Introduction
Hepatocellular carcinoma (HCC) is a malignancy
associated with a poor prognosis and has a heterogeneous
composition, with multiple variables that vary among different
regions. Although several novel treatment options have been
investigated and used in the clinical setting, hepatic resection
(HR) remains the primary treatment for HCC. However, even after
curative resection, recurrence is common and remains the main cause
of mortality. The 3-year tumor recurrence rate was reported to be
>60% following HR (1,2) and the 5-year overall survival (OS)
rate ranges between 39 and 50% (3,4).
Consequently, adequate and effective adjuvant therapy is essential
to improve the OS of HCC patients.
Various types of postoperative therapies, such as
transarterial therapy with or without embolization, systematic
therapy, interferon, lamivudine, vitamin A and K2 analog and
adoptive immunotherapy, have been reported for HCC patients
following curative treatment. However, interferon is frequently
associated with various adverse effects (5) and postoperative transarterial
chemoembolization appears to be promising only for HCC patients
with a high risk of recurrence (6). Adoptive immunotherapy, although
associated with a lower recurrence rate following surgery for HCC,
does not appear to increase OS (7), whereas the manufacturing operation
and therapeutic process are complex. The number of randomized
clinical trials (RCTs) investigating the effect of adjuvant vitamin
A or K2 analog and lamivudine therapy is limited. Orally
administered chemotherapy is a type of systemic treatment. As a
convenient and non-invasive therapy, oral chemotherapeutic drug
administration is easily adopted.
Although conventional systemic chemotherapy is well
tolerated by inoperable HCC patients (8,9), HCC
is widely considered to be chemotherapy-resistant, as the response
rates are ~20% for single-agent as well as combination chemotherapy
(10). One of the most widely used
chemotherapeutic agents is 5-fluorouracil. Several investigators
have attempted to improve the survival of HCC patients
postoperatively by conventional oral systemic chemotherapy (COSC)
over the last few decades. However, these trials reported
contradictory findings. Therefore, a systematic review is required
to provide a more comprehensive analysis and assess the efficacy of
COSC.
Materials and methods
Identification of trials
The Medline, Embase and Cochrane Library electronic
databases were systematically searched through to April, 2012 and
comparative studies investigating curative HR with and without COSC
were identified using any of the following key words:
hepatocellular carcinoma, hepatic tumor, liver tumor,
postoperative, adjuvant, chemotherapy, or oral. The search was not
limited to controlled or randomized trials for minimizing the
chances of missing a study. Manual search of relevant references
and review articles was also performed. There were no date and
language restrictions. Only RCTs were included.
RCTs which assessed the effect of COSC for
postoperative HCC were included in this review. Studies
investigating liver metastases or postoperative recurrent HCC were
excluded. The patients in the control group only received curative
HR. The studies identified during the search were screened
independently by two reviewers. Any disagreements were arbitrated
by a third reviewer.
Types of outcome measures
The primary outcomes evaluated in this review were
OS and recurrence rates. The secondary outcome was incidence of
adverse events attributable to COSC.
Quality assessment
Two reviewers independently evaluated the quality of
each retrieved trial in terms of randomization by sequence
generation, allocation concealment, blinding of outcome assessors
and reporting of intention-to-treat analysis. The trials were
considered to be of low quality if they reported none of the items,
of moderate quality if they reported on one or two items and of
good quality if they reported on three or four items. The reporting
of this systematic review is in accordance with the QUOROM
statement (11).
Data extraction
Two reviewers independently extracted data regarding
author details, methodological quality, number of patients, patient
characteristics, interventions and outcomes. Discrepancies were
resolved by consensus. When multiple publications of the same trial
were identified, data were extracted from the multiple publications
and reported as a single trial.
Statistical analysis
The data from each study were analyzed using the
RevMan 5.1 software package. Odds ratios (ORs) with 95% confidence
intervals (CIs) were calculated for OS and recurrence rate
outcomes. Fixed- and random-effects models were applied in an
‘intention-to-treat’ analysis, i.e., all the patients were
evaluated according to their group allocation. Patients whose
endpoint was unknown were considered to be deceased or to have
suffered tumor recurrence. Homogeneity between trials was analyzed
using the χ2 test, with significance set at P>0.1;
the extent of heterogeneity was assessed by calculating
I2. The point estimate of the OR was considered
statistically significant at P<0.05 if the 95% CI did not
include the value 1.
Results
Identification and characteristics of
selected studies
From 916 citations identified by database searches,
three eligible RCTs (12–14) involving a total of 286 patients
were included in this systematic review (Fig. 1). One study was conducted in China
(12) and two in Japan (13,14).
A definitive diagnosis of HCC was made based on histological
evidence or a combination of several imaging modalities, e.g.,
hepatic angiography, enhanced computed tomography and magnetic
resonance imaging. All the HCC patients underwent curative HR. No
patient in the control group received any type of chemopreventive
therapy prior to the discovery of recurrent HCC. The median
follow-up was >4 years in all three RCTs (12–14).
The patients in the treated group received oral capecitabine
(12), uracil-tegafur (13), or carmofur (14), which are fluoropyrimidine drugs.
Across the three studies, recurrence was measured and assessed in
the same way by at least two imaging methods. The characteristics
of the studies included in the review are summarized in Table I. In order to compare the efficacy
of adjuvant COSC of other studies to the included studies, three
other studies were systematically reviewed, including two RCTs
(15,16) and one case-control trial (17). All the HCC patients in these three
trials underwent curative HR (15–17).
The patients in the control group of two trials only received
supportive care following HR (15,17).
By contrast, the patients in the treated group received adjuvant
COSC, with or without other types of chemotherapy.
 | Table IBaseline characteristics of randomized
clinical trials included into the systematic review. |
Table I
Baseline characteristics of randomized
clinical trials included into the systematic review.
| Study | Mean follow-up, years
(range) | Child-Pugh
classification (A/B) | Sample size (n) | Tumor size (median,
cm) | Hepatitis (n)
(HBV/HCV) | Cirrhosis (%) | Vascular invasion
(n) | (Refs.) |
|---|
| Xia et al | 4.0 (0.3–5.4) | 60/0 | T:30 | 7.27 | 26/NR | 63.3 | 12 | (12) |
| | | C:30 | 6.34 | 24/NR | 70.0 | 10 | |
| Hasegawa et
al | 4.8 (0.5–7.9) | 138/21 | T:79 | 3.3 | 14/58 | 53.2 | 18 | (13) |
| | | C:80 | 3.4 | 15/56 | 47.5 | 17 | |
| Yamamoto et
al | 4–6 | NR | T:35 | NR | NR | 100.0 | NR | (14) |
| | | C:32 | NR | NR | 100.0 | NR | |
Quality of included RCTs
The risks of bias in the studies included in this
systematic review are detailed in Table II. The methodological quality was
considered to be high in the first (12), moderate in the second (13) and low in the third study (14).
 | Table IIMethodological quality assessment and
internal validity of the included randomized clinical trials. |
Table II
Methodological quality assessment and
internal validity of the included randomized clinical trials.
| Study | Random
allocation | Concealment of random
allocation | Blinding of persons
who assess treatment effects | Intention-to-treat
analysis | (Refs.) |
|---|
| Xia et
al | + | + | − | + | (12) |
| Hasegawa et
al | + | − | − | + | (13) |
| Yamamoto et
al | − | − | − | − | (14) |
Efficacy
Some of the efficacy of the included RCTs (12–14)
and other trials (15–17) of adjuvant COSC for HCC patients are
summarized in Table III.
 | Table IIIResults of randomized clinical trials
and other clinical trials of adjuvant conventional oral systemic
chemotherapy for hepatocellular carcinoma. |
Table III
Results of randomized clinical trials
and other clinical trials of adjuvant conventional oral systemic
chemotherapy for hepatocellular carcinoma.
| Study | Drugs and dose of
the treated and control groups | Outcomes | Treated group
(%) | Control group
(%) | P-value | (Refs.) |
|---|
| Xia et
al | Two weeks of
capecitabine (1,000 mg/m2) twice a day, followed by 1
week of rest, for 4–6 cycles | 5-year DFS | 46.7 | 23.3 | <0.05 | (12) |
| Supportive
care | 5-year OS | 62.5 | 39.8 | >0.05 | |
| Hasegawa et
al | Oral uracil-tegafur
(300 mg/day) for 1 year | 5-year DFS | 29 | 29 | >0.05 | (13) |
| Supportive
care | 5-year OS | 58 | 73 | >0.05 | |
| Yamamoto et
al | Oral carmofur (200
mg) twice daily for as long as possible | 5-year DFSa | 50 | 19 | <0.05 | (14) |
| Supportive
care | 5-year OSa | 72 | 49 | <0.05 | |
| Kohno et
al | Oral uracil-tegafur
(300 mg/day) for 1 year, plus transarterial chemotherapy once
(epirubicin 40 mg/m2) | 5-year DFS | 17 | 14 | >0.05 | (16) |
| Oral uracil-tegafur
(300 mg/day) for 1 year | 5-year OS | 35 | 30 | >0.05 | |
| Ono et
al | Transarterial
chemotherapy (epirubicin 40 mg/m2); then intravenous
chemotherapy (epirubicin 40 mg/m2), once every 3 months
for 2 years; in addition, oral carmofur (300 mg/day) for 2
years | 5-year DFS | 32 | 22.5 | >0.05 | (15) |
| Supportive
care | 5-year OS | 31.5 | 57 | >0.05 | |
| Takenaka et
al | Oral uracil-tegafur
or carmofur (300–400 mg/day) for 18 months | 3-year DFS | 50 | 15 | >0.05 | (17) |
| Supportive
care | | | | | |
OS
All the RCTs (12–14)
described the OS. In the study by Xia et al (12), the median OS time was longer in the
capecitabine group (60.0 vs. 52.5 months), but the difference was
not statistically significant (P=0.22). The study by Yamamoto et
al (14) concluded that the OS
of patients with stage I disease was higher in the oral carmofur
compared to that in the control group (P=0.08). However, in
patients with stage II disease, no significant difference was
observed (P=0.77). Interestingly, Hasegawa et al (13) drew an opposite conclusion,
reporting that OS was marginally but not significantly worse in the
uracil-tegafur compared to that in the control group (P=0.08).
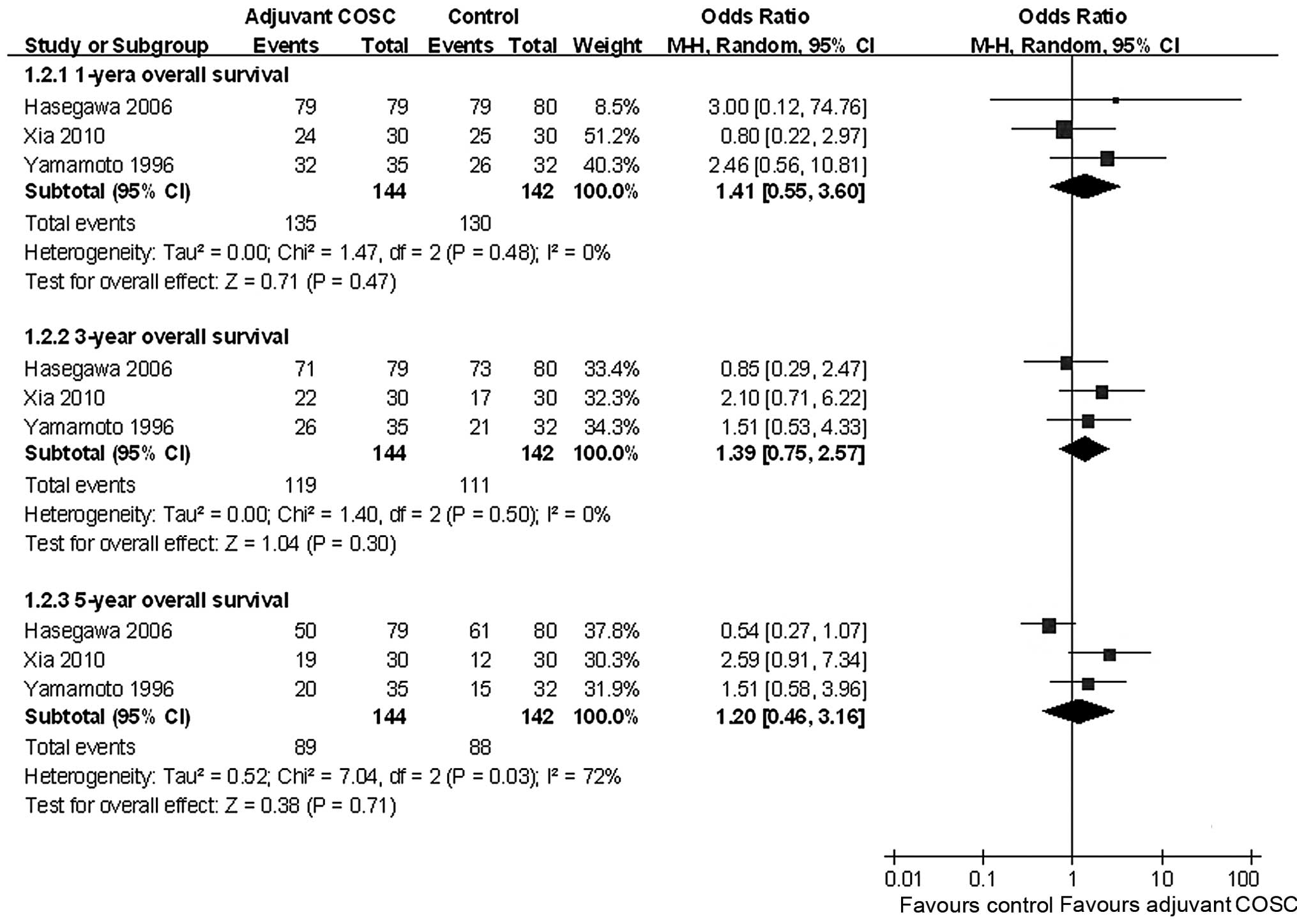
A meta-analysis revealed that adjuvant COSC did not
significantly increase the 1-, 3- and 5-year OS, with pooled ORs of
1.43 (95% CI: 0.58–3.56, P=0.44), 1.39 (95% CI: 0.75–2.55, P=0.29)
and 1.20 (95% CI: 0.46–3.16, P=0.71), respectively (Fig. 2).
There were no significant differences between the
two groups regarding long-term survival in the study by Kohno et
al (16) (P=0.22) and the
study by Ono et al (15)
(P=0.14).
Recurrence rates
All the three RCTs (12–14)
reported recurrence rates. Compared to supportive care,
capecitabine significantly decreased the recurrence rate (P=0.046)
(12). Carmofur also improved the
recurrence-free survival rates of patients with stage I disease
(P=0.04). However, in patients with stage II disease, no
significant difference was observed (P=1.00) (14). Yamamoto et al (14) concluded that the potential benefits
of carmofur on tumor recurrence must be weighed against the risks
of adverse reactions in patients with mild liver dysfunction. In
the third study, however, the recurrence-free survival curves were
similar between the groups (P=0.87) (13).
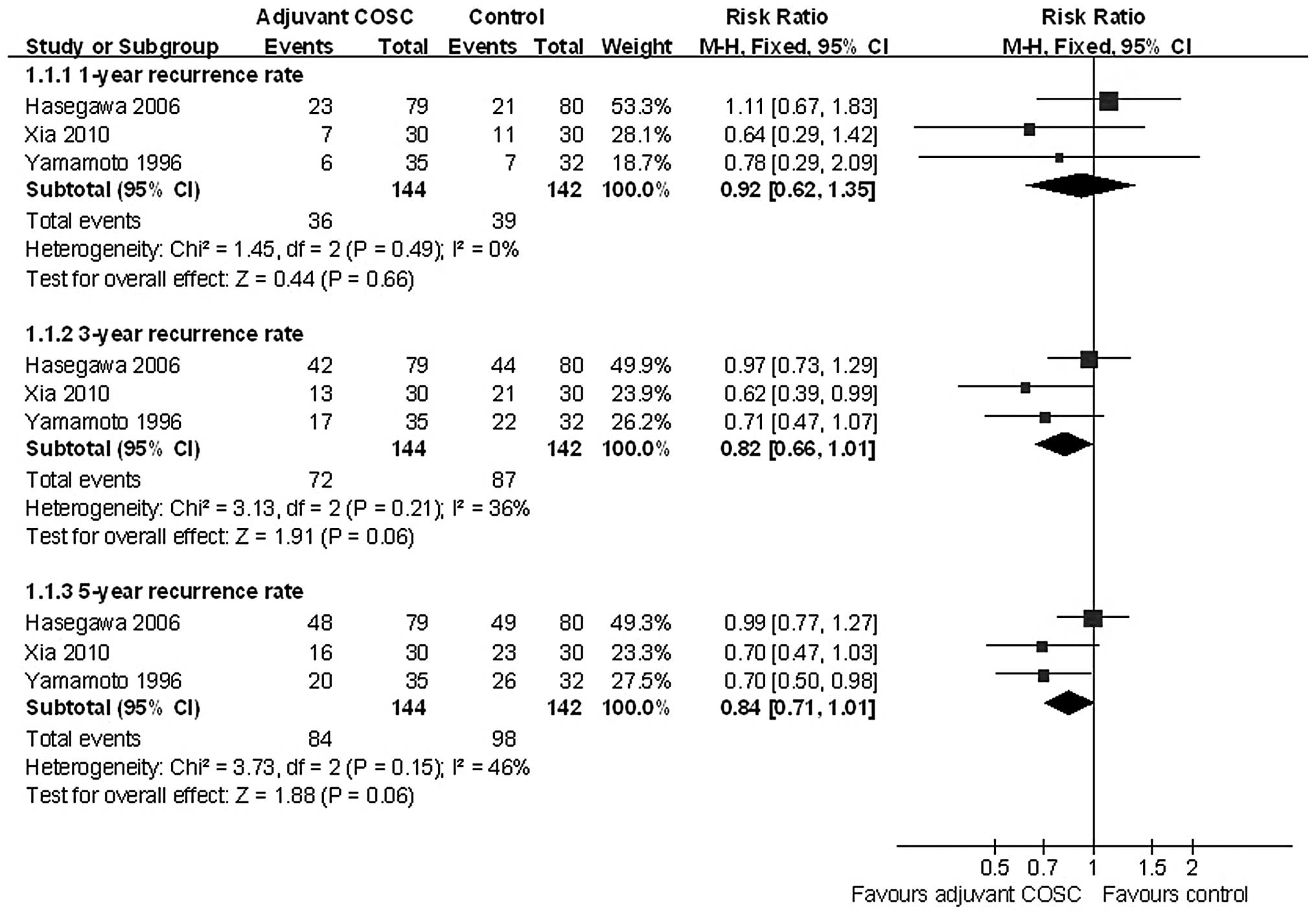
Although adjuvant COSC was expected to reduced
recurrence, a meta-analysis did not revealed a statistically
significant decrease in the incidence of the 1-, 3- and 5-year HCC
recurrence rate, with pooled ORs of 0.92 (95% CI: 0.62–1.35,
P=0.66); 0.82 (95% CI: 0.66–1.01, P=0.06); and 0.84 (95% CI:
0.71–1.01, P=0.06), respectively (Fig.
3).
All the three trials demonstrated no statistically
significant difference in the disease-free survival (DFS) curves
between the control and COSC with or without other type of
chemotherapy groups (15–17).
Adverse effects of adjuvant COSC
There was no reported treatment-related mortality.
In the study by Xia et al (12), the observed adverse reactions were
generally mild. Nausea (23.3%) and diarrhea (16.7%) were the most
common adverse effects associated with oral capecitabine. Two
patients (7%) withdrew from capecitabine therapy due to repeated
grade III nausea or low white blood cell and platelet counts.
Although treatment with uracil-tegafur was temporarily or
permanently discontinued in 41% of the patients due to adverse
effects, liver toxicity was negligible. Moreover, all the adverse
events responded to conservative therapy (13). However, carmofur administration was
suspended due to side effects in 9 of 21 patients (42.9%) with
clinical stage I and in 3 of 6 patients (50%) with stage II
cirrhosis, although the symptoms resolved within 2 months of
suspension of the drug. The most common adverse effects were
neuropathy (18.5%) and liver dysfunction (18.5%) (14).
Discussion
The aim of this systematic review was to assess the
available evidence regarding the effect of HR plus COSC on OS and
tumor recurrence in HCC patients. On the whole, adjuvant COSC with
capecitabine, uracil-tegafur, or carmofur was well tolerated by the
majority of the HCC patients. Two included RCTs indicated that
adjuvant COSC significantly decreased the recurrence rate of HCC
patients following curative HR (12,14).
However, the meta-analysis demonstrated that there was no
statistically significant benefit regarding OS and tumor
recurrence. Moreover, another two RCTs which investigated the
efficacy of adjuvant COSC plus transarterial chemotherapy (15,16)
and one case-control trial (17)
did not demonstrate a statistically significant benefit of adjuvant
COSC. These results were consistent with those of former studies
evaluating the efficacy of COSC (9,18)
and systemic intravenous chemotherapy for advanced HCC (19–22).
Conventional systemic therapy with a single agent or a combination
of agents has provided marginal survival benefits.
Theoretically, adjuvant COSC may prevent metastatic
recurrence caused by HCC cells present in the microcirculation,
which were not identified by preoperative imaging, with fewer
severe side effects on the liver. It is hypothesized that
tumoricidal effects on lesions in the precancerous stage may be
achieved by oral intake of anticancer agents, which induces a high
portal drug concentration (14).
Indeed, systemic chemotherapy is often difficult to perform on
cirrhotic HCC patients following HR. First, the majority of the HCC
patients are cirrhotic. Anticancer drugs may lead to further
impairement of liver function. Hence, cirrhosis exerts a
significant effect on the pharmacokinetics of systemic therapy for
HCC. Second, certain drug resistance genes of HCC cells, such as
P-glycoprotein, glutathione-S-transferase, heat shock proteins and
mutations in p53, are overexpressed. Unlike other cancers, the
majority of the reported deaths in HCC patients are attributed to
liver disease rather than to HCC (13). In cirrhotic patients, adjuvant
chemotherapy is associated with worse DFS and OS by negatively
affecting liver function (14,23).
Furthermore, accelerated repopulation of surviving tumor cells may
occur after sequential chemotherapy (24). A marginally higher incidence of
advanced recurrence in the adjuvant COSC group was reported by
Hasegawa et al (13) and
Lai et al (25) reported
that adjuvant chemotherapy was associated with more frequent
extrahepatic recurrences and a worse outcome. Therefore, systemic
chemotherapy is not widely used. The majority of the HCC patients
in this systematic review were infected by hepatitis B/C virus
and/or cirrhotic. The meta-analysis demonstrated that adjuvant COSC
was likely to decrease the recurrence rate and improve the OS.
However, no statistically significant benefit was observed.
Certain double-blind, multicenter, phase III RCTs
indicated that oral sorafenib is effective for the treatment of
advanced HCC (26–28). A phase III trial is ongoing to
evaluate the safety and efficacy of adjuvant sorafenib compared to
placebo in the treatment of HCC. Oral sorafenib may prove to be a
viable option for the treatment of HCC following curative HR.
A major drawback of this review is that the number
of the included patients, who were all Asian, was relatively small.
Although we systematically searched Medline, Embase and the
Cochrane Library databases and reviewed two RCTs and one
case-control trial investigating adjuvant systemic chemotherapy for
HCC, there is a risk of random errors. Furthermore, some of the
important patient characteristics, such as tumor size and hepatitis
status, were not described. In addition, the randomization
procedure and allocation concealment remained unclear in certain
studies. Due to these limitations, the results and the conclusions
of the present study should be interpreted with caution.
In summary, although adjuvant COSC appears to be
well tolerated, our results demonstrated only marginal benefits for
HCC patients undergoing curative HR. Further trials should be
conducted to investigate novel adjuvant therapies, such as
treatment with multikinase inhibitors.
Acknowledgements
This study was supported by the Self-Raised
Scientific Research Fund of the Ministry of Health of Guangxi
Province (nos. Z2012345 and Z2014241) and Youth Science Foundation
of Guangxi Medical University (no. GXMUYSF201302) to Z.J.H., and by
the Guangxi Natural Science Foundation (no. 2011GXNSFD 018032) to
L.L.Q.
References
|
1
|
Imamura H, Matsuyama Y, Tanaka E, et al:
Risk factors contributing to early and late phase intrahepatic
recurrence of hepatocellular carcinoma after hepatectomy. J
Hepatol. 38:200–207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhong C, Guo RP, Li JQ, et al: A
randomized controlled trial of hepatectomy with adjuvant
transcatheter arterial chemoembolization versus hepatectomy alone
for stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol.
135:1437–1445. 2009. View Article : Google Scholar
|
|
3
|
Capussotti L, Muratore A, Amisano M, et
al: Liver resection for hepatocellular carcinoma on cirrhosis:
analysis of mortality, morbidity and survival - a European single
center experience. Eur J Surg Oncol. 31:986–993. 2005. View Article : Google Scholar
|
|
4
|
Lang H, Sotiropoulos GC, Brokalaki EI, et
al: Survival and recurrence rates after resection for
hepatocellular carcinoma in noncirrhotic livers. J Am Coll Surg.
205:27–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong JH, Li H, Li LQ, et al: Adjuvant
therapy options following curative treatment of hepatocellular
carcinoma: a systematic review of randomized trials. Eur J Surg
Oncol. 38:286–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong JH and Li LQ: Postoperative adjuvant
transarterial chemoembolization for participants with
hepatocellular carcinoma: a meta-analysis. Hepatol Res. 40:943–953.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong JH, Ma L, Wu LC, et al: Adoptive
immunotherapy for postoperative hepatocellular carcinoma: a
systematic review. Int J Clin Pract. 66:21–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Llovet JM, Ruff P, Tassopoulos N, et al: A
phase II trial of oral eniluracil/5-fluorouracil in patients with
inoperable hepatocellular carcinoma. Eur J Cancer. 37:1352–1358.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benson AB III, Mitchell E, Abramson N,
Klencke B, Ritch P, Burnhan JP, McGuirt C, Bonny T, Levin J and
Hohneker J: Oral eniluracil/5-fluorouracil in patients with
inoperable hepatocellular carcinoma. Ann Oncol. 13:576–581. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson PJ: Hepatocellular carcinoma: is
current therapy really altering outcome? Gut. 51:459–462. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moher D, Cook DJ, Eastwood S, et al:
Improving the quality of reports of meta-analyses of randomised
controlled trials: the QUOROM statement. QUOROM Group. Br J Surg.
87:1448–1454. 2000. View Article : Google Scholar
|
|
12
|
Xia Y, Qiu Y, Li J, et al: Adjuvant
therapy with capecitabine postpones recurrence of hepatocellular
carcinoma after curative resection: a randomized controlled trial.
Ann Surg Oncol. 17:3137–3144. 2010. View Article : Google Scholar
|
|
13
|
Hasegawa K, Takayama T, Ijichi M, et al:
Uracil-tegafur as an adjuvant for hepatocellular carcinoma: a
randomized trial. Hepatology. 44:891–895. 2006. View Article : Google Scholar
|
|
14
|
Yamamoto M, Arii S, Sugahara K and Tobe T:
Adjuvant oral chemotherapy to prevent recurrence after curative
resection for hepatocellular carcinoma. Br J Surg. 83:336–340.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ono T, Nagasue N, Kohno H, et al: Adjuvant
chemotherapy with epirubicin and carmofur after radical resection
of hepatocellular carcinoma: a prospective randomized study. Semin
Oncol. 24(Suppl 6): 18–25. 1997.PubMed/NCBI
|
|
16
|
Kohno H, Nagasue N, Hayashi T, et al:
Postoperative adjuvant chemotherapy after radical hepatic resection
for hepatocellular carcinoma (HCC). Hepatogastroenterology.
43:1405–1409. 1996.PubMed/NCBI
|
|
17
|
Takenaka K, Yoshida K, Nishizaki T, et al:
Postoperative prophylactic lipiodolization reduces the intrahepatic
recurrence of hepatocellular carcinoma. Am J Surg. 169:400–405.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patt YZ, Hassan MM, Aguayo A, et al: Oral
capecitabine for the treatment of hepatocellular carcinoma,
cholangiocarcinoma, and gallbladder carcinoma. Cancer. 101:578–586.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edeline J, Raoul JL, Vauleon E, et al:
Systemic chemotherapy for hepatocellular carcinoma in non-cirrhotic
liver: a retrospective study. World J Gastroenterol. 15:713–716.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu AX: Systemic treatment of
hepatocellular carcinoma: dawn of a new era? Ann Surg Oncol.
17:1247–1256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uchino K, Obi S, Tateishi R, et al:
Systemic combination therapy of intravenous continuous
5-fluorouracil and subcutaneous pegylated interferon alfa-2a for
advanced hepatocellular carcinoma. J Gastroenterol. 47:1152–1159.
2012. View Article : Google Scholar
|
|
22
|
Cao H, Phan H and Yang LX: Improved
chemotherapy for hepatocellular carcinoma. Anticancer Res.
32:1379–1386. 2012.
|
|
23
|
Ono T, Yamanoi A, Nazmy El Assal O, et al:
Adjuvant chemotherapy after resection of hepatocellular carcinoma
causes deterioration of long-term prognosis in cirrhotic patients:
metaanalysis of three randomized controlled trials. Cancer.
91:2378–2385. 2001. View Article : Google Scholar
|
|
24
|
Davis AJ and Tannock JF: Repopulation of
tumour cells between cycles of chemotherapy: a neglected factor.
Lancet Oncol. 1:86–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lai EC, Lo CM, Fan ST, et al:
Postoperative adjuvant chemotherapy after curative resection of
hepatocellular carcinoma: a randomized controlled trial. Arch Surg.
133:183–188. 1998.PubMed/NCBI
|
|
26
|
Abou-Alfa GK, Johnson P, Knox JJ, et al:
Doxorubicin plus sorafenib vs. doxorubicin alone in patients with
advanced hepatocellular carcinoma: a randomized trial. JAMA.
304:2154–2160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar : PubMed/NCBI
|