Introduction
Hepatocellular carcinoma (HCC) is most prevalent in
developing countries; however, its incidence was reported to be on
the increase in North America (1).
HCC is one of the most common malignancies worldwide (2) and the third leading cause of
cancer-related mortality (3), with
an overall 5-year survival rate of merely 3–5% (4). It was reported that 10–20% of newly
diagnosed HCCs are >10 cm in diameter (5). Although there are various treatments
for HCC, including surgery (hepatic resection and liver
transplantation), percutaneous ethanol injection (PEI),
radiofrenquency ablation (RFA) and transarterial chemoembolization
(TACE), the treatment options for huge (≥10 cm) HCCs are limited.
PEI, RFA and liver transplantation are not considered suitable
treatment modalities for large HCCs (6–8).
Hepatic resection is considered the treatment of choice for HCC;
however, a tumor recurrence rate of 50–60% remains a significant
issue following curative resection (9). Furthermore, a number of patients have
unresectable HCCs (10,11). TACE alone is unsatisfactory,
particularly for large tumors (12). Therefore, there is a need for
effective treatments for huge HCCs. Despite the lack of randomized
controlled trials, radiotherapy is becoming recognized as a
potentially curative treatment option (13). Conformal radiotherapy (CRT) for HCC
was reported to exert a significant effect (14–16),
as was SBRT (17–20). However, the number of studies on
the application of CRT and SBRT for the treatment of huge HCCs is
limited. Thus, it remains to be determined whether SBRT is
feasible, safe and effective in the treatment of huge HCCs. In
order to expand the use of SBRT as an effective treatment for
patients with huge HCCs, in this study, we retrospectively analyzed
the clinical outcomes of 72 such patients treated with a
combination of SBRT and TACE.
Methods and materials
Patient eligibility
In this retrospective study, data were collected
from the Tumor Radiotherapy Center of Fuzhou General Hospital. A
total of 1,086 consecutive HCC patients were treated with gamma-ray
SBRT between May, 2006 and December, 2012 and 72 patients were
ultimately included in the study after a retrospective review
following Institutional Review Board approval. The inclusion
criteria were as follows: i) inoperable tumor; ii) tumor sized ≥10
cm; iii) Child-Pugh class A or B; iv) Eastern Cooperative Oncology
Group performance status (ECOG PS) of 0–2; v) no extrahepatic
metastasis; vi) treatment with SBRT combined with incomplete TACE;
vii) no history of hepatic radiotherapy. The included patients had
undergone contrast-enhanced computed tomography (CT), magnetic
resonance imaging (MRI) and/or positron emission tomography of the
abdomen. The blood tests included hepatitis B surface antigen,
antibody to hepatitis C virus, serum α-fetoprotein (AFP), serum
creatinine, albumin, alanine transaminase and total bilirubin. HCC
was diagnosed by cytological/histological evidence (n=65), one
radiological image showing the characteristic features of HCC
together with an elevated AFP level (>400 ng/ml) (n=5), or at
least 2 radiological images showing the characteristic features of
HCC (n=2). The 72 patients were classified into those with and
those without tumor encapsulation (group A, 33 patients and group
B, 39 patients, respectively).
Treatment
TACE was performed with infusion of a mixture of
5–10 ml of iodized oil (Lipiodol; Laboratoires Guerbet,
Roissy-Charles-de-Gaulle Cedex, France) and 1 mg/kg cisplatin
(Dong-A Pharmaceutical Co. Ltd., Seoul, Korea), followed by gelatin
sponge cubes (Gelfoam; Upjohn Co., Kalamazoo, MI, USA). The feeding
arteries of the tumor were carefully selected for TACE in order to
preserve the liver function as much as possible. TACE was performed
without Lipiodol to prevent severe damage to normal liver when
there was an arterial portal shunt. SBRT was administered using the
total body gamma-ray stereotactic radiotherapy system 2–4 weeks
after TACE. Briefly, the patients were immobilized by vacuum
cushions and underwent a CT scan in the supine or prostrate
position. The CT data were transferred to the SBRT Treatment
Planning System (SGI; Southeast University, Nanjing, China). The
body surface, tumor contour and important normal tissues were
reconstructed to display three-dimensional representation. The
clinical target volume (CTV) is defined as the macroscopic volume
of the tumor. The planning target volume (PTV) was created by
symmetrically expanding the CTV by 0.5 cm. The position, number and
size of focused fields were elaborately selected to enhance the
dose for the PTV but minimize the dose to the normal tissues and
the irradiated volumes. The generated dose-volume histogram and
isodose curves were used to evaluate the treatment planning. Dose
prescription was normalized at 50 or 55% isodose curve.
Verification films were taken to verify the tumor localization and
the patient’s position prior to SBRT. The median total dose of 35.6
Gy was delivered over 12–14 days with a fractional dose of 2.6–3.0
Gy and 6 fractions per week. The total and fractional dose depended
on the predicted toxicity of normal tissues and the functional
liver reserve. All the patients had one day of rest after every 6
consecutive fractions of treatment. Written informed consent was
obtained from each patient prior to treatment with TACE and
SBRT.
Evaluation of response, survival and
toxicity
The patients were weekly assessed by complete blood
counts and liver function tests during the course of the treatment.
Tumor response within the radiotherapy field was based on CT and/or
MRI scans 4 weeks after the completion of the treatment and at 1-
to 3-month intervals thereafter. According to the World Health
Organization criteria (21),
complete response (CR) was defined as disappearance of the tumor,
partial response (PR) as a >50% decrease in tumor size,
progressive disease (PD) as a >25% of in-field tumor growth and
stable disease (SD) as neither PR nor PD. The sum of CR and PR was
defined as objective response (OR). Survival time was estimated
from treatment initiation to the date of death or the last
follow-up.
Acute and late toxicities were assessed using the
National Cancer Institute Common Toxicity Criteria, version 2.0,
and the Late Radiation Morbidity Scoring Scheme of Radiotherapy
Oncology Group/European Organization for Research and Treatment of
Cancer, respectively.
Statistical analysis
The overall survival (OS) rate was calculated using
the Kaplan-Meier method. The log-rank test was used to identify the
predictive factors for survival. For multivariate analysis to
evaluate the association between the OS and various parameters, the
stepwise procedure was performed using the Cox regression model.
P<0.05 was considered to indicate a statistically significant
difference. The statistical analyses were conducted using the SPSS
16.0 statistical software package (SPSS Inc., Chicago, IL,
USA).
Results
Patient characteristics
The two groups of patients were compared by age,
gender, tumor size, ECOG PS, Child-Pugh classification, fractional
dose and delivered total dose. The characteristics of the
investigated patients are compared in Table I.
 | Table ICharacteristics of the included
patients. |
Table I
Characteristics of the included
patients.
| Group A | Group B |
|---|
|
|
|
|---|
| Variables | Values | No. of patients
(%) | Values | No. of patients
(%) |
|---|
| Age, years range
(mean) | 41–69 (54) | | 38–66 (51) | |
| Gender |
| Male | | 27 (81.8) | | 30 (76.9) |
| Female | | 6 (18.2) | | 9 (23.1) |
| ECOG PS |
| 0 | | 3 (9.1) | | 0 (0.0) |
| 1 | | 22 (66.7) | | 29 (74.4) |
| 2 | | 8 (24.2) | | 10 (25.6) |
| Child-Pugh class |
| A | | 24 (72.7) | | 28 (71.8) |
| B | | 9 (27.3) | | 11 (28.2) |
| AFP, ng/ml |
| ≥400 | | 27 (81.8) | | 32 (82.1) |
| <400 | | 6 (18.2) | | 7 (17.9) |
| HBsAg-positivity | | 25 (75.8) | | 29 (74.4) |
|
Anti-HCV-positivity | | 4 (12.1) | | 5 (12.8) |
| C/H confirmation |
| Yes | | 30 (90.9) | | 35 (89.7) |
| No | | 3 (9.1) | | 4 (10.3) |
| Delivered dose, Gy
range (median) | 33.8–39.0 (35.7) | | 33.8–39.0 (35.4) | |
| Fractional dose, Gy
range (median) | 2.6–3.0 (2.8) | | 2.6–3.0 (2.8) | |
| Tumor size, cm range
(median) | 10.8–16.5 (12.6) | | 10.2–17.6 (13.1) | |
Tumor response
Among the 72 patients, CR, PR, SD and PD were
observed in 6 (8.3%), 51 (70.8%), 9 (12.5%) and 6 patients (8.3%),
respectively, within a median follow-up of 18 months. The OR rate
was 79.1%. In group A, 2 patients (6.1%) had in-field recurrence
and 5 patients (15.2%) developed intra-and/or extrahepatic
metastases within 1 year. In group B, 4 patients (10.3%) had
in-field recurrence within 8 months and 13 patients (33.3%)
developed intra-and/or extrahepatic metastases within 1 year. None
of the patients in group B achieved a CR. The tumor responses in
the two groups are compared in Table
II.
 | Table IIResponse of huge HCCs treated by SBRT
combined with TACE. |
Table II
Response of huge HCCs treated by SBRT
combined with TACE.
| No. of patients
(%) |
|---|
|
|
|---|
| Type of response | Group A | Group B |
|---|
| Complete response
(CR) | 6 (18.2) | 0 (0.0) |
| Partial response
(PR) | 24 (72.7) | 27 (69.2) |
| Stable disease
(SD) | 1 (3) | 8 (20.5) |
| Progressive disease
(PD) | 2 (6.1) | 4 (10.3) |
| Objective response
(OR=CR+PR) | 31 (90.9) | 27 (69.2) |
Survival outcomes
The follow-up period ranged between 4 and 70 months
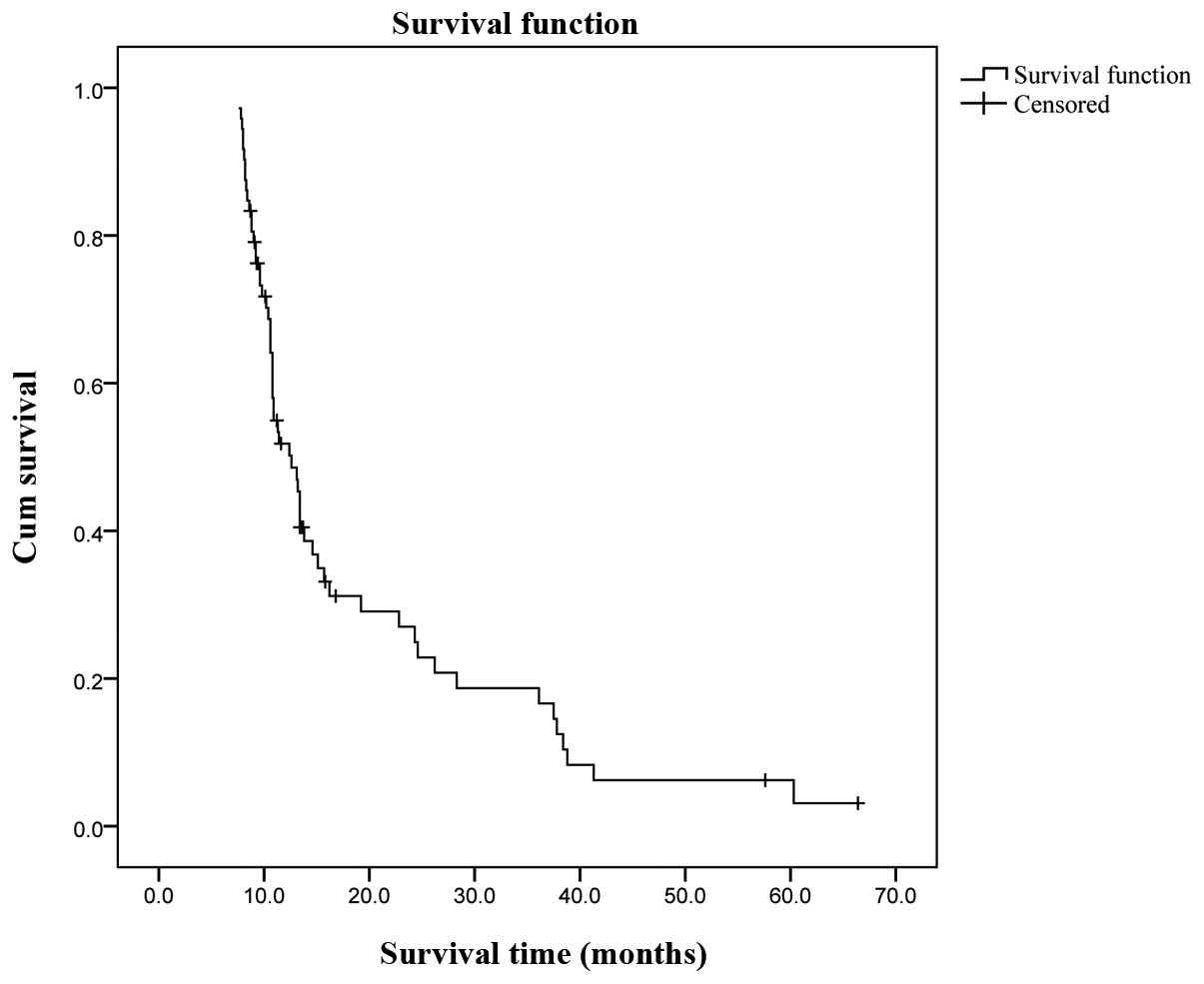
(median, 18 months). The Kaplan-Meier survival analysis indicated
an overall cumulative median survival of 12.2 months (range,
7.6–66.4 months; Fig. 1). The
overall cumulative 1-, 3- and 5-year survival rates assessed by the
life table (survival) analysis were 38, 12 and 3%, respectively. A
multivariate analysis was performed using the Cox regression model.
The results indicated that age, gender, ECOG PS, tumor size,
delivered dose and AFP level were not associated with overall
cumulative survival. However, patient grouping (A vs. B) was
associated with overall cumulative survival. In group A, the
overall cumulative 1-, 3- and 5-year survival rates were 56, 21 and
6%, respectively, with a median survival of 19 months. The results
were significantly better compared to those in group B, in which
the overall cumulative 1-, 3- and 5-year survival rates were 23, 4
and 0%, respectively, with a median survival of 10.8 months
(P=0.023). Child-Pugh class (A vs. B) was also associated with
overall cumulative survival; the overall cumulative 1-, 3- and
5-year survival rates of Child-Pugh class A patients were 57.2,
23.6 and 8.2%, respectively, vs. 35.4, 6.5 and 0% in Child-Pugh
class B patients. However, the difference was not statistically
significant (P=0.068). The overall cumulative survival of the two
groups (A vs. B) and Child-Pugh classes (A vs. B) is shown in
Figs. 2 and 3, respectively.
Toxicity
All the patients completed the treatment with no
severe radiation-induced liver disease (RILD) observed during the
median 18-month follow-up period (range, 4–70 months). Grade 1–2
liver and gastrointestinal toxicity were observed in 4 (5.6%) and 7
(9.8%) patients, respectively. The most common complication was
fatigue, which was observed in 28 patients (38.9%). Dermatitis was
also frequently encountered. The most severe complication was grade
3 dermatitis, which was observed in 3 patients (4.2%). The tumor
diameter in those 3 patients was ≥16 cm (16.5, 16.8 and 17.6 cm,
respectively) and the tumors were adjacent to the skin. The
complications and toxicity are summarized in Table III.
 | Table IIIComplications and toxicities due to
stereotactic body radiotherapy. |
Table III
Complications and toxicities due to
stereotactic body radiotherapy.
| Grade |
|---|
|
|
|---|
| 1 | 2 | 3 |
|---|
|
|
|---|
|
Complication/toxicity | No. (%) | No. (%) | No. (%) |
|---|
| Edema | 2 (2.8) | | |
| Anemia | 1 (1.4) | | |
| Gastrointestinal
toxicity | 3 (4.2) | 4 (5.6) | |
| Fatigue | 18 (25.0) | 10 (13.9) | |
| Nausea | 6 (8.3) | 4 (5.6) | |
| Dermatitis | 5 (6.9) | 13 (18.1) | 3 (4.2) |
| Elevated liver
function tests | 1 (1.4) | 3 (4.2) | |
Discussion
Treatment options for huge (≥10 cm) HCCs are
limited. Large tumors are more likely to recur (22–25),
as they harbor unrecognized small vessel tumor invasion (26). Furthermore, large tumors may
portend worse biological behavior due to genetic factors which are
currently unknown (27).
In this study, the outcomes of 72 patients with huge
HCCs treated with a combination of TACE and SBRT were
retrospectively analyzed. The rationale for this combined treatment
was based on the following evidence: First, the efficacy of TACE
alone for patients with unresectable HCC has been unsatisfactory
(12,28,29);
second, the deposit of iodized oil after TACE may help in more
accurate contouring of the margin of gross tumor volume (GTV) in
SBRT; and third, the irradiation dose delivered to the liver may be
reduced, as the tumor often shrinks due to TACE.
The outcomes in this study demonstrated that huge
HCCs treated with this combination therapy may achieve a high
objective response rate (79.1%) and a low incidence of recurrence
(8.3%). In addition, this combined modality may prolong patient
survival (the overall cumulative median survival was 12.2 months
and the overall cumulative 1-, 3- and 5-year survival rates were
38, 12 and 3%, respectively). Tumor encapsulation was found to be a
significant prognostic factor for survival, as encapsulated tumors
treated with this combined therapy achieved better results compared
to unencapsulated tumors, which is in accordance with previously
reported results (30,31). In contrast to other findings
(32), the Child-Pugh class was
associated with overall cumulative survival in this study, although
the difference was not statistically significant (P=0.068).
The survival of patients with encapsulated tumors in
this study was comparable to the survival of patients with huge
HCCs treated by resection; however, the incidence of recurrence of
huge HCCs treated with the current combined modality was
significantly lower compared to that following resection (33). In addition, the eligibility
criteria for patients were significantly different, as patients
treated by resection should be strictly selected (34). The outcomes in this study were also
comparable to those previously reported (35). Of note, there was no severe
toxicity observed in this study. This may be due to the fact that
gamma-ray SBRT is able to easily meet the requirement of limiting
the irradiation of normal liver; 30 beams of gamma-rays revolve
around an axis and then shape the focused field, leading to the
delivery of an increased dose to the target, while sparing the
uninvolved liver. Additionally, the PTV is encompassed by a
prescription isodose curve of 50 or 55%, but ≥70% isodose curves
are all in the GTV. The dose delivered to the GTV was significantly
higher compared to the dose delivered to normal tissue. The
striking difference of the dose between GTV and normal tissue is
considered to be the main reason that huge HCCs treated by this
combined therapy achieve high response rates without accompanying
severe toxicity. However, grade 3 dermatitis was observed in 3
patients (4.2%). The tumor diameters of those 3 patients were
>16 cm (16.5, 16.8 and 17.6 cm) and the tumors were adjacent to
the skin. Additionally, the fractional dose was as high as 3 Gy.
The development of grade 3 dermatitis reflects the shortcomings of
the technology in the treatment of such tumors (>16 cm) that are
adjacent to skin. Although the beams of gamma-rays revolve around
an axis and then shape the focused field, the direction of the axis
is perpendicular. Several fields are required for a huge tumor, so
that the prescription isodose curve (50 or 55%) encompasses the
PTV. The skin (including the subcutaneous tissue) is at the
entrance channel of each focused field. The dose delivered to the
skin by each focused field is quite small, but dermatitis of
proximal skin may be caused when numerous fields are merged.
Dermatitis, particularly grade 3 dermatitis, may be avoided by
lowering the fractional dose.
The delivered total dose and fractional dose in this
study depended on the predicted toxicity of normal tissues and the
functional liver reserve. In conventional radiotherapy, patients
generally receive the treatment over 35–49 days (5–7 weeks) and 5
consecutive fractions per week, with a daily fraction of 2 Gy.
Since the longer the treatment period, the more the dose is
reduced, Toya et al (36)
suggested that it may be more appropriate for HCC patients to be
treated with radiotherapy over a shorter period of time. In the
present study, all the patients received the SBRT treatment over
12–14 days, with a daily fraction of 2.6–3.0 Gy and 6 fractions per
week. Although the fractional dose was higher compared to
conventional radiotherapy, the treatment period was significantly
shorter.
There were certain limitations to this study, mainly
due to its retrospective design. For example, the treatment
schedules were mainly determined by disease progression. In
addition, the association between the radiation dose and the liver
volume was not investigated. Therefore, a randomized trial may be
required to determine the role of individual SBRT combined with
TACE in the treatment of huge HCCs.
In summary, the outcomes demonstrate that combined
treatment with SBRT and TACE is a safe, effective and promising
option for unresectable huge HCCs. Further randomized trials are
required to confirm the utility of this combined modality. Of note,
patients with tumors >16 cm that are adjacent to the skin should
be irradiated with a small fractional dose.
References
|
1
|
Sangiovanni A, Del Ninno E, Fasani P, et
al: Increased survival of cirrhotic patients with a hepatocellular
carcinoma detected during surveillance. Gastroenterology.
126:1005–1014. 2004. View Article : Google Scholar
|
|
2
|
Cormier JN, Thomas KT, Chari RS, et al:
Management of hepatocellular carcinoma. J Gastrointest Surg.
10:761–780. 2006. View Article : Google Scholar
|
|
3
|
Pakin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
4
|
Llovert JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
5
|
No authors listed. Predictive factors for
long term prognosis after partial hepatectomy for patients with
hepatocellular carcinoma in Japan. The Liver Cancer Group of Japan.
Cancer. 74:2772–2780. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases.
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
7
|
Chirica M, Scatton O, Massault PP, et al:
Treatment of stage IVA hepatocellular carcinoma: should we
reappraise the role of surgery? Arch Surg. 143:538–543. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mazzaferro V, Regalia E, Doci R, et al:
Liver transplantation for the treatment of small hepatocellular
carcinomas in patients with cirrhosis. N Engl J Med. 334:693–699.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yanaga K: Current status of hepatic
resection for hepatocellular carcinoma. J Gastroenterol.
39:919–926. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou ZD, Tang ZY, Yang BH, et al:
Experience of 1000 patients who underwent hepatectomy for small
hepatocellular carcinoma. Cancer. 91:1479–1486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mazzaferro V, Chun YS, Poon RT, et al:
Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol.
15:1001–1007. 2008. View Article : Google Scholar
|
|
12
|
Trevisani F, De Notariis S, Rossi C and
Bernardi M: Randomized control trials on chemoembolization for
hepatocellular carcinoma: Is there room for new studies? J Clin
Gastroenterol. 32:383–389. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hawkins MA and Dawson LA: Radiation
therapy for hepatocellular carcinoma: from palliation to cure.
Cancer. 106:1653–1663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng MB, Cui YL, Lu Y, et al:
Transcatheter arterial chemoembolization in combination with
radiotherapy for unresectable hepatocellular carcinoma: a
systematic review and meta-analysis. Radiother Oncol. 92:184–194.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren ZG, Zhao JD, Gu K, et al:
Three-dimensional conformal radiation therapy and
intensity-modulated radiation therapy combined with transcatheter
arterial chemoembolization for locally advanced hepatocellular
carcinoma: an irradiation dose escalation study. Int J Radiat Oncol
Biol Phys. 79:496–502. 2011. View Article : Google Scholar
|
|
16
|
Law AL, Ng WT, Lee MC, et al: Treatment of
primary liver cancer using highly-conformal radiotherapy with
kV-image guidance and respiratory control. Radiother Oncol.
102:56–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang ZX, Wang D, Wang G, et al: Clinical
study of recombinant adenovirus-p53 combined with fractionated
stereotactic radiotherapy for hepatocellular carcinoma. J Cancer
Res Clin Oncol. 136:625–630. 2010. View Article : Google Scholar
|
|
18
|
Price TR, Perkins SM, Sandrasegaran K, et
al: Evaluation of response after stereotactic body radiotherapy for
hepatocellular carcinoma. Cancer. 118:3191–3198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Son SH, Choi BO, Ryu MR, et al:
Stereotactic body radiotherapy for patients with unresectable
primary hepatocellular carcinoma: dose-volumetric parameters
predicting the hepatic complication. Int J Radiat Oncol Biol Phys.
78:1073–1080. 2010.PubMed/NCBI
|
|
20
|
Zhong NB, Chen ZH and LV GM: A clinical
study on stereotactic body radiotherapy for hepatocellular
carcinoma with portal vein tumor thrombosis. J Cancer Res Ther.
1:143–148. 2013. View Article : Google Scholar
|
|
21
|
WHO Handbook for Reporting Results of
Cancer Treatment. WHO Offset Publication No. 48. World Health
Organization; Geneva: 1979
|
|
22
|
Shah SA, Greig PD, Galllinger S, et al:
Factors associated with early recurrence after resection for
hepatocellular carcinoma and outcomes. J Am Coll Surg. 202:275–283.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Regimbeau JM, Abdalla Ek, Vauthey JN, et
al: Risk factors for early death due to recurrence after liver
resection for hepatocellular carcinoma: results of a multicenter
study. J Surg Oncol. 85:36–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh CN, Lee WC and Chen MF: Hepatic
resection and prognosis for patients with hepatocellular carcinoma
larger than 10 cm: two decades of experience at Chang Gung memorial
hospital. Ann Surg Oncol. 10:1070–1076. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shah SA, Wei AC, Cleary SP, et al:
Prognosis and results after resection of very large (>or=10 cm)
hepatocellular carcinoma. J Gastrointest Surg. 11:589–595. 2007.
View Article : Google Scholar
|
|
26
|
Pawlik TM, Delman KA, Vauthey JN, et al:
Tumor size predicts vascular invasion and histologic grade:
Implications for selection of surgical treatment for hepatocellular
carcinoma. Liver Transpl. 11:1086–1092. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Llovet JM, Schwartz M and Mazzaferro V:
Resection and liver transplantation for hepatocellular carcinoma.
Semin Liver Dis. 25:181–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harada T, Matsuo K, Inoue T, et al: Is
preoperative hepatic arterial chemoembolization safe and effective
for hepatocellular carcinoma? Ann Surg. 224:4–9. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JK, Chung YH, Song BC, et al:
Recurrence of hepatocellular carcinoma following initial remission
by transcatheter arterial chemoembolization. J Gastroenterol
Hepatol. 17:52–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mok KT, Wang BW, Lo GH, et al:
Multimodality management of hepatocellular carcinoma larger than 10
cm. J Am Coll Surg. 197:730–738. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu TH, Yu MC, Chen TC, et al:
Encapsulation is a significant prognostic factor for better outcome
in large hepatocellular carcinoma. J Surg Oncol. 105:85–90. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ng KM, Yan TD, Black D, et al: Prognostic
determinants for survival after resection/ablation of a large
hepatocellular carcinoma. HPB (Oxford). 11:311–320. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen XP, Qiu FZ, Wu ZD, et al: Long-term
outcome of resection of large hepatocellular carcinoma. Br J Surg.
93:600–606. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Poon RT, Fan ST and Wong J: Selection
criteria for hepatic resection in patients with large
hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll
Surg. 194:592–602. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee MT, Kim JJ, Dinniwell R, et al: Phase
I study of individualized stereotactic body radiotherapy of liver
metastases. J Clin Oncol. 27:1585–1591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Toya R, Murakami R, Baba Y, et al:
Conformal radiotherapy for portal vein tumor thrombosis of
hepatocellular carcinoma. Radiother Oncol. 84:266–271. 2007.
View Article : Google Scholar : PubMed/NCBI
|