Introduction
T-cell non-Hodgkin’s lymphomas (T-NHLs) are a group
of malignant types of NHL. The incidence rate of T-NHL has been
increasing worldwide over the last few years (1). In particular, the incidence rate of
T-NHL in Asia was found to be higher compared to that in the United
States and Europe (2–4). Chemotherapy or combined radiotherapy
are currently the main treatment options for NHL. The overall
survival (OS) of NHL has improved following the introduction of
molecular-targeted drugs. However, when compared to B-cell lymphoma
(B-NHL), most types of T-NHL remain difficult to diagnose, exhibit
a more aggressive behaviour and are associated with worse outcomes
(5). There is currently no
standard regimen for the treatment of T-NHL in the first- or
second-line setting (6,7) and the recommended treatment identify
and employ clinical trials as described in National Comprehensive
Cancer Network guideline.
Thalidomide, a synthetic glutamic acid derivative,
was widely used as a sedative and antiemetic in Europe and Canada
in the 1950s (8). However, it was
withdrawn from the market due to its teratogenic side effects that
caused limb deformities in tens of thousands of infants (9). It was recently demonstrated that
thalidomide exerts antitumor effects through inhibiting
angiogenesis (10), promoting
apoptosis (11) and
immunomodulatory activity. Thalidomide has been used in the
treatment of multiple myeloma (12,13),
Kaposi’s sarcoma (14), mantle
cell lymphoma (15), idiopathic
myelofibrosis (16) and other
diseases (17). However, all the
previous studies on the treatment of lymphoma with thalidomide
included patient samples of limited size. We conducted a randomized
trial on 46 cases of T-NHL patients to determine whether
thalidomide combined with chemotherapy may exhibit enhanced
efficacy in the clinical treatment of T-NHL.
Materials and methods
Patients
Eligible T-NHL patients were selected from the
Department of Medical Oncology of the First Affiliated Hospital of
Anhui Medical University (Hefei, Anhui, China) between January,
2009 and June, 2013. The inclusion criteria were as follows: i) The
patient diagnosis was confirmed by histological and
immunohistochemical examination according to the 2008 WHO
classification of haematopoietic and lymphoid tissue neoplasms
(18); ii) the patients were aged
18–75 years and had a total leukocyte count of
>4.0×109/l, a platelet count of
>100×109/l, serum bilirubin, alanine aminotransferase
and alkaline phosphatase levels less than two times the upper limit
of normal, a 24-h creatinine clearance of >60 ml/min and no
significant abnormalities on ECG; iii) the ECOG performance status
score was ≤2 (19) and the
expected survival time was >3 months; and iv) all the patients
received at least two cycles of chemotherapy and were allowed to
receive local radiotherapy (except to the target lesion). The
clinical data and follow-up information, including age, gender, B
symptoms, number of extranodal organ involvement, bone marrow
aspiration or biopsy results, lactate dehydrogenase (LDH) level, T
lymphocyte subsets and Ann Arbor clinical stage were obtained from
the hospital records or by telephone. Following treatment, the
clinical results were evaluated over time. Patients with lymphoma
of the central nervous system, bone marrow infiltration of >25%
as determined by histological examination, HIV infection or any
major organ dysfunction were excluded from this study.
The study protocol was approved by the local
Institutional Ethics Committee and informed consent was obtained
from all the participants.
Chemotherapy
The patients were randomized into i) the control
group (conventional combined chemotherapy) and ii) the thalidomide
group (thalidomide plus combined chemotherapy). In the control
group, the selection of the chemotherapeutic regimen was mainly
based on pathological and clinical stage; the standard CHOP regimen
(20) was mainly used, with
administration of one cycle every 3 weeks consisting of
cyclophosphamide, adriamycin, vincristine and prednisone.
Antiemetic treatment was administered prior to chemotherapy.
Certain patients were administered sodium bicarbonate to alkalinize
the urine. In the thalidomide group, thalidomide was synchronously
administered orally every night during and after chemotherapy. The
initial dose was 100 mg/day, increasing by 50 mg/day every week
(21). The selection of the
chemotherapeutic regimen and the management of chemotherapy-related
side effects were similar to the control group.
Clinical assessment
The size of the tumors was measured by computed
tomography (CT), positron emission tomography/CT and/or magnetic
resonance imaging at baseline, after every 2 cycles and every 9
weeks during follow-up. The response to treatment was evaluated
according to the Response Evaluation Criteria in Solid Tumors,
version 1.1 (22) as follows:
Complete response (CR): disappearance of all target lesions; any
pathological lymph nodes (target or non-target) were required to
exhibit a reduction in the short axis to <10 mm; partial
response (PR): at least 30% decrease in the sum of the diameters of
the target lesions, with the baseline sum diameters used as
reference and no appearance of new lesions; progressive disease
(PD): at least 20% increase in the sum of the diameters of the
target lesions, with the smallest sum on study (this includes the
baseline sum if that is the smallest on study) used as reference.
In addition to the relative increase of 20%, the sum was also
required to demonstrate an absolute increase of ≥5 mm; and stable
disease (SD): neither sufficient shrinkage to qualify for PR, nor
sufficient increase to qualify for PD, with the smallest sum
diameters while on study used as reference.
The overall response was determined as CR + PR + SD
and the objective response as CR + PR. Hematological and other
toxicities were evaluated for every treatment cycle according to
version 3.0 of the Common Toxicity Criteria of the National Cancer
Institute of Canada (23). If any
toxicity > grade 1 was observed in a former cycle, the dose of
chemotherapy was decreased in the subsequent cycle.
Follow-up
Clinical data and follow-up information were mainly
obtained from hospital records or by telephone. Other methods were
also used, including medical follow-up letters. OS was defined as
the time from treatment initiation until the date of death or last
follow-up. Progression-free survival (PFS) was defined as the time
from treatment initiation to disease progression or to termination
of the follow-up if no relapse occured or death from any cause.
Patients that were lost to the follow-up were excluded. Our
follow-up ended September 30, 2013 and three cases were lost
(6.5%).
Statistical analysis
The SPSS 18.0 statistical software package was used
for statistical processing. The survival rate was calculated and a
survival curve was drawn using the GraphPad Prism method. The
measurement data of the control and thalidomide treatment groups
were compared using the t-test and the count data were compared
using the Fisher’s exact probability test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical characteristics
A total of 46 patients were enrolled in this study,
with 24 patients assigned to the thalidomide group and 22 to the
control group (Table I). The
patients included 32 men and 14 women, with 36 patients undergoing
primary treatment and 10 undergoing treatment for recurrence. The
median age of the patients was 47.5 years (range, 18–77 years). The
T-NHL subtypes were as follows: 21 cases of natural killer/T-cell
lymphoma, 9 cases of peripheral T-cell lymphoma, 7 cases of
anaplastic lymphoma kinase-positive anaplastic large-cell lymphoma,
4 cases of angioimmunoblastic T-cell lymphoma (AITL), 2 cases of
precursor T-lymphoblastic lymphoma, 1 case of subcutaneous
panniculitis-like T-cell lymphoma, 1 case of primary cutaneous
anaplastic large-cell lymphoma and 1 case of adult T-cell
leukemia/lymphoma. Prior to treatment, 15 patients were diagnosed
with stage I, 8 with stage II, 10 with stage III and 13 with stage
IV disease.
 | Table IClinical characteristic of the 46
T-NHL patients. |
Table I
Clinical characteristic of the 46
T-NHL patients.
| No. | Gender | Age (yrs) | Type | Stage | Primary or
recurrence | Group | Combined with | Cycles | CAa | PFS (mo) | OS (mo) |
|---|
| 1 | M | 45 | NK/T | I | Primary | TLD | CHOPb+RTc | 6 | CR | >42 | >42 |
| 2 | M | 42 | NK/T | I | Primary | TLD | CHOPb+RTc | 6 | CR | >33 | >33 |
| 3 | F | 40 | ALCL | I | Primary | TLD | CHOPb | 6 | CR | >32 | >32 |
| 4 | F | 51 | NK/T | II | Recurrence | TLD | DICEd | 4 | PD | 3 | 5 |
| 5 | M | 77 | AITL | II | Primary | TLD | COPe | 4 | PR | 3 | 7 |
| 6 | M | 33 | PTCL | III | Recurrence | TLD | CHOPb+RTc | 4 | PR | 2 | >5 |
| 7 | M | 70 | AITL | III | Primary | TLD | CHOPb | 4 | PR | 5 | >12 |
| 8 | M | 41 | NK/T | I | Primary | TLD | CHOPb+Gem+RTc | 6 | CR | >26 | >26 |
| 9 | M | 45 | PTCL | II | Recurrence | TLD | CHOPb | 3 | CR | >15 | >15 |
| 10 | M | 53 | T-LBL | IV | Recurrence | TLD | FMDf | 2 | PR | 7.5 | 11.5 |
| 11 | M | 75 | AITL | IV | Primary | TLD | CHOPb | 2 | SD | 4 | 7 |
| 12 | M | 39 | PTCL | IV | Primary | TLD | CHOPb+DDP+RTc | 7 | PR | 9 | 21 |
| 13 | F | 32 | SPTCL | I | Primary | TLD | CHOPb+DDP+RTc | 6 | CR | >21 | >21 |
| 14 | M | 71 | NK/T | IV | Recurrence | TLD | CHOPb+GEM | 5 | CR | 19 | >21 |
| 15 | F | 50 | AITL | IV | Primary | TLD | CHOPb+GEM | 5 | CR | >18 | >18 |
| 16 | M | 48 | NK/T | IV | Recurrence | TLD | GEM+L-OHP | 7 | CR | >16 | >16 |
| 17 | F | 73 | C-ALCL | IIIE | Recurrence | TLD | CHOPb | 2 | PD | 1 | >8 |
| 18 | F | 54 | ALCL | III | Recurrence | TLD | DICEd | 4 | PR | 12 | >14 |
| 19 | F | 58 | NK/T | III | Primary | TLD | CHOPb+RTc | 2 | CR | >13 | >13 |
| 20 | F | 74 | NK/T | IV | Primary | TLD | GEM+L-OHP | 2 | PD | 2 | 3 |
| 21 | F | 60 | NK/T | I | Primary | TLD |
GEM+L-OHP+RTc | 4 | CR | >12 | >12 |
| 22 | M | 45 | NK/T | I | Primary | TLD | GEM+L-OHP | 2 | PD | 1 | 3 |
| 23 | M | 56 | NK/T | II | Primary | TLD | CHOPb+RTc | 2 | PR | 3 | 3 |
| 24 | F | 60 | NK/T | I | Primary | TLD |
GEM+L-OHP+RTc | 3 | CR | >8 | >8 |
| 25 | M | 23 | PTCL | IV | Primary | Control | CHOPb | 2 | PD | 2 | 4 |
| 26 | M | 47 | NK/T | I | Primary | Control | CHOPb+RTc | 6 | CR | >37 | >37 |
| 27 | M | 60 | PTCL | III | Primary | Control | CHOPb | 6 | CR | >36 | >36 |
| 28 | F | 40 | PTCL | IV | Primary | Control | CHOPb+GEM | 5 | PR | 6 | 10 |
| 29 | M | 72 | ALCL | IV | Primary | Control | CHOPb+RTc | 6 | SD | 6 | 8 |
| 30 | M | 58 | PTCL | III | Primary | Control | CHOPb | 2 | PR | >3 | >3 |
| 31 | F | 35 | NK/T | II | Primary | Control | CHOPb | 2 | PD | 0 | 3 |
| 32 | M | 37 | NK/T | I | Primary | Control | CHOPb+RTc | 4 | CR | >34 | >34 |
| 33 | M | 29 | ALCL | III | Recurrence | Control | CHOPb+RTc | 6 | SD | 6 | >11 |
| 34 | M | 65 | ATCL | IV | Recurrence | Control | GEM+L-OHP | 3 | SD | 5 | 7 |
| 35 | M | 65 | NK/T | II | Primary | Control | CHOPb | 2 | PR | 5 | 6 |
| 36 | M | 58 | NK/T | I | Primary | Control | CHOPb+RTc | 2 | PR | 2 | 8 |
| 37 | M | 38 | NK/T | I | Primary | Control | GPh+DXM | 2 | PD | 2 | 4 |
| 38 | F | 62 | PTCL | I | Primary | Control | CHOPb+RTc | 6 | CR | >26 | >26 |
| 39 | M | 61 | NK/T | I | Primary | Control | CHOPb+RTc | 6 | CR | >23 | >23 |
| 40 | M | 40 | NK/T | II | Primary | Control | CHOPb | 6 | CR | >21 | >21 |
| 41 | M | 69 | PTCL | IV | Primary | Control | CHOPb | 8 | CR | 11 | 17 |
| 42 | M | 65 | ALCL | III | primary | Control | CHOPb | 2 | PD | 1 | 3 |
| 43 | M | 18 | ALCL | IV | Primary | Control | CHOPb | 2 | PR | 1 | >6 |
| 44 | M | 65 | ALCL | III | Primary | Control | CHOPb | 2 | PD | 1 | 3 |
| 45 | F | 68 | T-LBL | I | Primary | Control | CHOPb | 4 | PR | 15 | >15 |
| 46 | M | 36 | NK/T | II | Primary | Control | EPg | 5 | CR | >7 | >7 |
Clinical response
Each patient completed at least two cycles of
treatment. In the 24 cases included in the thalidomide group, the
median dose of thalidomide was 200 mg (range, 150–400 mg) every
night, without reported severe side effects. The response was CR in
12 cases, PR in 7, SD in 1 and PD in 4 cases. In the 22 cases of
the control group, the response was CR in 8 cases, PR in 6, SD in 3
and PD in 5 cases. The CR rate was 50 and 36.4% in the thalidomide
and the control groups, respectively (P<0.05). The overall
response rate (CR + PR) was 79.2 and 63.6% in the thalidomide and
the control groups, respectively (χ2=1.90,
P>0.05).
The PFS curves are shown in Fig. 1. The median PFS was 12 months in
the thalidomide group and 6 months in the control group. Therefore,
the PFS was longer by 6 months in the thalidomide group compared to
that in the control group.
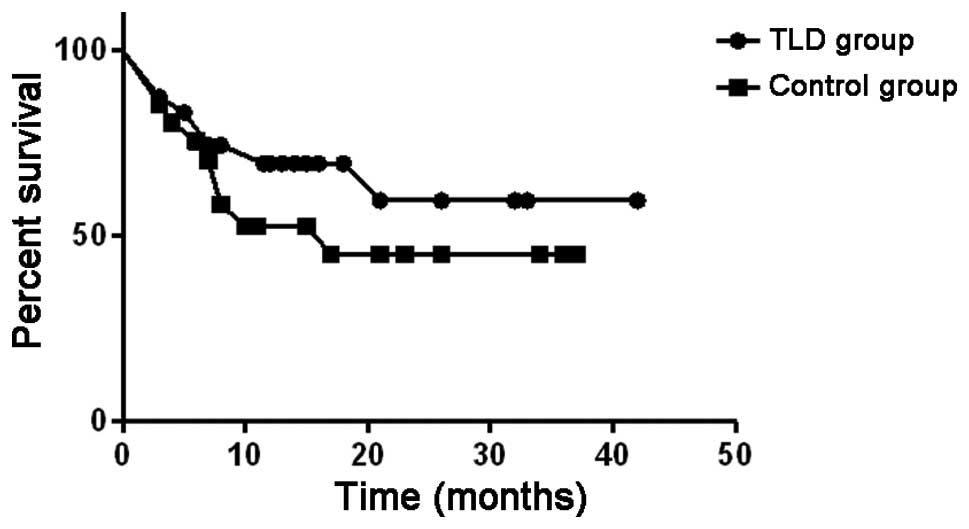
The OS curves are shown in Fig. 2. The median OS in the thalidomide
group was undefined and in the control group it was 17 months.
Therefore, the OS was longer in the thalidomide group compared to
that in the control group.
LDH level and changes in the T
lymphocytic subsets prior to and after chemotherapy
Prior to chemotherapy, the LDH level was
753.32±482.58 and 811.25±424.67 U/l in the control and the
thalidomide groups, respectively, without a significant difference
between the two groups. After chemotherapy, the LDH level was
415.54±150.36 U/l in the control group and 180.62±131.17 U/l in the
thalidomide group in the proportion of CR + PR patients. The
difference between the two groups was statistically significant
(P<0.05) (data not shown). When we compared the changes in T
lymphocyte subsets prior to and after chemotherapy, we observed no
statistically significant difference between the two groups
(P>0.05) (data not shown).
Evaluation of side effects and safety in
the two treatment groups
The majority of the patients (n=36) developed
chemotherapy-related side effects. A total of 15 patients developed
grade 2 gastrointestinal reactions, 6 exhibited weakness, 5 had an
infection (severe in 1 patient), 5 developed limb peripheral
neuritis, 3 exhibited mild liver function abnormalities and 1
patient had drowsiness. A total of 3 patients previously had sleep
abnormalities, but improved after taking thalidomide for 2 weeks.
In addition, 2 patients developed grade 1 oral mucositis and there
was 1 case of cerebral thrombosis. There was no reported renal or
cardiac toxicity, no drug-induced fever or rash and no
treatment-related deaths in the two groups.
Discussion
In this study, of the 46 patients with T-NHL
lymphoma, 24 were randomly selected and received treatment with
thalidomide combined with chemotherapy. We observed that the CR
rate and overall response rate (CR + PR) were higher in the
thalidomide group (50 and 79.2%, respectively) compared to those in
the control group (36.4 and 63.6%, respectively). The median PFS
and OS were also longer in thalidomide group. Our results
demonstrated that thalidomide combined with chemotherapy enhances
the clinical efficacy of T-NHL treatment.
Over the last few years, an increasing number of
studies reported that vascular endothelial growth factor was
associated with the incidence, development and angiogenesis of
malignant tumors (24) and
anti-angiogenic therapy has become one of the most important
methods in antitumor therapy. Thalidomide was gradually introduced
in the treatment of malignant tumors due to its anti-angiogenic
properties (10). Clinical and
experimental data demonstrated that thalidomide effectively
inhibited tumor growth, invasion and metastasis in multiple
myeloma, colon cancer (25) and
NHL (17). Therefore, thalidomide
plus dexamethasone was approved by the Food and Drug Administration
as a first-line regimen for multiple myeloma in May, 2006 (13).
The mechanism of action of thalidomide is complex
and may involve the suppression of tumor necrosis factor-α (TNF-α)
and interferon-γ secretion (26),
which are known to upregulate endothelial cell integrin expression,
a process crucial for new vessel formation (27). Thalidomide inhibits the secretion
of basic fibroblast growth factor, an angiogenic factor secreted by
human tumors (28), thus reducing
tumor-associated macrophage infiltration, possibly through
suppressing the expression of endothelial cell adhesion molecules
(29). Thalidomide also suppresses
TNF-α-induced intercellular adhesion molecule-1 expression through
inhibiting nuclear factor-κB (30). A recent study (31) identified cereblon (CRBN) as the
primary teratogenic target of thalidomide. CRBN is also required
for the antimyeloma activity of thalidomide and other related
drugs, referred to as immune-modulatory drugs (IMiDs). Low CRBN
expression was found to be correlated with drug resistance in
myeloma cell lines and primary myeloma cells. CRBN was also
implicated in several effects of IMiDs, such as downregulation of
TNF-α and T-cell activity.
Strupp et al (32) were the first to report the
application of thalidomide in the treatment of lymphoma. Two AILT
patients were treated with thalidomide as anti-angiogenic therapy
and achieved complete remission. Subsequently, Wilson et al
(33) reported one case of mantle
cell lymphoma treated with thalidomide. That patient had
experienced several tumor relapses and treatment by chemotherapy,
steroids and rituximab had failed. The patient received daily
single-agent thalidomide by oral ingestion at a dose of 800 mg and
achieved a partial remission, which was maintained for 6 months.
Additional studies also reported cases of refractory mantle cell
lymphoma or recurrent lymphoma successfully treated with
thalidomide (15,34,35),
achieving an overall response rate of 81%, with a CR rate of 31%,
when thalidomide was used in combination with rituximab in 16
relapsed mantle cell lymphoma patients.
In China, Su et al (36) reported that the overall response
rate using the CHOP regimen with etoposide, with vs. without
thalidomide, was 73.91 vs. 39.13%, respectively, in advanced NHL.
The 1- and 2-year survival rates were 86.96 and 56.52%,
respectively. There was no difference in the 1-year survival rate
between the two groups; however, there was a significant difference
in the 2-year survival rate. The major adverse reactions associated
with the combination of chemotherapy and thalidomide were
leucopenia, drowsiness, constipation, bloating and fatigue. Grade
3–4 leucopenia occurred in ~15.0% of the patients who developed
chemotherapy-related reactions. The results suggested that
thalidomide may be used in the treatment of lymphoma.
The majority of the T-cell lymphoma types are
aggressive. Therefore, they are not easily cured, even with
autologous stem cell transplantation. Damaj et al (37) reported three cases of relapsed
refractory T-cell lymphoma that exhibited a good tumor response
under treatment with thalidomide: One patient achieved a transient
PR and the remaining two patients achieved a complete resolution of
the symptoms and the tumor masses. One of these two patients
remained alive on low-dose thalidomide maintenance therapy, whereas
the other succumbed to an aggressive relapse, while off
thalidomide, 1 month after intensive chemotherapy-radiotherapy.
Dogan et al (38) reported
a case of a 78-year-old female patient with AITL confirmed by lymph
node biopsy. After declining the standard treatment options, the
patient agreed to treatment with 200 mg daily thalidomide, which
was maintained for 15 months and was well tolerated, except for a
mild sensory neuropathy.
A certain dosage range of thalidomide is considered
to be safe, as well as effective. It was previously reported
(39) that thalidomide increased
the patients’ appetite and body weight, improved sleep and relieved
the stress associated with advanced cancer. Oral thalidomide is
easy to administer, cost-effective and has tolerable side effects;
therefore, its application in clinical practice is feasible and
convenient. It has been, however, associated with an increased risk
of venous thromboembolism (40).
In our study, the median dose of oral thalidomide was 200 mg daily.
Other side effects reported in the literature, such as rash,
bradycardia, hypothyroidism and peripheral neuropathy, were not
recorded in our study. We hypothesized that thalidomide may be used
over a longer period of time, due to its slower anti-angiogenic
effect.
In the present study, we demonstrated that
thalidomide effectively reduced LDH in the blood prior to and after
chemotherapy, suggesting that it may improve the prognosis of T-NHL
patients. However, the T cell subsets did not change significantly
prior to and after chemotherapy, possibly due to the limited effect
of thalidomide on the immune system. Newly developed drugs that are
similar to thalidomide, such as lenalidomide (Revlimid, CC-5013)
and actimid (CC-4047) have been confirmed to exert significant
regulatory effects on the human immune system (41). These results suggested that drugs
similar to thalidomide may have a place in cancer treatment.
In conclusion, we randomly selected 24 patients
among 46 cases of T-NHL lymphoma and administered treatment with
thalidomide combined with chemotherapy, achieving a significant
improvement in the CR rate and overall response rate (CR + PR). In
addition, the PFS and OS were prolonged, with no associated
increase in toxicity. Although the differences were not considered
statistically significant, it may be of value to investigate
further by expanding the sample size or grouping by thalidomide
dose and certain subtypes of T-NHL. Thalidomide combined with
chemotherapy may reduce the incidence of gastrointestinal reactions
and the psychological fear of chemotherapy in cancer patients and
may represent a viable treatment option for T-NHL.
Acknowledgements
This study was supported by the Health Bureau of
Anhui Province (grant no. 09B114).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Ekström-Smedby K: Epidemiology and
etiology of non-Hodgkin lymphoma - a review. Acta Oncol.
45:258–271. 2006.
|
|
3
|
Vose J, Armitage J and Weisenburger D;
International T-Cell Lymphoma Project. International peripheral
T-cell and natural killer/T-cell lymphoma study: pathology findings
and clinical outcomes. J Clin Oncol. 26:4124–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shiels MS, Engels EA, Linet MS, et al: The
epidemic of non-Hodgkin lymphoma in the United States:
disentangling the effect of HIV, 1992–2009. Cancer Epidemiol
Biomarkers Prev. 22:1069–1078. 2013.PubMed/NCBI
|
|
5
|
Gisselbrecht C, Gaulard P, Lepage E, et
al: Prognostic significance of T-cell phenotype in aggressive
non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte
(GELA). Blood. 92:76–82. 1998.
|
|
6
|
Reimer P, Rüdiger T, Geissinger E, et al:
Autologous stem cell transplantation as first-line therapy in
peripheral T-cell lymphomas: results of a prospective multicenter
study. J Clin Oncol. 27:106–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jagadeesh D and Smith MR: Novel targeted
therapies in peripheral T cell lymphoma. Discov Med. 15:367–378.
2013.PubMed/NCBI
|
|
8
|
Somers GF: Pharmacological properties of
thalidomide (alpha-phthalimido glutarimide), a new sedative
hypnotic drug. Br J Pharmacol Chemother. 15:111–116. 1960.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tseng S, Pak G, Washenik K, Pomeranz MK
and Shupack JL: Rediscovering thalidomide: a review of its
mechanism of action, side effects, and potential uses. J Am Acad
Dermatol. 35:969–979. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kruse FE, Joussen AM, Rohrschneider K,
Becker MD and Völcker HE: Thalidomide inhibits corneal angiogenesis
induced by vascular endothelial growth factor. Graefes Arch Clin
Exp Ophthalmol. 236:461–466. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aseffa A, Dietrich MA and Shannon EJ:
Effect of thalidomide on apoptosis of lymphocytes and neutrophils.
Immunopharmacol Immunotoxicol. 19:313–326. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singhal S, Mehta J, Desikan R, et al:
Antitumor activity of thalidomide in refractory multiple myeloma. N
Engl J Med. 341:1565–1571. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
FDA. FDA Approves Thalomid (thalidomide)
to Treat Multiple Myeloma. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107296.htm.
Accessed May 18, 2014
|
|
14
|
Krown SE: Management of Kaposi sarcoma:
the role of interferon and thalidomide. Curr Opin Oncol.
13:374–381. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaufmann H, Raderer M, Wöhrer S, et al:
Antitumor activity of rituximab plus thalidomide in patients with
relapsed/refractory mantle cell lymphoma. Blood. 104:2269–2271.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strupp C, Germing U, Scherer A, et al:
Thalidomide for the treatment of idiopathic myelofibrosis. Eur J
Haematol. 72:52–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramírez J, Wu K, Janisch L, et al: The
effect of thalidomide on the pharmacokinetics of irinotecan and
metabolites in advanced solid tumor patients. Cancer Chemother
Pharmacol. 68:1629–1632. 2011.PubMed/NCBI
|
|
18
|
Swerdlow SH, Campo E, Harris NL, et al:
WHO Classification of Tumours of Haematopoietic and Lymphoid
Tissues. 4th edition. International Agency for Research on Cancer
Press; Lyon: 2008
|
|
19
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang HQ, Peng YL, Lin XB, et al: Clinical
outcomes of 106 patients with peripheral T-cell lymphoma treated by
standard CHOP regimen. Chin J Cancer. 23(Suppl 11): S1443–S1447.
2004.(In Chinese).
|
|
21
|
Haas PS, Denz U, Ihorst G and Engelhardt
M: Thalidomide in consecutive multiple myeloma patients:
single-center analysis on practical aspects, efficacy, side effects
and prognostic factors with lower thalidomide doses. Eur J
Haematol. 80:303–309. 2008. View Article : Google Scholar
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
23
|
NCI. Cancer therapy evaluation program,
common terminology criteria for adverse events. Version 3.0. Dec
12–2003, Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
Accessed May 18, 2014
|
|
24
|
Rapisarda A and Melillo G: Role of the
VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res.
114:237–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Govindarajan R: Irinotecan/thalidomide in
metastatic colorectal cancer. Oncology (Williston Park). 16(Suppl
3): S23–S26. 2002.
|
|
26
|
Majumder S, Sreedhara SR, Banerjee S and
Chatterjee S: TNF α signaling beholds thalidomide saga: a review of
mechanistic role of TNF-α signaling under thalidomide. Curr Top Med
Chem. 12:1456–1467. 2012.
|
|
27
|
Ladizinski B, Shannon EJ, Sanchez MR and
Levis WR: Thalidomide and analogues: potential for immunomodulation
of inflammatory and neoplastic dermatologic disorders. J Drugs
Dermatol. 9:814–826. 2010.PubMed/NCBI
|
|
28
|
Mei SC and Wu RT: The G-rich promoter and
G-rich coding sequence of basic fibroblast growth factor are the
targets of thalidomide in glioma. Mol Cancer Ther. 7:2405–2414.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rafiee P, Stein DJ, Nelson VM, Otterson
MF, Shaker R and Binion DG: Thalidomide inhibits inflammatory and
angiogenic activation of human intestinal microvascular endothelial
cells (HIMEC). Am J Physiol Gastrointest Liver Physiol.
298:G167–G176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin YC, Shun CT, Wu MS and Chen CC: A
novel anticancer effect of thalidomide: inhibition of intercellular
adhesion molecule-1-mediated cell invasion and metastasis through
suppression of nuclear factor-kappaB. Clin Cancer Res.
12:7165–7173. 2006. View Article : Google Scholar
|
|
31
|
Zhu YX, Kortuem KM and Stewart AK:
Molecular mechanism of action of immune-modulatory drugs
thalidomide, lenalidomide and pomalidomide in multiple myeloma.
Leuk Lymphoma. 54:683–687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strupp C, Aivado M, Germing U, Gattermann
N and Haas R: Angioimmunoblastic lymphadenopathy (AILD) may respond
to thalidomide treatment: two case reports. Leuk Lymphoma.
43:133–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilson EA, Jobanputra S, Jackson R, Parker
AN and McQuaker IG: Response to thalidomide in
chemotherapy-resistant mantle cell lymphoma: a case report. Br J
Haematol. 119:128–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Damaj G, Lefrère F, Delarue R, Varet B,
Furman R and Hermine O: Thalidomide therapy induces response in
relapsed mantle cell lymphoma. Leukemia. 17:1914–1915. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pro B, Younes A, Albitar M, et al:
Thalidomide for patients with recurrent lymphoma. Cancer.
100:1186–1189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Su X, Zhang R, Liu H, et al: Clinical
study of E-CHOP plus thalidomide in treatment of advanced
non-Hodgkin’s lymphoma. China Pharmaceuticals. 17:53–55. 2008.
|
|
37
|
Damaj G, Bouabdallah R, Vey N, Bilger K,
Mohty M and Gastaut JA: Single-agent thalidomide induces response
in T-cell lymphoma. Eur J Haematol. 74:169–171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dogan A, Ngu LS, Ng SH and Cervi PL:
Pathology and clinical features of angioimmunoblastic T-cell
lymphoma after successful treatment with thalidomide. Leukemia.
19:873–875. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tassinari D, Santelmo C, Tombesi P and
Sartori S: Thalidomide in the treatment of cancer cachexia. J
Palliat Care. 24:187–189. 2008.PubMed/NCBI
|
|
40
|
Carrier M, Le Gal G, Tay J, Wu C and Lee
AY: Rates of venous thromboembolism in multiple myeloma patients
undergoing immunomodulatory therapy with thalidomide or
lenalidomide: a systematic review and meta-analysis. J Thromb
Haemost. 9:653–663. 2011. View Article : Google Scholar
|
|
41
|
Zhu D, Corral LG, Fleming YW and Stein B:
Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce
apoptosis of both hematological and solid tumor cells through NK
cell activation. Cancer Immunol Immunother. 57:1849–1859. 2008.
View Article : Google Scholar : PubMed/NCBI
|