit the in vivo growth
of SNU-16 and OCUM-2M gastric cancer cells with FGFR2 gene
amplification (13). Our results
suggested that a selective inhibitor of FGFR1, FGFR2 and FGFR4 or
the combined use of anti-FGFR1, -FGFR2 and -FGFR4 monoclonal
antibodies may represent an effective FGFR-targeted therapy for
gastric cancer.
In conclusion, high expression of FGFR1, FGFR2 or
FGFR4 may be associated with tumor progression and poor survival in
patients with gastric cancer. Similar to FGFR2, FGFR1 and FGFR4 may
represent future prognostic factors and treatment targets in
gastric cancer.
Acknowledgements
K.M., M.I. and K.S. were responsible for drafting
the manuscript. K.M., K.K. and Y.T. contributed to the
immunohistochemical analysis. M.I. and K.K. contributed to the
analysis and interpretation of data.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar
|
|
3
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar
|
|
4
|
Cunningham D, Starling N, Rao S, et al:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
6
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: a new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hofmann M, Stoss O, Shi D, et al:
Assessment of a HER2 scoring system for gastric cancer: results
from a validation study. Histopathology. 52:797–805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kimelman D and Kirschner M: Synergistic
induction of mesoderm by FGF and TGF-beta and the identification of
an mRNA coding for FGF in the early Xenopus embryo. Cell.
51:869–877. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Moerlooze L, Spencer-Dene B, Revest JM,
Hajihosseini M, Rosewell I and Dickson C: An important role for the
IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in
mesenchymal-epithelial signalling during mouse organogenesis.
Development. 127:483–492. 2000.PubMed/NCBI
|
|
10
|
Turner N and Grose R: Fibroblast growth
factor signalling: from development to cancer. Nat Rev Cancer.
10:116–129. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kunii K, Davis L, Gorenstein J, et al:
FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3
signaling for growth and survival. Cancer Res. 68:2340–2348. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toyokawa T, Yashiro M and Hirakawa K:
Co-expression of keratinocyte growth factor and K-sam is an
independent prognostic factor in gastric carcinoma. Oncol Rep.
21:875–880. 2009.PubMed/NCBI
|
|
13
|
Zhao WM, Wang L, Park H, et al: Monoclonal
antibodies to fibroblast growth factor receptor 2 effectively
inhibit growth of gastric tumor xenografts. Clin Cancer Res.
16:5750–5758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao G, Li WY, Chen D, et al: A novel,
selective inhibitor of fibroblast growth factor receptors that
shows a potent broad spectrum of antitumor activity in several
tumor xenograft models. Mol Cancer Ther. 10:2200–2210. 2011.
View Article : Google Scholar
|
|
15
|
Brooks AN, Kilgour E and Smith PD:
Molecular pathways: fibroblast growth factor signaling: a new
therapeutic opportunity in cancer. Clin Cancer Res. 18:1855–1862.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shin EY, Lee BH, Yang JH, et al:
Up-regulation and co-expression of fibroblast growth factor
receptors in human gastric cancer. J Cancer Res Clin Oncol.
126:519–528. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oki M, Yamamoto H, Taniguchi H, Adachi Y,
Imai K and Shinomura Y: Overexpression of the receptor tyrosine
kinase EphA4 in human gastric cancers. World J Gastroenterol.
14:5650–5656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye Y, Shi Y, Zhou Y, et al: The fibroblast
growth factor receptor-4 Arg388 allele is associated with gastric
cancer progression. Ann Surg Oncol. 17:3354–3361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye YW, Zhou Y, Yuan L, et al: Fibroblast
growth factor receptor 4 regulates proliferation and antiapoptosis
during gastric cancer progression. Cancer. 117:5304–5313. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eswarakumar VP, Lax I and Schlessinger J:
Cellular signaling by fibroblast growth factor receptors. Cytokine
Growth Factor Rev. 16:139–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vainikka S, Joukov V, Wennström S, Bergman
M, Pelicci PG and Alitalo K: Signal transduction by fibroblast
growth factor receptor-4 (FGFR-4). Comparison with FGFR-1. J Biol
Chem. 269:18320–18326. 1994.PubMed/NCBI
|
|
22
|
Wang JK, Gao G and Goldfarb M: Fibroblast
growth factor receptors have different signaling and mitogenic
potentials. Mol Cell Biol. 14:181–188. 1994.PubMed/NCBI
|
|
23
|
Shaoul E, Reich-Slotky R, Berman B and Ron
D: Fibroblast growth factor receptors display both common and
distinct signaling pathways. Oncogene. 10:1553–1561.
1995.PubMed/NCBI
|
|
24
|
Xian W, Schwertfeger KL and Rosen JM:
Distinct roles of fibroblast growth factor receptor 1 and 2 in
regulating cell survival and epithelial mesenchymal transition. Mol
Endocrinol. 21:987–1000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Turner N, Pearson A, Sharpe R, et al:
FGFR1 amplification drives endocrine therapy resistance and is a
therapeutic target in breast cancer. Cancer Res. 70:2085–2094.
2010. View Article : Google Scholar
|
|
26
|
Theillet C, Adelaide J, Louason G, et al:
FGFRI and PLAT genes and DNA amplification at 8p12 in breast and
ovarian cancers. Genes Chromosomes Cancer. 7:219–226. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simon R, Richter J, Wagner U, et al:
High-throughput tissue microarray analysis of 3p25 (RAF1) and 8p12
(FGFR1) copy number alterations in urinary bladder cancer. Cancer
Res. 61:4514–4519. 2001.PubMed/NCBI
|
|
28
|
Weiss J, Sos ML, Seidel D, et al: Frequent
and focal FGFR1 amplification associates with therapeutically
tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl
Med. 2:62ra932010.PubMed/NCBI
|
|
29
|
Sato T, Oshima T, Yoshihara K, et al:
Overexpression of the fibroblast growth factor receptor-1 gene
correlates with liver metastasis in colorectal cancer. Oncol Rep.
21:211–216. 2009.PubMed/NCBI
|
|
30
|
Sugiura K, Ozawa S, Kitagawa Y, Ueda M and
Kitajima M: Co-expression of aFGF and FGFR-1 is predictive of a
poor prognosis in patients with esophageal squamous cell carcinoma.
Oncol Rep. 17:557–564. 2007.PubMed/NCBI
|
|
31
|
Bange J, Prechtl D, Cheburkin Y, et al:
Cancer progression and tumor cell motility are associated with the
FGFR4 Arg(388) allele. Cancer Res. 62:840–847. 2002.PubMed/NCBI
|
|
32
|
Thussbas C, Nahrig J, Streit S, et al:
FGFR4 Arg388 allele is associated with resistance to adjuvant
therapy in primary breast cancer. J Clin Oncol. 24:3747–3755. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marmé F, Hielscher T, Hug S, et al:
Fibroblast growth factor receptor 4 gene (FGFR4) 388Arg allele
predicts prolonged survival and platinum sensitivity in advanced
ovarian cancer. Int J Cancer. 131:E586–E591. 2012.PubMed/NCBI
|
|
34
|
Frullanti E, Berking C, Harbeck N, et al:
Meta and pooled analyses of FGFR4 Gly388Arg polymorphism as a
cancer prognostic factor. Eur J Cancer Prev. 20:340–347. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leung HY, Gullick WJ and Lemoine NR:
Expression and functional activity of fibroblast growth factors and
their receptors in human pancreatic cancer. Int J Cancer.
59:667–675. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takahashi A, Sasaki H, Kim SJ, et al:
Identification of receptor genes in renal cell carcinoma associated
with angiogenesis by differential hybridization technique. Biochem
Biophys Res Commun. 257:855–859. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hattori Y, Odagiri H, Nakatani H, et al:
K-sam, an amplified gene in stomach cancer, is a member of the
heparin-binding growth factor receptor genes. Proc Natl Acad Sci
USA. 87:5983–5987. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heiskanen M, Kononen J, Bärlund M, et al:
CGH, cDNA and tissue microarray analyses implicate FGFR2
amplification in a small subset of breast tumors. Anal Cell Pathol.
22:229–234. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jang JH, Shin KH and Park JG: Mutations in
fibroblast growth factor receptor 2 and fibroblast growth factor
receptor 3 genes associated with human gastric and colorectal
cancers. Cancer Res. 61:3541–3543. 2001.PubMed/NCBI
|
|
40
|
Davies H, Hunter C, Smith R, et al:
Somatic mutations of the protein kinase gene family in human lung
cancer. Cancer Res. 65:7591–7595. 2005.PubMed/NCBI
|
|
41
|
Byron SA, Gartside MG, Wellens CL, et al:
FGFR2 mutations are rare across histologic subtypes of ovarian
cancer. Gynecol Oncol. 117:125–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pollock PM, Gartside MG, Dejeza LC, et al:
Frequent activating FGFR2 mutations in endometrial carcinomas
parallel germline mutations associated with craniosynostosis and
skeletal dysplasia syndromes. Oncogene. 26:7158–7162. 2007.
View Article : Google Scholar
|
|
43
|
Gartside MG, Chen H, Ibrahimi OA, et al:
Loss-of-function fibroblast growth factor receptor-2 mutations in
melanoma. Mol Cancer Res. 7:41–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hattori Y, Itoh H, Uchino S, et al:
Immunohistochemical detection of K-sam protein in stomach cancer.
Clin Cancer Res. 2:1373–1381. 1996.PubMed/NCBI
|
|
45
|
Nord H, Segersten U, Sandgren J, et al:
Focal amplifications are associated with high grade and recurrences
in stage Ta bladder carcinoma. Int J Cancer. 126:1390–1402.
2010.PubMed/NCBI
|
|
46
|
Khnykin D, Troen G, Berner JM and Delabie
A: The expression of fibroblast growth factors and their receptors
in Hodgkin’s lymphoma. J Pathol. 208:431–438. 2006.
|
|
47
|
Rosty C, Aubriot MH, Cappellen D, et al:
Clinical and biological characteristics of cervical neoplasias with
FGFR3 mutation. Mol Cancer. 4:152005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hernández S, de Muga S, Agell L, et al:
FGFR3 mutations in prostate cancer: association with low-grade
tumors. Modern Pathol. 22:848–856. 2009.PubMed/NCBI
|
|
49
|
Goriely A, Hansen RMS, Taylor IB, et al:
Activating mutations in FGFR3 and HRAS reveal a shared genetic
origin for congenital disorders and testicular tumors. Nat Genet.
41:1247–1252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
50
|
van Oers JM, Wild PJ, Burger M, et al:
FGFR3 mutations and a normal CK20 staining pattern define low-grade
noninvasive urothelial bladder tumours. Eur Urol. 52:760–768.
2007.
|
|
51
|
Mhawrech-Fauceglia P, Cheney RT, Fischer
G, Beek A and Herrmann FR: FGFR3 and p53 protein expressions in
patients with pTa and pT1 urothelial bladder cancer. Eur J Surg
Oncol. 32:231–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qiu WH, Zhou BS, Chu PGG, et al:
Over-expression of fibroblast growth factor receptor 3 in human
hepatocellular carcinoma. World J Gastroenterol. 11:5266–5272.
2005.PubMed/NCBI
|
|
53
|
Kuroso K, Imai Y, Kobayashi M, et al:
Immunohistochemical detection of fibroblast growth factor receptor
3 in human breast cancer: correlation with
clinicopathological/molecular parameters and prognosis.
Pathobiology. 77:231–240. 2010. View Article : Google Scholar
|
|
54
|
Katoh M and Nakagama H: FGF receptors:
cancer biology and therapeutics. Med Res Rev. 34:280–300. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qiu H, Yashiro M, Zhang X, Miwa A and
Hirakawa K: A FGFR2 inhibitor, Ki23057, enhances the
chemosensitivity of drug-resistant gastric cancer cells. Cancer
Lett. 307:47–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xie L, Su X, Zhang L, et al: FGFR2 gene
amplification in gastric cancer predicts sensitivity to the
selective FGFR inhibitor AZD4547. Clin Cancer Res. 19:2572–2583.
2013. View Article : Google Scholar : PubMed/NCBI
|