Introduction
Lung cancer, which is usually diagnosed at an
advanced stage, is a major cause of mortality from malignant
diseases due to its high incidence, malignant behavior and lack of
major advancements in treatment strategy (1). Lung cancer was the leading cause for
respiratory surgery (48.9%) in 2011 in Japan (2) and >33,000 patients underwent
surgery for lung cancer at Japanese institutions in the same year
(2). The clinical behavior of
non-small cell lung cancer (NSCLC) is largely associated with its
stage, and surgery can only cure the early stage NSCLC disease
(3). Chemotherapy is the
therapeutic mainstay for squamous NSCLC, however, recent advances
in target therapy for NSCLC have increased the treatment choices
for non-squamous NSCLC. One novel promising therapeutic approach
may be immunotherapy (4).
Circulating immune cells may be able to identify,
infiltrate and eliminate certain incipient cancer cells, however,
some may bypass the immune surveillance and immune system-mediated
cell death (5). Recent studies
have enhanced the understanding of the molecular basis for this
phenomenon and have facilitated in the identification of anticancer
approaches that act by modulating the immune system. An imbalance
of the immune system regulation changes the tumor-specific T-cell
immunity in the cancer microenvironment and adjusts the tumor
progression and metastasis (6).
Limited immunostimulatory activation can be detrimental if it
impedes the immune responses against cancer (7). Numerous receptor-ligand interactions
are known to trigger anti-apoptotic pathways that prevent the
activation-induced T-cell death (8,9). The
programmed death 1 (PD-1) protein, a T-cell co-inhibitory receptor,
plays a central role in the ability of the tumor cells to escape
the host immune system. PD-L1, one of the ligands, is selectively
expressed in a number of tumors (10–12).
Inhibition of the interactions between PD-1 and PD-L1 improves the
immune function in vitro and mediates antitumor activity in
preclinical models (10,11). Recent studies have indicated that
the antibody-mediated blockade of PD-1 (13) and PD-L1 (14) induced durable tumor regression and
prolonged stabilization of disease in certain patients with
advanced cancers, including NSCLC. The PD-1 −606 G allele at
the promoter showed a significant correlation with Japanese
subacute sclerosing panencephalitis (SSPE) (15). This −606 G/A single-nucleotide
polymorphism (SNP) resides in the putative binding site for UCE-2
transcription regulators (GGCCG at position −610 to −606). A
haplotype of the −606 G allele with a high promoter activity was
correlated with the development of SSPE (15). The relative PD-1 expression was
higher in SSPE patients compared to the control (15), however, the correlation between
this Asian-specific PD-1 SNP and NSCLC has not been well
investigated.
In the present study, the PD-1 and cytotoxic
T-lymphocyte-associated antigen 4 (CTLA-4) gene
polymorphisms were investigated in Japanese patients with NSCLC
using TaqMan genotyping quantitative polymerase chain reaction
(qPCR) in surgically-treated cases. The findings were compared to
the clinicopathological features of NSCLC and the PD-1 or
CTLA-4 gene SNP statuses.
Patients and methods
Patient samples
The study group included NSCLC patients who had
undergone surgery at the Department of Surgery, Nagoya City
University Hospital (Nagoya, Japan) between 1997 and 2012. All the
tumor samples were immediately frozen and stored at −80°C until
analysis. The patient consent was obtained from all the patients.
The study was approved by the Ethics Committee of the University.
The clinical and pathological characteristics of the 583 NSCLC
patients for PD-1 gene analyses were as follows: 399 males
(68.4%), 184 females (31.6%), 366 diagnosed with adenocarcinomas
(adeno) (62.8%) and 161 with squamous cell carcinomas (SCC)
(27.6%), 395 smokers (67.8%), 188 non-smokers (32.2%) and 348 with
pathological stage I (59.7%).
qPCR assay for the PD-1 gene
Genomic DNA was extracted from peripheral blood or
thymus tissues using Wizard SV Genomic DNA Purification system
(Promega, Madison, WI, USA) according to the manufacturer’s
instructions. The DNA concentration was determined by a NanoDrop
ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Rockland,
DE, USA). The primers and TaqMan probes for PD-1 (−606 G/A,
codon −606 of promoter, rs36084323; +6371 G/A, intron 2,
rs34819629) and CTLA-4 (+49A/G, codon 17 of exon 1,
rs231775) were designed at Applied Biosystems (Foster City, CA,
USA). For the SNP genotyping, one pair of TaqMan probes and one
pair of PCR primers were used. The two TaqMan probes differed at
the polymorphic site, with one probe complementary to the wild-type
and the other complementary to the variant allele. TaqMan PCR and
genotyping analysis were performed on an Applied Biosystems 7500
Real-Time PCR system. The reaction mixture were amplified in 1 μl
template DNA (10 ng/μl), 12.5 μl 2X TaqMan Universal Master mix,
0.625 μl 20X primer/probe mix and 10.875 μl ddH2O in a
total volume of 25 μl. The cycling conditions were as follows:
Initial denaturation at 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec and 58°C for 1 min. The results were analyzed on
the Applied Biosystems 7500 Real-Time PCR system using the alleic
discrimination assay program.
Immunohistochemistry
The specimens were cut into 4-μm sections and were
deparaffinized by xylene and alcohol. Endogenous peroxidase
activity was blocked by the peroxidase blocking reagent (R&D
Systems, Minneapolis, MN, USA) for 5 min. Subsequently, the
sections were washed three times in phosphate-buffered saline
(PBS). The nonspecific binding was blocked with serum-blocking
reagent D (Cell Tissue Staining kit, no. 008) for 15 min and the
sections were incubated with avidin-blocking reagent for 15 min and
biotin-blocking reagent for 15 min. The sections were incubated
with the primary anti-PD-1 antibody (R&D Systems) in a humid
chamber at 4°C overnight. Following three washes with PBS, the
sections were incubated with biotinylated secondary antibodies for
45 min, washed three times in PBS and incubated with
streptavidin-conjugated peroxidase for 30 min. Subsequent to three
additional washes in PBS, 3,3′-diaminobenzidine tetrahydrochloride
chromogen buffer was applied and the sections were counterstained
with hematoxylin. The sections were determined as positive if
>10% of the tumor-infiltrating lymphocytes (TILs) were stained
(16).
Statistical analysis
Statistical analyses were performed using the
Student’s t-test for unpaired samples and χ2 test for
paired samples. Correlation coefficients were determined using
χ2 test. The overall survival time of the lung cancer
patients was examined by the Kaplan-Meier methods and the
differences were examined by the log-rank test. All the analyses
were conducted using the StatView software package (Abacus
Concepts, Inc., Berkeley, CA, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
PD-1 DNA status in Japanese lung cancer
patients
The PD-1 gene promoter polymorphism status
was genotyped for 583 NSCLC samples. The PD-1 −606 SNP
statuses at the promoter region (rs36084323) were 146 AA (25.0%),
293 GA (50.3%) and 144 GG (24.7%). The ratios were extremely
similar to the healthy control of the Asian population in a
previous study: 24.9% AA, 47.8% GA and 27.3% GG. The PD-1
rs36084323 were 87 AA (23.8%), 186 AG (50.8%), 93 GG (25.4%) in
adeno; and 48 AA (29.8%), 74 AG (46.0%), 39 GG (24.2%) in SCC. The
ratio of the GG phenotype was not significantly different between
adeno vs. others (P=0.6057) or SCC vs. others (P=0.8692). The GG
ratio was not correlated with gender (male vs. female, 25.1% vs.
23.9%; P=0.7648), ages (≤65 vs. >65 years, 25.5 vs. 24.1%;
P=0.6987), smoking status (smoker vs. non-smoker, 26.1 vs. 21.8%;
P=0.2641) and EGFR mutations status (wild type vs. mutant
patients, 24.7 vs. 24.7%; P=0.9934). The GG ratio was not
correlated with lymph node metastasis (positive vs. negative, 28.1
vs. 23.3%; P=0.2218). The GG ratio of PD-1 −606 according to
the pathological T stages were as follows: 24.6% pT1, 24.6% pT2,
25% pT3 and 25% pT4. The GG ratio was not significantly different
(pT1 vs. pT2-4, P=0.9565). The PD-1 −606 were 80 AA (23.0%),
184 GA (52.9%), 84 GG (24.1%) at stage I; 28 AA (29.2%), 44 GA
(45.8%), 24 GG (25%) at stage II; and 38 AA (27.3%), 65 GA (46.8%),
36 GG (25.9%) at stage III–IV. The ratio of the GG phenotype was
not significantly different between stage I vs.stages II–IV
(P=0.7018) (Table I).
 | Table IClinicopathological data of 583 lung
cancer patients. |
Table I
Clinicopathological data of 583 lung
cancer patients.
| PD-1 |
|---|
|
|
|---|
| Factors | AA+GA patients, n
(%) | GG patients, n
(%) | P-value |
|---|
Mean age
66.7±9.3 years | 439 (66.7±9.4) | 144 (66.3±9.5) | 0.5726 |
| Stage | | | |
| I | 264 (60.1) | 84 (58.3) | II–IV vs. I |
| II | 72 (16.4) | 24 (16.7) |
| III–IV | 103 (23.5) | 36 (25.0) | 0.7018 |
| Tumor status | | | |
| pT1 | 181 (41.2) | 59 (41.0) | T2-4 vs. T1 |
| pT2 | 174 (39.6) | 57 (39.6) |
| pT3 | 51 (11.6) | 17 (11.8) | 0.9934 |
| pT4 | 33 (7.5) | 11 (7.6) | |
| Lymph node
metastasis | | | |
| Negative | 319 (72.7) | 97 (67.4) | 0.2218 |
| Positive | 120 (27.3) | 47 (32.6) | |
| Age, years | | | |
| ≤65 | 184 (41.9) | 63 (43.8) | 0.6987 |
| >65 | 255 (58.1) | 81 (56.3) | |
| EGFR
mutation | | | |
| Positive | 116 (26.4) | 38 (26.4) | 0.9934 |
| Negative | 323 (73.6) | 106 (73.6) | |
| Smoking | | | |
| BI=0 | 147 (33.5) | 41 (28.5) | 0.2641 |
| BI>0 | 292 (66.5) | 103 (71.5) | |
| Pathological
subtypes | | | |
| Adeno | 273 (62.2) | 93 (64.6) | SCC vs. others |
| Squamous | 122 (27.8) | 39 (27.1) |
| Others | 44 (10.0) | 12 (8.3) | 0.8692 |
| Gender | | | |
| Male | 299 (68.1) | 100 (69.4) | 0.7648 |
| Female | 140 (31.9) | 44 (30.6) | |
The overall survival time of 583 lung cancer
patients from Nagoya City University, with a follow-up until August
31, 2013, was studied in reference to the PD-1 gene −606 SNP
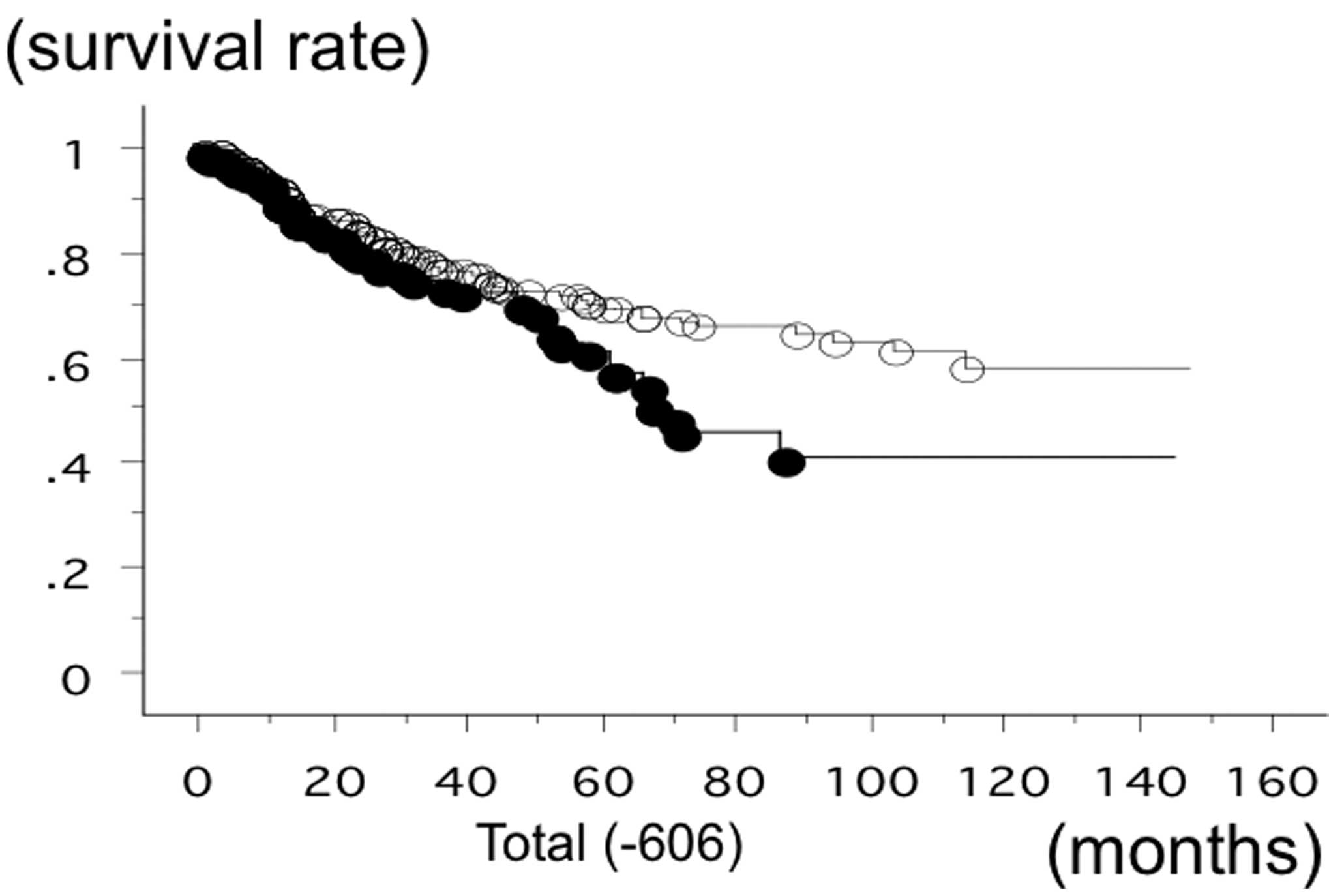
statuses. The survival time of the patients with the −606 GG
phenotype of PD-1 was significantly lower (n=147; 50
succumbed; mean survival, 60.6 months) compared to the patients
with −606 GA or −606 AA (n=436; 110 succumbed; mean survival, 84.6
months) (log-rank test, P=0.0183) (Fig. 1). The survival rate of the SCC
patients with the −606 GG phenotype of PD-1 was also
significantly lower (n=42; 18 succumbed; mean survival, 43.5
months) compared to the patients with −606 GA or −606 AA (n=124; 33
succumbed; mean survival, 84.3 months) (log-rank test, P=0.0090)
(Fig. 2). The survival time of the
stage I SCC patients with the −606 GG phenotype of PD-1 was
significantly worse (n=19; 8 succumbed; mean survival, 47 months)
compared to the patients with −606 GA or −606 AA (n=66; 12
succumbed; mean survival, 95.2 months; P=0.0091). However, the
survival time of the stage II–IV SCC patients with the −606 GG and
GA or AA was not significantly different (P=0.2840). The survival
time of the adeno patients with the −606 GG phenotype of
PD-1 (n=93; 28 succumbed; mean survival, 65.1 months) and
with the patients with −606 GA or −606 AA (n=273; 68 succumbed;
mean survival, 79.2 months) was not significantly different
(P=0.2718). Univariate analysis demonstrated that pathological
stage (I vs. II–IV, P<0.0001), smoking status (non-smoker vs.
smoker, P=0.007) and gender (P=0.006) were the prognostic factors
for NSCLC in the present cohort. Multivariate analysis showed that
pathological stage (hazard ratio, 2.751; P<0.0001), gender
(hazard ratio, 1.795; P=0.0204) and PD-1 −606 GG (hazard
ratio, 1.431; P=0.037) were the independent prognostic factors.
Disease-free survival (DFS) was evaluated for 325 NSCLC cases. The
DFS of PD-1 −606 GG (21/80 succumbed; mean survival, 52.6
months) and −606 GA or AA (59/245 succumbed; mean survival, 56.4
months) was not significantly different (P=0.7178).
PD-L1/β-actin mRNA levels in NSCLC were evaluated in
our previous study (17).
PD-L1/β-actin mRNA levels were AA, 5.838±8.265 (n=34); GA,
3.113±7.317 (n=64); and GG, 45.791±238.663 (n=36). There was a
tendency towards higher PD-L1/β-actin mRNA levels in GG compared to
GA+AA (4.059±7.726; P=0.0842). The PD-1 protein expression was
evaluated by immunohistochemistry. Only one case (GG case) showed
extremely high PD-1 signals (Fig.
3). The PD-1-positive sections (TILs) were GG, 7/21 (66.7%);
GA, 8/9 (47.1%); and AA, 2/10 (20%). There was a tendency towards
higher PD-1-positive sections in GG compared to GA or AA
(P=0.0809).
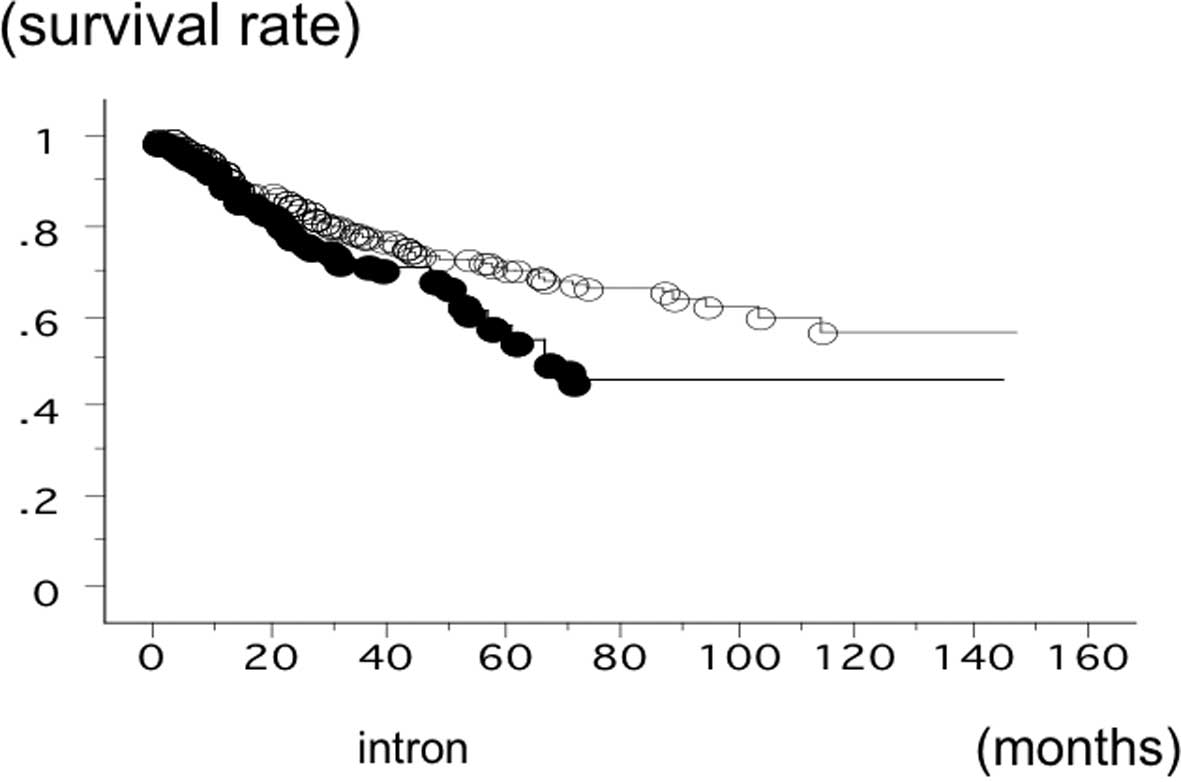
The PD-1 +6371 SNP statuses at the intron 2
region (rs34819629) were 134 AA (23.0%), 298 GA (51.2%) and 150 GG
(25.8%). The ratio was extremely similar to the promoter −606 SNP
and 89.7% was identical. The PD-1 rs34819629 were 83 AA
(22.7%), 186 AG (50.8%), 97 GG (26.5%) in adeno; and 42 AA (26.3%),
76 AG (47.5%), 42 GG (26.3%) in SCC. The ratio of the GG phenotype
was not significantly different between adeno vs. others (P=0.6004)
or SCC vs. others (P=0.8714). The GG ratio was not correlated with
gender (male vs. female, 26.9 vs. 23.4%; P=0.3674), ages (≤65 vs.
>65 years, 29.2 vs. 23.3%; P=0.1098), smoking status (smoker vs.
non-smoker, 27.4 vs. 22.8%; P=0.1909) and EGFR mutations
status (wild type vs. mutant patients, 26.2 vs. 24.7%; P=0.7164).
The GG ratio was not correlated with lymph node metastasis
(positive vs. negative, 26.9 vs. 25.3%; P=0.6815). The GG ratio of
PD-1 +6371 according to the pathological T stages were as
follows: 25.5% pT1, 25.1% pT2, 29.4% pT3 and 25% pT4. The GG ratio
was not significantly different (pT1 vs. pT2-4; P=0.9083). The
PD-1 +6371 were 73 AA (21.0%), 185 GA (53.3%), 89 GG (25.6%)
in stage I; 26 AA (26.8%), 47 GA (48.5%), 24 GG (24.7%) at stage
II; and 35 AA (25.5%), 66 GA (48.2%), 36 GG (26.3%) at stages
III–IV. The ratio of the GG phenotype was not significantly
different between stage I vs.stages II–IV (P=0.9333) (Table II).
 | Table IIClinicopathological data of 582 lung
cancer patients. |
Table II
Clinicopathological data of 582 lung
cancer patients.
| PD-1 +6371
(intron 2) |
|---|
|
|
|---|
| Factors | AA+GA patients, n
(%) | GG patients, n
(%) | P-value |
|---|
Mean age
66.7±9.3 years | 432 (66.8±9.4) | 150 (65.9±9.3) | 0.2320 |
| Stage | | | |
| I | 258 (59.7) | 89 (59.3) | II–IV vs. I |
| II | 73 (16.9) | 25 (16.7) |
| III–IV | 101 (23.4) | 36 (24.0) | 0.9333 |
| Tumor status | | | |
| pT1 | 178 (41.2) | 61 (40.7) | T2-4 vs. T1 |
| pT2 | 173 (40.0) | 58 (38.7) |
| pT3 | 48 (11.1) | 20 (13.3) | 0.9083 |
| pT4 | 33 (7.6) | 11 (7.3) | |
| Lymph node
metastasis | | | |
| Negative | 310 (71.8) | 105 (70.0) | 0.6815 |
| Positive | 122 (28.2) | 45 (30.0) | |
| Age, years | | | |
| ≤65 | 175 (40.5) | 72 (48.0) | 0.1098 |
| >65 | 257 (59.5) | 78 (52.0) | |
| EGFR
mutation | | | |
| Positive | 116 (26.9) | 38 (25.3) | 0.7164 |
| Negative | 316 (73.1) | 112 (74.7) | |
| Smoking | | | |
| BI=0 | 146 (33.8) | 42 (28.0) | 0.1909 |
| BI>0 | 286 (66.2) | 108 (72.0) | |
| Pathological
subtypes | | | |
| Adeno | 269 (62.3) | 97 (64.7) | SCC vs. others |
| Squamous | 118 (27.3) | 42 (28.0) |
| Others | 45 (10.4) | 11 (7.3) | 0.8714 |
| Gender | | | |
| Male | 291 (67.4) | 107 (71.3) | 0.3674 |
| Female | 141 (32.6) | 43 (28.7) | |
The overall survival time of 582 lung cancer
patients was studied in reference to the PD-1 gene +6371 SNP
status. The survival time of the patients with the +6371 GG
phenotype of PD-1 was significantly lower (n=151; 52
succumbed; mean survival, 52.8 months) compared to the patients
with +6371 GA or AA (n=431; 108 succumbed; mean survival, 84.6
months) (log-rank test, P=0.0103) (Fig. 4). The survival time of the SCC
patients with the +6371 GG phenotype of PD-1 was also
significantly worse (n=42; 19 succumbed; mean survival, 42.9
months) compared to the patients with +6371 GA or +6371 AA (n=123;
33 succumbed; mean survival, 84.6 months) (log-rank test,
P=0.0063). However, the survival time of the adeno patients with
the +6371 GG phenotype of PD-1 (n=98; 30 succumbed; mean
survival, 56.3 months) and with the patients with +6371 GA or AA
(n=268; 66 succumbed; mean survival, 79.6 months) was not
significantly different (P=0.1492). Multivariate analysis showed
pathological stage (hazard ratio, 2.775; P<0.0001), gender
(hazard ratio, 1.779; P=0.0224) and PD-1 +6371 GG (hazard
ratio, 1.516; P=0.0141) were the independent prognostic
factors.
CTLA-4 +49 SNP status in Japanese lung
cancer patients
The CTLA-4 +49 SNP statuses at exon 1
(rs231775) were 86 AA (14.8%), 284 GA (48.7%) and 213 GG (36.5%).
The ratio was extremely similar to the Chinese lung cancer cohort
in a previous study: 13.6% AA; 42.0% GA and 44.0% GG. The
CTLA-4 +49 were 55 AA (15.0%), 184 AG (50.3%), 127 GG
(34.7%) in adeno; and 22 AA (13.7%) 80 AG (49.7%), 59 GG (36.6%) in
SCC. The ratio of the AA phenotype was not significantly different
between adeno vs. others (P=0.8072) or SCC vs. others (P=0.6477).
The AA ratio was not correlated with gender (male vs. female, 14.8
vs. 14.7%; P=0.9715), ages (≤65 vs. >65 years, 15.4 vs. 14.3%;
P=0.7116), smoking status (smoker vs. non-smoker, 14.2 vs. 16.0%;
P=0.5710) and EGFR mutations status (wild type vs. mutant
patients, 14.2 vs. 16.2%; P=0.5453). The AA ratio had a lower
tendency in the patients with lymph node metastasis (10.8%)
compared to the patients without lymph node metastasis (16.3%)
(P=0.0865). The AA ratio of CTLA-4 +49 according to the
pathological T stages were as follows: 14.6% pT1, 16.4% pT2, 10.3%
pT3 and 13.6% pT4. The AA ratio was not significantly different
(pT1 vs. pT2-4, P=0.9238). The CTLA-4 +49 were 57 AA
(16.4%), 160 GA (46.0%), 131 GG (37.6%) at stage I; 12 AA (12.2%),
55 GA (56.1%), 31 GG (31.6%) at stage II; and 17 AA (12.4%), 69 GA
(50.4%), 51 GG (37.2%) at stage III–IV. The ratio of the AA
phenotype was not significantly different between stage I vs. II–IV
(P=0.1774) (Table III).
 | Table IIIClinicopathological data of 583 lung
cancer patients. |
Table III
Clinicopathological data of 583 lung
cancer patients.
| CTLA-4 +49
(exon 1) |
|---|
|
|
|---|
| Factors | AA patients, n
(%) | AG+GG patients, n
(%) | P-value |
|---|
Mean age
66.7±9.3 years | 86 (67.9±9.1) | 497 (66.3±9.4) | 0.2878 |
| Stage | | | |
| I | 57 (66.3) | 291 (58.6) | II–IV vs. I |
| II | 12 (14.0) | 86 (17.3) |
| III–IV | 17 (19.8) | 120 (24.1) | 0.1774 |
| Tumor status | | | |
| pT1 | 35 (40.7) | 205 (41.2) | T2-4 vs. T1 |
| pT2 | 38 (44.2) | 193 (38.8) |
| pT3 | 7 (8.1) | 61 (12.3) | 0.3205 |
| pT4 | 6 (7.0) | 38 (7.6) | |
| Lymph node
metastasis | | | |
| Negative | 68 (79.1) | 348 (70.0) | 0.0865 |
| Positive | 18 (20.9) | 149 (30.0) | |
| Age, years | | | |
| ≤65 | 38 (44.2) | 209 (42.1) | 0.7116 |
| >65 | 48 (55.8) | 288 (57.9) | |
| EGFR
mutation | | | |
| Positive | 25 (29.1) | 129 (26.0) | 0.5453 |
| Negative | 61 (70.9) | 368 (74.0) | |
| Smoking | | | |
| BI=0 | 30 (34.9) | 158 (31.8) | 0.5710 |
| BI>0 | 56 (65.1) | 339 (68.2) | |
| Pathological
subtypes | | | |
| Adeno | 55 (64.0) | 311 (62.6) | SCC vs. others |
| Squamous | 22 (25.6) | 139 (28.0) |
| Others | 9 (10.5) | 47 (9.5) | 0.6477 |
| Gender | | | |
| Male | 59 (68.6) | 340 (68.4) | 0.9715 |
| Female | 27 (31.4) | 157 (31.6) | |
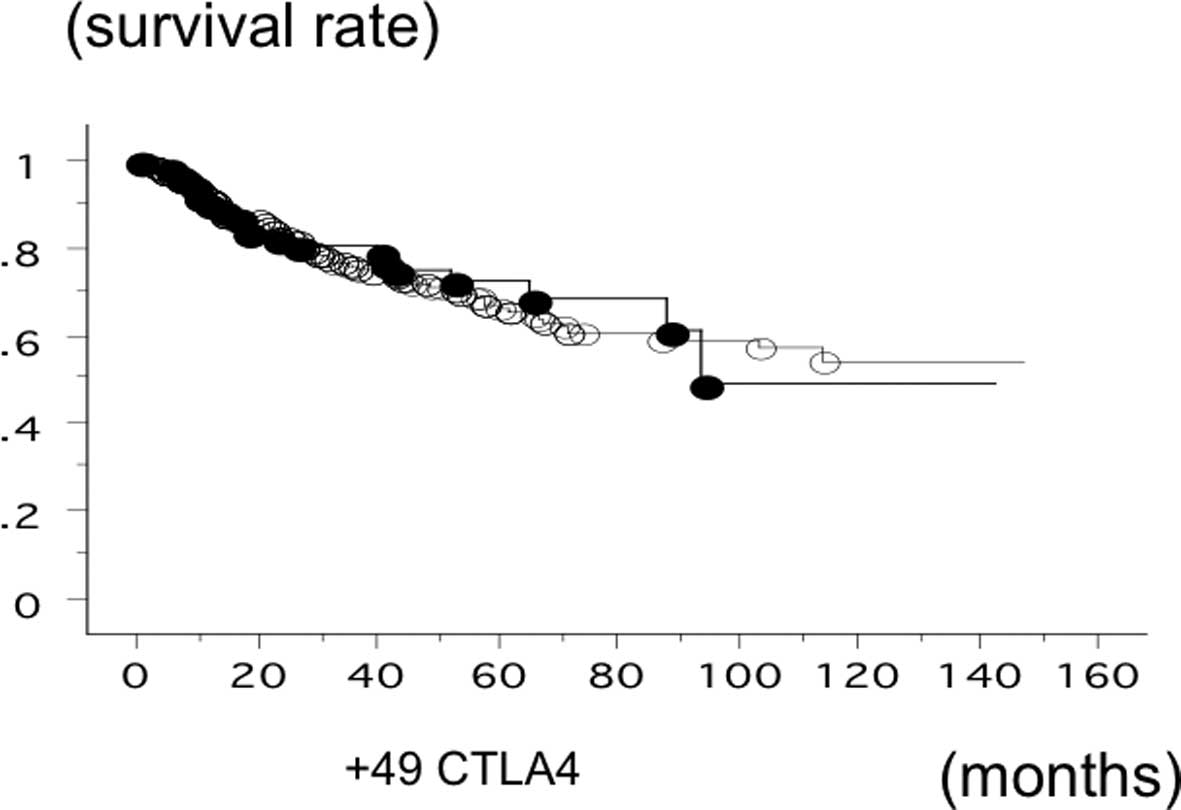
The overall survival time of 583 lung cancer was
studied in reference to the CTLA-4 gene +49 SNP status. The
survival of the patients with the AA phenotype of CTLA-4
(n=83; 21 500, 140 succumbed; mean survival, 81.0 months) was not
significantly different (log-rank test, P=0.6861) (Fig. 5). The survival of the SCC patients
with the +49 AA phenotype (n=21; 9 succumbed; mean survival, 58.6
months) and the patients with GA or GG (n=146; 42 succumbed; mean
survival, 80.3 months) was also not significantly different
(log-rank test, P=0.2591). In addition, the survival time of the
adeno patients with the +49 AA phenotype (n=127; 37 succumbed; mean
survival, 54.5 months) and the patients with GA or GG (n=240; 39
succumbed; mean survival, 79.2 months) was not significantly
different (P=0.2629).
Discussion
The focus of PD-1 in the present study, PD-1 was to
investigate whether it may be a novel molecular target for NSCLC.
The PD-1 gene promoter SNP was found to be correlated with a
poor prognosis in surgically-resected NSCLC.
Human cancers retain a number of genetic and
epigenetic changes, which can produce neoantigens that are
potentially recognizable by the immune system (18). Multistep-resistance systems,
including local immuno-suppression, induction of tolerance and
systemic dysfunction in T-cell signaling, are initiated by tumors
(19–22). Additionally, numerous pathways are
utilized by tumors to avoid immune destruction. There are a number
of checkpoints in place to modulate this nascent immune response
and to avoid the antitumor immune responses. Immune-checkpoint
pathways with therapeutic anticancer targeting potential include
PD-1, a member of the B7-CD28 family that regulates T-cell
activation, peripheral tolerance and the prevention of bystander
tissue damage during immune responses, and CTLA-4 pathways, which
control the early stages of T-cell activation. Intensive efforts
for developing immunotherapeutic approaches for cancer treatment
have evolved from these findings, including
immune-checkpoint-pathway inhibitors, such as anti-CTLA-4 antibody
(23,24) and anti-PD-L1 therapy (11,12).
These initial clinical studies using antibodies have shown a
promising safety profile and notable antitumor activity in the
subsets of patients with metastatic disease.
With regards to tumor immunology, CTLA-4 signaling
is more marked in limiting the initiation of a T-cell response in
the lymph nodes, whereas the engagement of PD-1 is clearer later in
the process and serves to limit the T-cell activity in the tumor
microenvironment (25). The +49
adenine-guanine CTLA-4 SNP has been found to increase the
risk of cancer (26) and is
classified as a prognostic predictor for advanced NSCLC (27). However, in the present study
analysis, the CTLA-4 +49 polymorphism did not correlate with
the survival rate of surgically-removed NSCLC cases. The
discrepancy may be due to the population difference, such as
advanced cases. The +49 AA ratio had a lower tendency in the
patients with lymph node metastasis. In addition, the presence of
the +49 AA allele is small in the Asian population, and therefore,
a larger cohort would determine the exact influence of this SNP. By
contrast, the role of the PD-1 SNP in NSCLC is not well
known. PD-1 is a key immune-checkpoint receptor that is expressed
by activated T-cells and mediates immunosuppression. The PD-1
ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), are expressed by tumor
and stroma cells (8,28–30).
Therefore, PD-1 may also act as a molecular target for tumor
progression in cancers. In vitro, the inhibition of the
interaction between PD-1 and PD-L1 enhanced T-cell responses and
mediated preclinical antitumor activity (10,11).
The use of an anti-PD-1 antibody has been initiated in advances for
solid tumors in a study (31). The
recent studies by Brahmer et al (14) and Topalian et al (13) have reported the safety and activity
of anti-PD-L1 or PD-1 immunotherapy in cancers, including NSCLC.
For NSCLC, 10% of patients responded to the anti-PD-L1 antibody
(14)and 18% responded to the
anti-PD-1 antibody (13). Notably,
in the study by Topalain et al (13), the expression of PD-L1 correlated
with the response. The majority of large retrospective studies
showed that PD-1 expression was associated with a poor prognosis
and/or more aggressive disease; however, numerous studies have
indicated that there is an inadequate association (16,32,33).
This may be due to the heterogeneity in the expression within tumor
tissue, the requirement to assess membrane PD-1 protein expression
rather than intracellular protein or mRNA, the limited specificity
of commercially available antibodies and the significant problems
associated with developing methods for the detection of PD-1
expression in formalin-fixed, paraffin-embedded tissue. PD-1
contains only two small linear hydrophilic regions.
A previous study has shown that −606 G/A PD-1
is associated with rheumatoid arthritis in the Chinese population
(34), but this SNP is rare in
Europeans (1%) and Africans (4%) (34). Ishizaki et al (15) demonstrated that a haplotype with
the −606 G allele and a high promoter activity was associated with
the development of Japanese SSPE (15). The haplotype frequency of
PD-1 containing the −606 G allele was significantly higher
in SSPE patients compared to controls in the Japanese population.
The promoter activity was significantly higher in the −606 G
construct compared to the −606 A allele. PD-1 expression levels
were significantly higher in SSPE patients compared to the
controls. The role of rs34819629 in intron 2 is not well known.
However, 89.7% linkage disequilibrium with rs36084323 may indicate
the genetic correlation of these SNPs.
In the analysis of the present study, the
PD-1 SNP statuses correlated with the SCC prognosis, but not
adeno. SCC exhibits a highly consistent immune profile, regarding
the expression of such molecules as p63/cytokeratin 5/6/34bE-12 and
non-expression of thyroid transcription factor-1. However, a higher
heterogeneity is shown for these and other immune elements in adeno
patients (35). Several analyses
have identified that squamous tumors more frequently express tumor
antigens, including melanoma-associated antigen or NY-ESO-1,
compared with non-squamous tumors (36,37). Thus, it is hypothesized
that the possibility of establishing the patient selection for
PD-1 SNPs on PD-1 expression in tumors requires
prospective assessment. In addition, the development and validation
of strategies to effectively improve the identification of the
high-responder patient population with anti-PD-1 strategies are
significant and likely to assume a place in the clinical
practice.
In conclusion, PD-1 may promote the tumor prognosis
of NSCLC, particularly in the early stage SCC patient population,
and provide a candidate for blockade of its function as a strategy
to antagonize the progression process.
Acknowledgements
The authors thank Miss Yuka Toda and Ito Yamamoto
for their excellent technical assistances. The present study was
supported by Grants-in-Aid for Scientific Research, Japan Society
for the Promotion of Science (JSPS) (grant nos. 25293303, 24592097
and 23659674) and the Health and Labour Sciences Research Grant on
Intractable Diseases (Neuroimmunological Diseases) from the
Ministry of Health, Labour and Welfare of Japan.
References
|
1
|
Ginsberg RJ, Kris MK and Armstrong G:
Cancer of the lung. Principles and Practice of Oncology. 4th
edition. Lippincott; Philadelphia: pp. 673–682. 1993
|
|
2
|
Amano J, Kuwano H and Yokomise H: Thoracic
and cardiovascular surgery in Japan during 2011: Annual report by
The Japanese Association for Thoracic Surgery. Gen Thorac
Cardiovasc Surg. 61:578–607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Postmus PE: Chemotherapy for non-small
cell lung cancer: the experience of the Lung Cancer Cooperative
Group of the European Organization for Research and Treatment of
Cancer. Chest. 113(Suppl 1): 28S–31S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zielinski C, Knapp S, Mascaux C and Hirsch
F: Rationale for targeting the immune system through checkpoint
molecule blockade in the treatment of non-small-cell lung cancer.
Ann Oncol. 24:1170–1179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou W: Immunosuppressive networks in the
tumour environment and their therapeutic relevance. Nature Rev
Cancer. 5:263–274. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen L, Linsley PS and Hellström KE:
Costimulation of T cells for tumor immunity. Immunol Today.
14:483–486. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boise LH, Noel PJ and Thompson CB: CD28
and apoptosis. Curr Opin Immunol. 7:620–625. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watts TH and DeBenedette MA: T cell
co-stimulatory molecules other than CD28. Curr Opin Immunol.
11:286–293. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong H, Strome SE, Salomao DR, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: a potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwai Y, Ishida M, Tanaka Y, et al:
Involvement of PD-L1 on tumor cells in the escape from host immune
system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad
Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Topalian SL, Hodi FS, Brahmer JR, et al:
Safety, activity, and immune correlates of anti-PD-1 antibody in
cancer. N Engl J Med. 366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brahmer JR, Tykodi SS, Chow LQ, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishizaki Y, Yukaya N, Kusuhara K, et al:
PD1 as a common candidate susceptibility gene of subacute
sclerosing panencephalitis. Hum Genet. 127:411–419. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Konishi J, Yamazaki K, Azuma M, et al:
B7-H1 expression on non-small cell lung cancer cells and its
relationship with tumor-infiltrating lymphocytes and their PD-1
expression. Clin Cancer Res. 10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasaki H, Suzuki A, Shitara M, et al:
PD-L1 gene expression in Japanese lung cancer patients. Biomed Rep.
1:93–96. 2013.PubMed/NCBI
|
|
18
|
Sjöblom T, Jones S, Wood LD, et al: The
consensus coding sequences of human breast and colorectal cancers.
Science. 314:268–274. 2006.
|
|
19
|
Topalian SL, Weiner GJ and Pardoll DM:
Cancer immunotherapy comes of age. J Clin Oncol. 29:4828–4836.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Drake CG, Jaffee E and Pardoll DM:
Mechanisms of immune evasion by tumors. Adv Immunol. 90:51–81.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizoguchi H, O’Shea JJ, Longo DL, et al:
Alterations in signal transduction molecules in T lymphocytes from
tumor-bearing mice. Science. 258:1795–1798. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hodi FS, O’Day SJ, McDermott DF, et al:
Improved survival with ipilimumab in patients with metastatic
melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar
|
|
24
|
Robert C, Thomas L, Bondarenko I, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fife BT and Bluestone JA: Control of
peripheral T-cell torelance and autoimmunity via the CTLA-4 and
PD-1 pathways. Immunol Rev. 224:166–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zhang J, Deng Y, et al:
Polymorphisms in the cytotoxic T-lymphocyte antigen 4 gene and
cancer risk: a meta-analysis. Cancer. 117:4312–4324. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song B, Liu Y, Liu J, et al: CTLA-4
+49A>G polymorphism is associated with advanced non-small cell
lung cancer prognosis. Respiration. 82:439–444. 2011.
|
|
28
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Freeman GJ, Long AJ, Iwai Y, et al:
Engagement of the PD-1 immunoinhibitory receptor by a novel B7
family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Topalian SL, Drake CG and Pardoll DM:
Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor
immunity. Curr Opin Immunol. 24:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brahmer JR, Drake CG, Wollner I, et al:
Phase I study of single-agent anti-programmed death-1 (MDX-1106) in
refractory solid tumors: safety, clinical activity,
pharmacodynamics, and immunologic correlates. J Clin Oncol.
28:3167–3175. 2010. View Article : Google Scholar
|
|
32
|
Karim R, Jordanova ES, Piersma SJ, et al:
Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell
infiltration and survival of patients with cervical carcinoma. Clin
Cancer Res. 15:6341–6347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sznol M and Chen L: Antagonist antibodies
to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human
cancer. Clin Cancer Res. 19:1021–1034. 2013. View Article : Google Scholar
|
|
34
|
Kong EK, Prokunina-Olsson L, Wong WH, et
al: A new haplotype of PDCD1 is associated with rheumatoid
arthritis in Hong Kong Chinese. Arthritis Rheum. 52:1058–1062.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rekhtman N, Ang DC, Sima CS, et al:
Immunohistochemical algorithm for differentiation of lung
adenocarcinoma and squamous cell carcinoma based on large series of
whole-tissue sections with validation in small specimens. Mod
Pathol. 24:1348–1359. 2011. View Article : Google Scholar
|