Introduction
In the 2008 World Health Organization (WHO)
classification system for malignant lymphoma, extranodal natural
killer (NK)/T-cell lymphomas (ENKTCL) are considered to be a
distinct entity. ENKTCLs are significantly more common in Asia and
Latin America compared to Europe and North America (1,2). We
collected a total of 4,801 cases with lymphoma in South China and
963 cases fulfilled the diagnostic criteria of mature T-cell or
NK/T-cell lymphoma and accounted for 20.1% of all cases of lymphoma
encountered during the same period. Of these cases, 281 (29.2%)
were diagnosed as ENKTCL, nasal type (3). Radiotherapy was the treatment
selection for early-stage nasal ENKTCL. However, distant and local
relapse often occurred and the 5-year overall survival (OS) was low
(29.8–66%) (4–7). An optimal treatment for ENKTCL has
not yet been established, particularly for advanced-stage disease.
The low treatment efficacy may be mainly attributed to the fact
that this disease is resistant to several chemotherapeutic agents,
due to the expression of P-glycoprotein (P-gp) (8,9).
Therefore, investigations have been focused on improving the
efficacy of chemotherapy and reducing the risk of disease
recurrence. It has been reported that L-asparaginase (L-ASP) and
gemcitabine (GEM) may achieve satisfactory response and survival
rates, as they are not regulated by P-gp in relapsed and refractory
ENKTCL (10–14). GEM combined with oxaliplatin
(L-OHP) has also been verified to be an effective salvage regimen
for patients with relapsed or refractory B-cell lymphoma, with
acceptable toxicity (15–17). Nowak-Göttl et al (18) reported that the use of
dexamethasone (DXM) instead of prednisone significantly reduced the
incidence of venous thromboembolism, which is a common adverse
reaction of L-ASP. Therefore, we investigated the efficacy and
toxicity of GEM combined with L-OHP, L-ASP and DXM (GOLD regimen)
in patients with newly-diagnosed ENKTCL.
Patients and methods
Patient eligibility
A total of 55 patients were recruited at the Henan
Province Cancer Hospital and the Cancer Center of Sun Yat-sen
University between May, 2008 and August, 2012. Only patients aged
>15 years were considered to be eligible for this study.
Additional eligibility criteria included ENKTCL histologically
confirmed by biopsy, with measurable lesions radiologically; normal
hepatic and renal function; and adequate bone marrow reserve
without previous treatment. Patients were excluded if they had
severe organ dysfunction or concomitant malignant tumors. The
diagnosis was established according to the WHO classification
criteria. Aggressive NK/T-cell leukemia was excluded. The lesions
were staged according to the Ann Arbor staging system. Additional
examinations included complete blood count, serum biochemistry and
lactate dehydrogenase (LDH) levels (19); in addition, the Eastern Cooperative
Oncology Group performance status (ECOG PS) and presence of
hemophagocytic syndrome (HPS) and/or B symptoms were assessed. All
the patients underwent bone marrow aspiration and biopsy, computed
tomographic scanning of the involved organ(s), chest and abdomen
and nasal endoscopic examination.
Informed consent was obtained from all the subjects
and the study was approved by the Medical Ethics Committee of the
Henan Province Cancer Hospital.
Chemotherapeutic regimen
This treatment scheme was a 14-day cycle and
included four drugs, namely GEM, L-OHP, L-ASP and DXM. The patients
received GEM (1,000 mg/m2) on day 1, L-OHP (100
mg/m2) on day 1, L-ASP (Changzhou Qianhong Bio-pharma
Co., Ltd, Changzhou, China) (10,000 U/m2) on days 1–5
and DXM (20 mg b.i.d.) on days 1–4. An intradermal test was
required prior to the administration of L-ASP. Every patient
received chemotherapy for ≥4 cycles.
All the patients with Ann Arbor stage I/II disease
received involved-field radiation (IFRT) following chemotherapy.
The decision was made at the discretion of the treating physician.
Three-dimensional conformal radiotherapy was performed with a
linear accelerator at a daily fraction of 2.0 Gy. The total dose
was ≥50 Gy.
Response to treatment and toxicity
assessment
Tumor response was classified as complete response
(CR), partial response (PR), stable disease (SD), or progressive
disease (PD), according to the report of an International Workshop
to standardize the response criteria for non-Hodgkin’s lymphomas.
All the adverse reactions were evaluated according to the National
Cancer Institute Common Toxicity Criteria, version 3 (20,21).
Statistical analysis
OS was calculated from the date of treatment
initiation to the date of the last follow-up or death from any
cause. Progression-free survival (PFS) was measured from the date
of diagnosis to relapse, death or last follow-up visit. Survival
was estimated using Kaplan-Meier curves and compared by the
log-rank test. P<0.05 with two-side test was considered to
indicate a statistically significant difference.
Results
Patient characteristics
A total of 55 patients were recruited from two
centers. The median age was 41 years (range, 15–69 years) and the
patients were mainly young adults. The male:female ratio was 3:1.
The majority of the patients had a good ECOG PS and international
prognostic index and early-stage disease by the Ann Arbor staging
system. B symptoms were present in 58% of the patients at diagnosis
and HPS occurred in 18% patients. The majority of the lesions (87%)
were located in the nasal cavity or nasopharynx (Table I).
 | Table IPatient characteristics (n=55). |
Table I
Patient characteristics (n=55).
| Characteristics | Patient no. | Percentage |
|---|
| Gender |
| Male | 41 | 75 |
| Female | 14 | 25 |
| Age (years) |
| 14–20 | 6 | 10 |
| 21–60 | 41 | 75 |
| 60–80 | 8 | 15 |
| ECOG PS |
| 0 | 42 | 76 |
| 1 | 9 | 16 |
| 2 | 4 | 8 |
| B symptoms |
| Yes | 32 | 58 |
| No | 23 | 42 |
| Ann Arbor stage |
| I/II | 45 | 82 |
| III/IV | 10 | 18 |
| IPI |
| 1 | 29 | 53 |
| 2 | 16 | 29 |
| 3 | 8 | 15 |
| 4 | 2 | 3 |
| LDH level |
| Elevated | 12 | 22 |
| Normal | 43 | 78 |
| HPS |
| Yes | 10 | 18 |
| No | 45 | 82 |
| Involved sites |
| Nasal
cavity/nasopharynx | 48 | 87 |
| Other | 7 | 13 |
Response to treatment and survival
outcomes
A total of 48 patients (91%) responded to the GOLD
regimen, with 34 cases (62%) achieving CR and 16 (29%) a PR,
whereas 2 patients achieved SD and 3 developed PD (Table II). Of the patients with CR, 25
received radiotherapy sequentially. Of the patients with PR, 10
were treated with radiotherapy, of whom 6 achieved a CR, 3 a PR and
1 exhibited PD. Two patients with SD received radiotherapy, with
one patient achieving a CR and the other SD. Three patients with PD
refused the second-line treatment.
 | Table IIResponse rate after the GOLD
regimen. |
Table II
Response rate after the GOLD
regimen.
| Type of
response | Patient no. | Percentage |
|---|
| Complete
response | 34 | 62 |
| Partial
response | 16 | 29 |
| Stable disease | 2 | 4 |
| Progressive
disease | 3 | 5 |
For all patients, the 1-, 2- and 3-year PFS was 86,
64 and 57% and the OS was 91, 80 and 74%, respectively (Fig. 1). Eleven patients were relapse.
Eight patients presented with systemic recurrence and 3 patients
with local recurrence. The 1-year PFS in patients with stage I/II
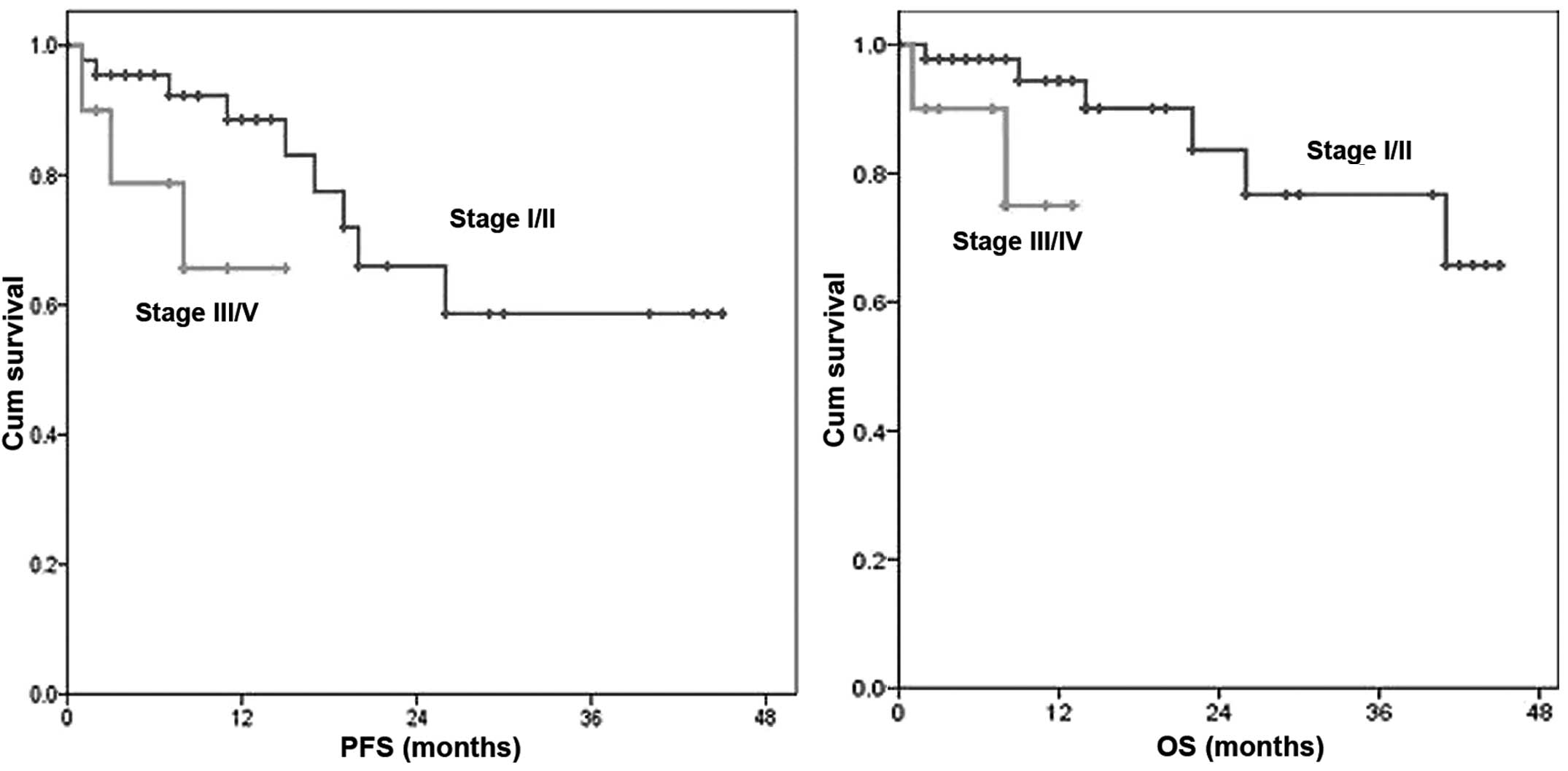
vs. those with III/IV disease was 87 vs. 66% (P<0.001) and the
1-year OS was 98 vs. 75%, respectively (P<0.001) (Fig. 2).
Toxicity
In our study, myelosuppression was observed in 16
patients, with grade 1/2 neutropenia and/or thrombocytopenia in 5
cases, grade 3/4 neutropenia and/or thrombocytopenia in 9 cases and
febrile neutropenia in 2 cases. Hepatic dysfunction was observed in
2 patients. The levels of aminotransferases were >200 U/l and
returned to normal following treatment with liver protectants. Mild
coagulation abnormalities were observed in 19 patients, reflected
by a prolongation of the activated partial thromboplastin time and
D-dimer elevation. One patient with elevated D-dimers and decreased
fibrinogen levels prior to treatment returned to normal levels
after chemotherapy and treatment with low-molecular weight heparin
(LMWH). Another patient developed left lower limb deep venous
thromboembolism and revascularization was achieved following
treatment with urokinase and LMWH. The consumption of fatty foods
was prohibited to lower the risk of pancreatitis and no such events
were reported. All the patients exhibited some peripheral nervous
system toxicity and grade 1/2 gastrointestinal reactions, such as
anorexia and vomiting. There were no hypersensitivity reactions or
chemotherapy-related mortality.
Discussion
It was reported that the median OS and PFS for
patients with ENKTCL was only 7.8 and 5.8 months, respectively.
According to the International Peripheral T-cell Lymphoma Project
(22), ENKTCL exhibited the worst
survival time among all peripheral T-cell lymphoma categories
(19). The authors of that study
concluded that radiotherapy was efficient for localized nasal
disease, whereas extranasal disease appeared to be less amenable.
For patients with advanced-stage nasal disease and those with
extranasal disease, the survival was dismal (<10%). In our
study, we investigated the efficacy and safety of the GOLD regimen
for patients with ENKTCL. The overall response rate (ORR) was 91%
(CR, 62%; and PR, 29%). The 1-, 2- and 3-year PFS and OS were 86,
64 and 57% and 91, 80 and 74%, respectively. The median follow-up
for all the patients was 22 months and the median PFS and OS were
not attained. Wang et al (8) reported that 67% of the patients were
positive for P-gp expression and the CR rate achieved in
P-gp-positive patients was significantly lower compared to that in
P-gp-negative patients (20 vs. 60%, respectively; P=0.045) when
treated with a cyclophosphamide, adriamycin, vincristine and
prednisone (CHOP)-like regimen. As adriamycin and vincristine are
substrates for P-gp, Yong et al (23) reported that ENKTCL patients treated
with CHOP regimen followed by IFRT exhibited a CR rate of only 27%.
It is crucial to attain CR in patients with aggressive lymphoma.
Several studies demonstrated that a CR with induction chemotherapy
followed by radiotherapy may prolong survival in ENKTCL cases
(24–26). Therefore, efforts have been
focusing on designing novel chemotherapeutic regimens, effective in
achieving a high CR rate. Nagafuji et al (12) first reported the case of a
60-year-old Japanese woman with stage IV ENKTCL who was treated
with L-ASP and achieved a CR without disease progression for 18
months. Yamaguchi et al (27) conducted a phase II study of SMILE
chemotherapy for ENKTCL and reported an ORR and CR of 79 and 45%,
respectively, with a 1-year survival rate of only 55%. These
results were inferior to those achieved with our regimen, possibly
due to the fact that all the patients enrolled in the Yamaguchi
et al study had relapsed or refractory disease. Of note, the
SMILE regimen comprised the steroid dexamethasone, the multidrug
resistance-unrelated agents methotrexate, ifosfamide and
L-asparaginase, and etoposide, which was found to be efficient
in vitro and in vivo against Epstein-Barr
virus-associated lymphoproliferative disorders. Further analysis of
that study found that the treatment-related toxicity was severe,
with grade 4 neutropenia and infection in 92 and 61% of the cases,
respectively (27). Considering
the highly aggressive nature of ENKTCL, we designed a scheme of 14
days per cycle. We found that toxicity was alleviated when not
using GEM on the eighth day. The safety of the GOLD regimen was
also satisfactory. Grade 3/4 hematological toxicity was observed in
only 16% of the cases (9/55). Leucopenia and thrombocytopenia were
the major manifestations and liver injury occurred in only 2
patients. All the adverse reactions were manageable with
symptomatic treatment and there were no severe complications. In
the 2013 ASCO Annual Meeting, Lin et al (24) reported the results of a phase
II/III study on ENKTCL treated with CID-ATT, with a 1- and 3-year
OS of 80.2 and 68% and a PFS of 74.9 and 60.5%, respectively. The
survival results of that study were similar to ours. The CID-ATT
regimen includes administration of CHOP-B, ifosfamide +
methotraxate + etoposide + dexamethasone (IMVD), and dexamethasone
+ cytarabine + cisplatin, in an alternating sequence, for a total
of 6 courses (2 cycles). Further analysis in our study revealed
significant differences in OS and PFS between early and advanced
stages. Several previous studies had reported that Ann Arbor stage
was a prognostic factor for OS and/or PFS (24,28,29),
although there remain controversies regarding the Ann Arbor staging
system.
In conclusion, our study demonstrated that the GOLD
regimen was highly effective and safe for patients with
newly-diagnosed ENKTCL. However, the short follow-up and the
retrospective design of the study may have affected the results to
a certain extent. Therefore, a prospective trial should be
conducted to further evaluate the efficacy and safety of the GOLD
regimen for the treatment of ENKTCL patients.
Acknowledgements
This study was supported by grants from the National
Natural Foundation of China (no. 81101797) and the Project of Henan
Health Department (no. 2011020158).
Abbreviations:
|
ENKTCL
|
extranodal NK/T-cell lymphoma
|
|
GEM
|
gemcitabine
|
|
L-OHP
|
oxaliplatin
|
|
L-ASP
|
L-asparaginase
|
|
DXM
|
dexamethasone
|
|
LDH
|
lactate dehydrogenase
|
|
ECOG PS
|
Eastern Cooperative Oncology Group
performance status
|
|
HPS
|
hemophagocytic syndrome
|
|
IFRT
|
involved-field radiation therapy
|
|
LMWH
|
low-molecular weight heparin
|
References
|
1
|
Suzuki R, Takeuchi K, Ohshima K and
Nakamura S: Extranodal NK/T-cell lymphoma: diagnosis and treatment
cues. Hematol Oncol. 26:66–72. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oshimi K: Progress in understanding and
managing natural killer-cell malignancies. Br J Haematol.
139:532–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang Q, Ye ZY, Su ZL, et al:
Clinicopathologic study of 963 cases of mature T-cell and natural
killer/T-cell lymphoma with respect to 2008 WHO classification of
lymphoid neoplasms. Chin J Pathol. 39:291–295. 2010.(In
Chinese).
|
|
4
|
Li YX, Wang H, Jin J, et al: Radiotherapy
alone with curative intent in patients with stage I extranodal
nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys.
82:1809–1815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li YX, Yao B, Jin J, Wang WH, et al:
Radiotherapy as primary treatment for stage IE and IIE nasal
natural killer/T-cell lymphoma. J Clin Oncol. 24:181–189. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Isobe K, Uno T, Tamaru J, et al:
Extranodal natural killer/T-cell lymphoma, nasal type: the
significance of radiotherapeutic parameters. Cancer. 106:609–615.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koom WS, Chung EJ, Yang WI, et al:
Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic
viewpoints. Int J Radiat Oncol Biol Phys. 59:1127–1137. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang B, Li XQ, Ma X, Hong X, Lu H and Guo
Y: Immunohistochemical expression and clinical significance of
P-glycoprotein in previously untreated extranodal NK/T-cell
lymphoma, nasal type. Am J Hematol. 83:795–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drénou B, Lamy T, Amiot L, et al:
CD3− CD56+ non-Hodgkin’s lymphomas with an
aggressive behavior related to multidrug resistance. Blood.
89:2966–2974. 1997.
|
|
10
|
Jaccard A, Petit B, Girault S, et al:
L-asparaginase-based treatment of 15 western patients with
extranodal NK/T-cell lymphoma and leukemia and a review of the
literature. Ann Oncol. 20:110–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Obama K, Tara M and Niina K:
L-asparaginase-based induction therapy for advanced extranodal
NK/T-cell lymphoma. Int J Hematol. 78:248–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagafuji K, Fujisaki T, Arima F and
Ohshima K: L-asparaginase induced durable remission of relapsed
nasal NK/T-cell lymphoma after autologous peripheral blood stem
cell transplantation. Int J Hematol. 74:447–450. 2001. View Article : Google Scholar
|
|
13
|
Yong W, Zheng W, Zhu J, et al:
L-asparaginase in the treatment of refractory and relapsed
extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 88:647–652.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahn HK, Kim SJ, Hwang DW, Ko YH, Tang T,
Lim ST and Kim WS: Gemcitabine alone and/or containing chemotherapy
is efficient in refractory or relapsed NK/T-cell lymphoma. Invest
New Drugs. 31:469–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mounier N, El Gnaoui T, Tilly H, et al:
Rituximab plus gemcitabine and oxaliplatin in patients with
refractory/relapsed diffuse large B-cell lymphoma who are not
candidates for high-dose therapy. A phase II Lymphoma Study
Association trial. Haematologica. 98:1726–1731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
López A, Gutiérrez A, Palacios A, et al:
GEMOX-R regimen is a highly effective salvage regimen in patients
with refractory/relapsing diffuse large-cell lymphoma: a phase II
study. Eur J Haematol. 80:127–132. 2008.PubMed/NCBI
|
|
17
|
El Gnaoui T, Dupuis J, Belhadj K, et al:
Rituximab, gemcitabine and oxaliplatin: an effective salvage
regimen for patients with relapsed or refractory B-cell lymphoma
not candidates for high-dose therapy. Ann Oncol. 18:1363–1368.
2007.PubMed/NCBI
|
|
18
|
Nowak-Göttl U, Ahlke E, Fleischhack G, et
al: Thromboembolic events in children with acute lymphoblastic
leukemia (BFM protocols): prednisone versus dexamethasone
administration. Blood. 101:2529–2533. 2003.
|
|
19
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the Committee on Hodgkin’s
Disease Staging Classification. Cancer Res. 31:1860–1861. 1971.
|
|
20
|
Cheson BD, Horning SJ, Coiffier B, et al:
Report of an international workshop to standardize response
criteria for non-Hodgkin’s lymphomas. NCI Sponsored International
Working Group. J Clin Oncol. 17:12441999.PubMed/NCBI
|
|
21
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Au WY, Weisenburger DD, Intragumtornchai
T, et al; International Peripheral T-Cell Lymphoma Project.
Clinical differences between nasal and extranasal natural
killer/T-cell lymphoma: a study of 136 cases from the International
Peripheral T-Cell Lymphoma Project. Blood. 113:3931–3937. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yong W, Zheng W and Zhang Y: Clinical
characteristics and treatment of midline nasal and nasal type NK/T
cell lymphoma. Zhonghua Yi Xue Za Zhi. 81:773–775. 2001.(In
Chinese).
|
|
24
|
Lin N, Song Y, Zheng W, et al: A
prospective phase II study of L-asparaginase- CHOP plus radiation
in newly diagnosed extranodal NK/T-cell lymphoma, nasal type. J
Hematol Oncol. 6:442013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Au WY, Lie AK, Liang R, et al: Autologous
stem cell transplantation for nasal NK/T-cell lymphoma: a progress
report on its value. Ann Oncol. 14:1673–1676. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HJ, Bang SM, Lee J, et al: High-dose
chemotherapy with autologous stem cell transplantation in
extranodal NK/T-cell lymphoma: a retrospective comparison with
non-transplantation cases. Bone Marrow Transplant. 37:819–824.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaguchi M, Kwong YL, Kim WS, et al:
Phase II study of SMILE chemotherapy for newly diagnosed stage IV,
relapsed, or refractory extranodal natural killer (NK)/T-cell
lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin
Oncol. 29:4410–4416. 2011. View Article : Google Scholar
|
|
28
|
Wu X, Li P, Zhao J, et al: A clinical
study of 115 patients with extranodal natural killer/T-cell
lymphoma, nasal type. Clin Oncol (R Coll Radiol). 20:619–625. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li YX, Fang H, Liu QF, et al: Clinical
features and treatment outcome of nasal-type NK/T-cell lymphoma of
Waldeyer ring. Blood. 112:3057–3064. 2008. View Article : Google Scholar : PubMed/NCBI
|