Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common cancer and the third leading cause of cancer-related
mortality worldwide (1). Over
600,000 new cases of HCC are officially reported annually
worldwide. HCC most commonly arises on a background of chronic
liver disease secondary to viral hepatitis, specifically hepatitis
B virus (HBV) and hepatitis C virus (HCV) infection, as well as
alcoholic and non-alcoholic fatty liver disease (2). Significant geographical variations in
the incidence of HCC have been documented, with the highest
incidence observed in Asia (3,4). HCC
may present with different morphological subtypes, including
‘focal/nodular’, ‘massive’ and ‘diffuse/infiltrating’ (5,6).
This gross classification of HCC is primarily based on radiological
characteristics. Focal/nodular HCC most commonly presents as an
arterially enhancing mass with well-defined margins and a washout
pattern during the portal venous phase (7,8). By
contrast, infiltrating HCC may be difficult to identify, since it
presents as a spreading, ill-defined mass that may blend into the
background cirrhotic liver on cross-sectional imaging (7,8).
Patients with infiltrating HCC are not good candidates for curative
treatment, such as liver resection, liver transplantation or local
ablation (9). Sorafenib is the
first targeted therapeutic agent approved for systemic treatment of
advanced HCC, on the basis of two randomized, double-blind,
placebo-controlled, phase III trials that demonstrated prolonged
overall survival (10,11). Sorafenib is recommended for the
treatment of advanced and unresectable HCC (12). However, other modalities, such as
transarterial chemoembolization (TACE) or transarterial
radioembolization using yttrium-90 microspheres are also used to
treat infiltrating HCC due to the modest efficacy and high cost of
sorafenib treatment (13–15). TACE is currently considered to be
one of the standard treatments for patients with unresectable HCC.
According to previous randomized controlled studies, TACE exhibited
clear survival benefits and improved the quality of life for
patients with unresectable HCC when compared to symptomatic
supportive care (16,17). Infiltrating HCC cases have seldom
been studied as candidates for TACE due to poor demarcation and
difficulty in defining the extent of infiltrating HCC on
cross-sectional imaging. Recently, a prospective comparative study
documented TACE to have worse efficacy for infiltrative compared to
focal nodular HCC (18). However,
some authors believe that TACE may be beneficial for carefully
selected patients with infiltrative HCC (13–15).
To the best of our knowledge, the number of comparative studies
that have been published to compare TACE with conservative
treatment for such patients is limited. We conducted this study to
determine whether TACE confers a survival benefit to patients with
infiltrative HCC and to uncover the prognostic factors of overall
survival.
Patients and methods
TACE group
Between January, 2007 and January, 2012, 131
consecutive patients with infiltrating HCC underwent TACE as
initial treatment at the Cancer Center, Sun Yat-sen University.
During the same period, 3,914 patients with HCC were treated at the
hospital. The patient and tumor characteristics and the presence of
underlying liver diseases are summarized in Table I.
 | Table IPatient and tumor characteristics. |
Table I
Patient and tumor characteristics.
| Variables | TACE group
(n=131) | Conservative group
(n=156) | P-value |
|---|
| Age, years [median
(range)] | 55 (20–75) | 55 (23–75) | 0.654 |
| Gender
(male/female) | 125/6 | 149/7 | 0.999 |
| HBV (yes/no) | 126/5 | 150/6 | 0.999 |
| HCV (yes/no) | 129/2 | 154/2 | 0.503 |
| AFP, ng/ml [median
(range)] | 1,060
(0–138,400) | 1,120
(0–138,400) | 0.078 |
| GGT, U/l (mean ±
SD) | 198.0±124.0 | 243±170 | 0.094 |
| AST, U/l (mean ±
SD) | 39.2±13.0 | 45.1±17.4 | 0.287 |
| ALT, U/l (mean ±
SD) | 65.3±13.5 | 67.7±15.8 | 0.513 |
| ALB, g/l (mean ±
SD) | 39.9±7.3 | 37.9±4.9 | 0.060 |
| TBIL, μmol/l (mean ±
SD) | 16.8±6.9 | 17.7±5.5 | 0.159 |
| PT, sec (mean ±
SD) | 12.4±0.7 | 12.8±1.0 | 0.364 |
| PLT, 10E9/l (mean ±
SD) | 1,120±100 | 101±77 | 0.500 |
| Cirrhosis
(yes/no) | 74/57 | 90/66 | 0.999 |
| Child-Pugh
classification (A/B) | 109/22 | 123/43 | 0.474 |
| ECOG score
(0–1/2) | 109/21 | 120/36 | 0.238 |
| BCLC staging
(B/C) | 12/119 | 13/153 | 0.851 |
| CLIP score
(2/3/4/5) | 6/36/70/19 | 10/45/72/29 | 0.759 |
Inclusion criteria
i) Patient age, 18–75 years; ii) Child-Pugh class A
or B liver function (19); iii)
Eastern Cooperative Oncology Group (ECOG) performance score ≤2; and
iv) HCC with no previous treatment.
Exclusion criteria
i) Severe coagulopathy (prothrombin activity <40%
or a platelet count <40,000/mm3); ii) Child-Pugh
class C liver function or evidence of hepatic decompensation,
including ascites, esophageal or gastric variceal bleeding, or
hepatic encephalopathy; iv) ECOG scores 3–4; and v) concomitant
serious diseases of other organs.
Diagnosis
Contrast-enhanced computed tomography (CT) and
magnetic resonance imaging (MRI) scans were used to diagnose
infiltrating HCC, as ultrasound was inadequate (20). The diagnosis of infiltrating HCC
was established by agreement between two radiologists coming from
the two centers participating in this study who performed
independent reviewing of the cross-sectional imagings of all the
patients.
TACE
TACE was performed as previously described (21). In brief, a selective 5 Fr catheter
was introduced and visceral angiography was performed to assess the
arterial blood supply to the liver and to confirm patency of the
portal vein. All the patients underwent a distal super-selective
catheterization of the hepatic arteries using a coaxial technique
and 2.9 Fr microcatheters (Terumo Corporation, Tokyo, Japan).
Subsequently, three chemotherapeutic agents at the same dosage were
used throughout this study, regardless of tumor number and size.
Hepatic artery infusion chemotherapy was first performed using
carboplatin 300 mg (Bristol-Myers Squibb, New York, NY, USA),
followed by chemolipiodolization using epirubicin 50 mg
(Pharmorubicin; Pfizer, Wuxi, China) and mitomycin C 8 mg (Zhejiang
Hisun Pharmaceutical Co., Ltd., Taizhou, China) mixed with 5 ml
lipiodol (Lipiodol Ultra-Fluide; Andre Guerbet Laboratories,
Aulnay-sous-Bois, France). If the territory of the
chemolipiodolized artery did not show stagnant flow, pure lipiodol
was then injected. For all cases, embolization was finally
performed with absorbable 1–2-mm gelatin sponge particles (Gelfoam;
Hangzhou alc Ltd., Hangzhou, China) or 350–560-μm polyvinyl alcohol
particles (Alicon Pharm SCT & TEC Co., Ltd., Hangzhou, China)
until stasis was achieved in the tumor-feeding arteries.
Conservative treatment group
During the same study period (January, 2007–January,
2012), 156 consecutive patients with infiltrating HCC who had
declined sorafenib treatment received conservative treatment (best
supportive care) at another cancer center. During the same period,
3,845 patients with HCC were treated in The First Affiliated
Hospital of Sun Yat-sen University. The inclusion, exclusion and
diagnostic criteria were identical to those in the TACE group. The
patient and tumor characteristics and the presence of underlying
liver diseases are summarized in Table
I.
Assessment of response
The response of the tumors to TACE was evaluated
using contrast-enhanced CT or MRI at 1 month after treatment. The
presence of non-enhanced tumoral areas reflected tissue necrosis.
The modified Response Evaluation Criteria in Solid Tumors on CT or
MRI were used to measure tumor response (22).
Follow-up
Patients in the TACE and conservative treatment
groups were followed up monthly for the first year and once every
three months thereafter in the outpatient setting using clinical
examination, biochemistry and serum α-fetoprotein (AFP)
measurements. Contrast-enhanced CT or MRI scans were performed once
every 1–2 months for the first year and every 2–3 months
thereafter. Bone metastases were excluded by bone scintigraphy on
clinical suspicion. In addition, data on the patients’ Child-Pugh
class and ECOG scores were recorded.
In the TACE group, hepatocellular injury was
monitored by serum bilirubin, alanine transaminase, serum albumin
(ALB) and prothrombin time. TACE-related complications were
evaluated at the end of the first month after treatment.
Complications were reported using the National Cancer Institute
Common Toxicity Criteria grading, version 4.0 (23). Another session of TACE was
performed once every 2–3 months until one of the following end
points was reached: i) complete devascularization of the tumor; ii)
technical impossibility to embolize the residual tumor, e.g., tumor
only supplied by extrahepatic collateral arteries; iii)
contraindications to TACE; and iv) total resection or ablation of
tumor by subsequent surgery or local ablation. Hepatic resection or
local ablation were performed as previously described (24,25).
In cases with ii) or iii), it was recommended that the patients
received sorafenib. If they refused, conservative treatment was
administered.
Statistical analysis
Statistical analyses were performed using the SPSS
10.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Comparisons between the two groups were performed using the
Student’s t-test for continuous data and the Chi-square test for
categorical data. Overall survival was calculated using a life
table method and compared with the Mantel-Cox test. The survival
curves were constructed with the Kaplan-Meier method and compared
using the log-rank test. The relative prognostic significance of
the variables in predicting overall survival rates was assessed
using the multivariate Cox proportional hazards regression
analysis. The results are presented as means ± standard deviation,
or median and range. All the statistical tests were two-sided and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 287 patients were recruited in this study
(TACE group, n=131; and conservative treatment group, n=156). The
characteristics of the patients are summarized in Table I. The two groups were comparable
regarding patient characteristics, preoperative liver function and
general condition (Table I).
Following treatment, 47 patients in the TACE and 62 patients in the
conservative treatment group received nucleoside-analog treatment
for HBV (P=0.735).
Radiographic characteristics
In all infiltrating HCCs, the margins of the tumors
were poorly demarcated. The median infiltrating HCC diameter was
9.0 and 9.8 cm for the TACE and conservative treatment groups,
respectively. The majority of the patients in the two groups had
radiographic evidence of macrovascular invasion at the time of the
diagnosis of infiltrating HCC (TACE vs. conservative treatment
group, 89/131 vs. 126/156, respectively; P=0.364). All patients
with macrovascular invasion had some degree of portal vein tumor
thrombosis (PVTT). In the TACE group, 22.9% of the patients had
main portal vein involvement, whereas 45% had involvement of the
right and/or the left hemihepatic portal and/or sectional/segmental
portal vein. In the conservative treatment group, 23.1% of the
patients had main portal vein involvement, whereas 57.7% had
involvement of the right and/or left hemihepatic portal and/or
sectional/segmental portal vein. In addition to portal vein tumor
thrombi, 12 and 16 of the patients in the TACE and conservative
treatment groups exhibited tumor invasion of the hepatic vein(s),
respectively (P=0.844). On further analysis, 6 and 9 of the
patients had the tumor thrombi extending into the main hepatic
vein(s), 6 and 7 into the inferior vena cava and 3 and 4 extended
into the right atrium in the TACE and the conservative treatment
groups, respectively. A total of 89 and 97 infiltrating HCC lesions
displayed early arterial hyper-enhancement in the TACE and the
conservative treatment groups (67.9 vs. 62.2%, respectively;
P=0.706). All these lesions demonstrated washout during the portal
venous phase. At the time of diagnosis of infiltrating HCC, 51.1
and 50.64% of the patients exhibited intrahepatic satellite lesions
and 29.8 and 30.1% had extrahepatic metastases, respectively
(P=0.999 and P=0.999, respectively). The most common metastatic
sites in the TACE and conservative treatment groups were the lungs
(18.3 vs. 19.2%, respectively) and lymph nodes (10.8 vs. 10.3%,
respectively). Intrahepatic biliary ductal dilatation was found in
9.9 and 9.6% in the TACE and conservative treatment groups,
respectively (P=0.999). There were 30 and 45 patients in the TACE
and the conservative treatment groups who received a liver MRI
(P=0.528). Among these patients, 22 (73.0%) in the TACE and 31
(69.0%) tumors in the conservative treatment group exhibited
relative homogeneity and mild hyperintensity on T2-weighted images.
The remaining tumors exhibited isointensity to the surrounding
liver parenchyma (Table II).
 | Table IIRadiographic and pathological
characteristics of patients with infiltrating HCC at the time of
diagnosis. |
Table II
Radiographic and pathological
characteristics of patients with infiltrating HCC at the time of
diagnosis.
| Variables | TACE group
(n=131) | Conservative group
(n=156) | P-value |
|---|
| Maximum tumor size,
cm (mean ± SD) | 9.0±2.5 | 9.8±1.0 | 0.070 |
| Vascular
invasion | 89 | 126 | 0.364 |
| Portal vein | | | 0.488 |
| Main | 30 | 36 | |
| Hemihepatic | 37 | 61 | |
|
Sectional/segmental | 22 | 29 | |
| Main/hemihepatic
portal vein obstruction (yes/no) | 19/112 | 23/134 | 0.999 |
| Hepatic vein
invasion | 12 | 16 | 0.844 |
| Hepatic vein
only | 6 | 9 | |
| Inferior vena
cava | 6 | 7 | |
| Right atrium | 3 | 4 | |
| Arterial
hyper-enhancement (yes/no) | 89/42 | 97/59 | 0.706 |
| Intrahepatic
metastases (yes/no) | 67/64 | 79/77 | 0.999 |
| Distant
metastases | 39 | 47 | 0.999 |
| Lung | 24 | 30 | |
| Lymph nodes | 13 | 15 | |
| Bone | 1 | 1 | |
| Adrenal | 1 | 1 | |
| Biliary duct
dilation | 13 | 14 | 0.999 |
| Hemihepatic | 9 | 7 | |
| Segmental | 2 | 3 | |
| Whole liver | 2 | 4 | |
| MRI T2 signal
appearance | 30 | 45 | 0.528 |
| Hyperintense | 22 | 31 | |
| Isointense | 8 | 14 | |
Outcomes of TACE
In the TACE group, 131 patients received a mean of
1.5 sessions (range, 1–4 sessions) of TACE. Of those patients, 52
(39.7%) received one session and 79 (60.3%) received more than one
sessions of TACE. The initial TACE consisted of the injection of
anticancer drugs, lipiodol and gelatin sponge particles in 10 of 19
(52.6%) patients with main/hemihepatic portal vein invasion and
portal vein obstruction, 43 of 70 (61.4%) patients with
main/hemihepatic portal vein invasion, but without portal vein
obstruction, and 27 of 59 (45.8%) patients with sectional/segmental
PVTT. The remaining 42 patients received anticancer drugs and
lipiodol injection only.
The tumor response and complications in the two
groups are shown in Tables III
and IV, respectively. All the
TACE-related complications were successfully managed with
conservative treatment. The 1-month mortality rate was 0.8 and 3.8%
in the TACE and conservative groups, respectively (P=0.134).
 | Table IIITumor response in the transarterial
chemoembolization (TACE) and conservative treatment groups. |
Table III
Tumor response in the transarterial
chemoembolization (TACE) and conservative treatment groups.
| Type of
response | TACE group
(n=131) | Conservative group
(n=156) | P-value |
|---|
| Complete
response | 0 | 0 | - |
| Partial
response | 21 | 0 | <0.001 |
| Stable disease | 52 | 33 | 0.014 |
| Progressive
disease | 58 | 123 | 0.004 |
 | Table IVComplications in the transarterial
chemoembolization (TACE) and conservative groups. |
Table IV
Complications in the transarterial
chemoembolization (TACE) and conservative groups.
| Complications | TACE group
(n=131) | Conservative group
(n=156) | P-value |
|---|
| TACE-related |
| Postembolization
syndrome | 97 | 0 | <0.001 |
| Cholecystitis | 1 | 0 | 0.452 |
|
Anemia/thrombocytopenia | 1 | 0 | 0.452 |
| Temporary liver
decompensation | 42 | 0 | <0.001 |
| Disease-related (at
1 month) |
| Spontaneous
rupture | 0 | 1 | 0.999 |
| Variceal
bleeding | 0 | 1 | 0.999 |
| Progressive liver
failure | 0 | 1 | 0.999 |
| Procedure-related
mortality | 0 | 0 | 0.999 |
| 1-month
mortality | 1 | 6 | 0.134 |
Following TACE, the tumors in 6 patients were
downstaged and suitable for partial hepatectomy (n=6) or local
ablative therapy (radiofrequency ablation, n=1; or radiofrequency +
percutaneous ethanol injection, n=1). Thirteen patients with tumor
progression following TACE received sorafenib treatment.
Survival outcomes
At a median follow-up of 6.0 months (range, 1–59
months), 285 patients (94.9%) had succumbed to the disease. The
overall median survival was 5.0±0.35 months [95% confidence
interval (CI): 4.32–5.68 months]. The 6-, 12- and 24-month overall
survival rates for all the patients were 41.9, 12.9 and 1.1%,
respectively. The median survival for the TACE and the conservative
treatment groups was 7.0±0.3 and 3.0±0.1 months, respectively
(P<0.001). The 6-, 12- and 24-month overall survival rates for
the TACE and the conservative treatment groups were 61.7, 18.5 and
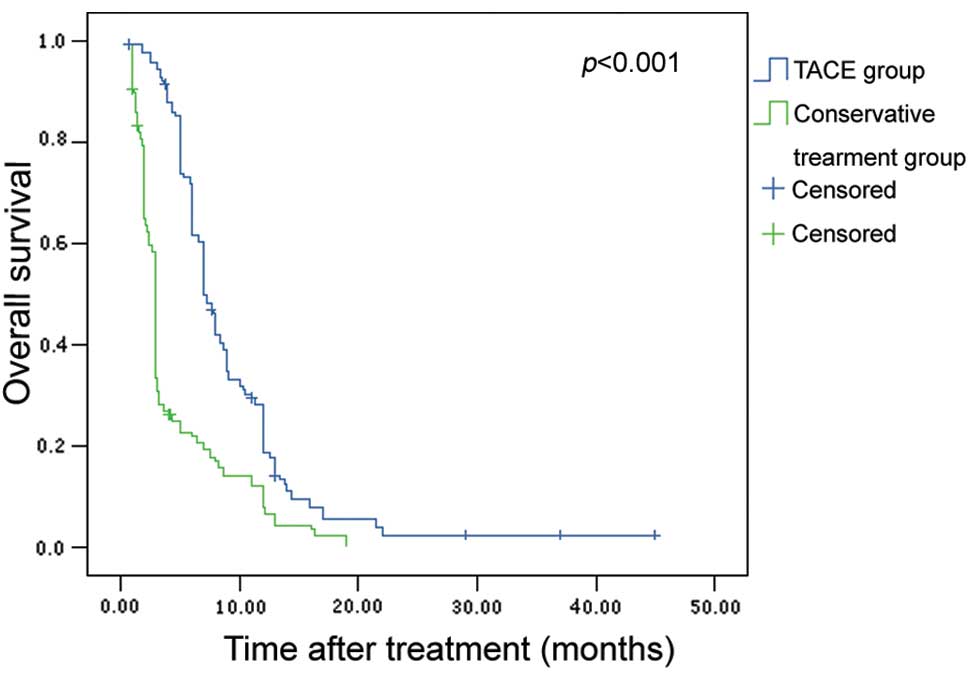
2.3% vs. 22.7, 12.1 and 0%, respectively. The TACE group exhibited
significantly better overall survival compared to the conservative
group (P<0.001, Fig. 1).
In the TACE group, the median survival for the 8
patients who were downstaged to receive potentially curative
treatments and the remaining 123 patients was 13.0±3.07 and
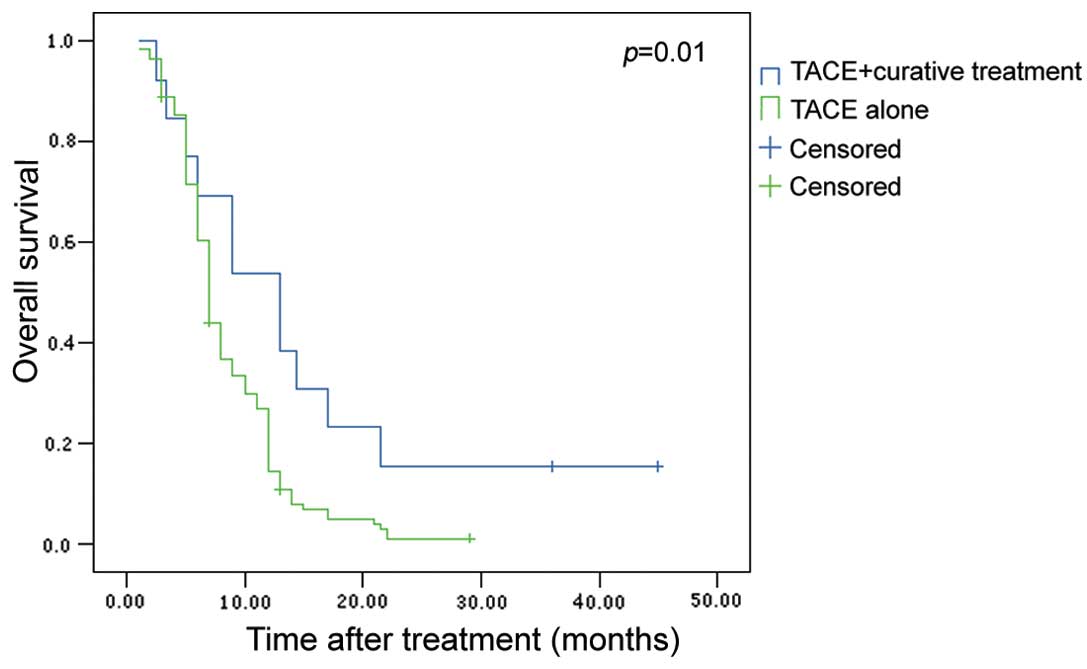
7.0±0.27 months, respectively. The 6-, 12- and 24-month overall
survival rates for TACE + curative treatment and TACE alone were
69.2, 53.8 and 15.4 vs. 60.4, 14.4 and 1%, respectively. The
difference was significant (P=0.01, Fig. 2). The median survival for the 13
patients who received sorafenib and the remaining 118 patients was
7.1±0.86 and 6.9±0.42 months, respectively (P=0.563).
Survival factor analysis
On univariate analysis, 6 factors were correlated
with survival, namely age, serum γ-glutamyl transpeptidase, serum
ALB, PVTT type, maximum tumor size and treatment allocation
(Table V). On multivariate
analysis, only treatment allocation [odds ratio (OR)=1.777; 95% CI:
1.499–2.107; P<0.001] and PVTT type (OR=1.721; 95% CI:
1.504–1.907; P<0.001) were independent predictors of overall
survival.
 | Table VUnivariate and multivariate analysis
of prognostic factors. |
Table V
Univariate and multivariate analysis
of prognostic factors.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | P-value | OR | 95% CI | P-value |
|---|
| Age, years (60 vs.
>60) | 0.01 | | | |
| Gender (male vs.
female) | | | | |
| HBV (yes vs.
no) | | | | |
| HCV (yes vs.
no) | | | | |
| AFP, ng/ml (≤400
vs. >400) | | | | |
| GGT, U/l (≤50 vs.
>50) | 0.009 | | | |
| AST, U/l (≤40 vs.
>40) | | | | |
| ALT, U/l (≤40 vs.
>40) | | | | |
| ALB, g/l (≤35 vs.
>35) | 0.018 | | | |
| TBIL, μmol/l (≤20
vs. >20) | | | | |
| PT, sec (≤13.5 vs.
>13.5) | | | | |
| PLT, 109/l (≤100
vs. >100) | | | | |
| PVTT type
(segmental vs. main/hemiliver) | <0.001 | 1.721 | 1.504–1.907 | <0.001 |
| Maximum tumor size,
cm (≤10.0 vs. >10.1) | <0.001 | | | |
| Cirrhosis (yes vs.
no) | | | | |
| ECOG (0–1 vs.
2) | | | | |
| Child-Pugh
classification (A vs. B) | | | | |
| Treatment
allocation (TACE vs. conservative treatment) | <0.001 | 1.777 | 1.499–2.107 | <0.001 |
Discussion
Infiltrating HCC has not been adequately
investigated, as it is difficult to diagnose and measure on
cross-sectional images. However, infiltrating HCC is not rare
(5,6). As liver resection and transplantation
are not treatment options for the majority of patients with
infiltrating HCC, TACE and other locoregional treatments have been
advocated as potential therapeutic options (15,18).
Lopez et al (18) reported
on a small series (n=19) of patients with infiltrating HCC who
underwent TACE. In that study, the authors compared patients with
focal vs. those with infiltrating HCC who underwent conventional
TACE. Of note, the authors reported more procedure-related
mortalities among patients with infiltrating HCC (16% of the
patients succumbed within 30 days of TACE) and recommended caution
in utilizing intra-arterial therapy (IAT) for patients with
infiltrating HCC due to the high periprocedural mortality rate. By
contrast, in this study, TACE was found to be relatively safe and
well-tolerated. By using a large cohort of patients with
infiltrating HCC, this study was the first comparative study to
demonstrate a significantly improved overall survival for patients
treated with TACE when compared to patients treated conservatively
(P<0.001).
Of the 131 patients, 8 (6.1%) underwent potentially
curative treatment after tumor downstaging and their survival was
significantly superior to that of the remaining 123 patients in the
TACE group (P=0.01). This result indicated that salvage procedures
after tumor downstaging are beneficial for those patients who
present initially with unresectable HCC (26–28).
The main problem with tumor downstaging in infiltrating HCC is that
only a small proportion of patients respond well enough to
treatment to allow salvage liver resection or percutaneous ablative
procedures and the responders cannot be predicted. In our study, 13
patients with tumor progression after TACE received sorafenib
treatment. Patients who received combined TACE and sorafenib did
not exhibit a survival superior to that of the remaining 118
patients who received TACE alone (P=0.542). However, it is
difficult to determine the true role of sorafenib in this study,
since it was used as a salvage treatment for patients with
infiltrating HCC when there was tumor progression after TACE. In
addition, only a small number of patients received sorafenib after
TACE in this study.
The combination of carboplatin, doxorubicin and
mitomycin C is the most commonly used drug combination regimen used
in TACE (29). In this study,
there was no significant difference in the 1-month mortality rate
between the TACE (0.8%) and the conservative groups (3.8%,
P=0.134). TACE-related complications were adequately managed using
non-operative treatment, thus suggesting that TACE is a safe
treatment option for patients with infiltrating HCC.
Recently, a study by Kneuertz et al (14) on patients treated with IAT,
reported that their median overall survival was longer compared to
that of patients who received best supportive care (12 vs. 3
months, respectively; P=0.001), with a periprocedural mortality of
2.7% after TACE. In addition, the survival of patients after IAT
was similar for patients with infiltrating or multifocal HCC
(P=0.27). The authors concluded that IAT for infiltrating HCC was
safe and was associated with a survival comparable to that of
patients with multifocal HCC. Thus, infiltrating HCC is no longer
considered a contraindication to IAT in selected patients. The
survival benefit after TACE in the Kneuertz et al (14) study was better compared to that in
our study. However, in that study, the IAT group had significantly
lower AFP levels (244 vs. 1,563 ng/ml) and 25 of the 48 patients
(52.1%) received periprocedural sorafenib in addition to IAT. As
low AFP levels and sorafenib are associated with improved survival,
these factors were likely to contribute to the 9-month survival
benefit as observed among patients with infiltrating HCC who
received IAT in the Kneuertz et al study (14). In another study conducted by Mehta
et al (15), the outcomes,
effects of treatment and prognostic factors were assessed in a
large cohort of patients with infiltrating HCC (n=155). In that
study, 11.8% (18/152) patients received TACE and these patients
exhibited a significantly better survival (P=0.0002) compared to
those who did not receive tumor-directed therapy (n=109). The
authors concluded that patients may derive survival benefit from
TACE, although further investigations are required (15).
Our study had several limitations. The main
limitation was the retrospective, non-randomized study design.
Several confounding factors may have affected our findings.
Furthermore, only a small number of patients received sorafenib in
this study and patients may achieve better results with sorafenib
therapy. It is also possible that our results may not apply to
patients with infiltrating HCC in other countries, due to
differences in demographics and underlying causes of liver disease.
Despite these limitations, however, our data represent the largest
patient cohort in the literature that allows better
characterization of the clinical and radiological characteristics,
outcomes and prognostic factors associated with unresectable
infiltrating HCC treated with TACE or conservative treatment.
In conclusion, the present study demonstrated that
TACE is a safe treatment option for patients with unresectable
infiltrating HCC and patients achieved better survival with TACE
rather than with conservative treatment. However, further
prospective studies are required to confirm the efficacy and safety
of TACE for patients with infiltrating HCC.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81301842), the
Outstanding Young Scientist Award of First Affiliated Hospital of
Sun Yat-sen University (2013–2017), the Outstanding Young Scientist
Award of Guangzhou (2014), the State Key Project on Infectious
Diseases of China (no. 2012ZX10002-016), the 5010 Foundation of Sun
Yat-sen University (no. 2007043) and the Science and Technology
Planning Project of Guangdong Province (no. 2012B031800032).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
4
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trevisani F, Caraceni P, Bernardi M, et
al: Gross pathologic types of hepatocellular carcinoma in Italian
patients. Relationship with demographic, environmental and clinical
factors. Cancer. 72:1557–1563. 1993. View Article : Google Scholar
|
|
6
|
Okuda K, Peters RL and Simson IW: Gross
anatomic features of hepatocellular carcinoma from three disparate
geographic areas. Proposal of new classification. Cancer.
54:2165–2173. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lencioni R, Crocetti L, Della Pina MC and
Cioni D: Guidelines for imaging focal lesions in liver cirrhosis.
Expert Rev Gastroenterol Hepatol. 2:697–703. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanematsu M, Semelka RC, Leonardou P,
Mastropasqua M and Lee JK: Hepatocellular carcinoma of diffuse
type: MR imaging findings and clinical manifestations. J Magn Reson
Imaging. 18:189–195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Demirjian A, Peng P, Geschwind JF, et al:
Infiltrating hepatocellular carcinoma: seeing the tree through the
forest. J Gastrointest Surg. 15:2089–2097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Llovet JM, Ricci S, Mazzaferro V, et al;
SHARP Investigators Study Group. Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar
|
|
12
|
Forner A, Reig ME, de Lope CR and Bruix J:
Current strategy for staging and treatment: the BCLC update and
future prospects. Semin Liver Dis. 30:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jang ES, Yoon JH, Chung JW, et al:
Survival of infiltrative hepatocellular carcinoma patients with
preserved hepatic function after treatment with transarterial
chemoembolization. J Cancer Res Clin Oncol. 139:635–643. 2013.
View Article : Google Scholar
|
|
14
|
Kneuertz PJ, Demirjian A, Firoozmand A, et
al: Diffuse infiltrative hepatocellular carcinoma: assessment of
presentation, treatment, and outcomes. Ann Surg Oncol.
19:2897–2907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mehta N, Fidelman N, Sarkar M and Yao FY:
Factors associated with outcomes and response to therapy in
patients with infiltrative hepatocellular carcinoma. Clin
Gastroenterol Hepatol. 11:572–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lo CM, Ngan H, Tso WK, et al: Randomized
controlled trial of transarterial lipiodol chemoembolization for
unresectable hepatocellular carcinoma. Hepatology. 35:1164–1171.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Llovet JM, Real MI, Montana X, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: a
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar
|
|
18
|
Lopez RR Jr, Pan SH, Hoffman AL, et al:
Comparison of transarterial chemoembolization in patients with
unresectable, diffuse vs. focal hepatocellular carcinoma. Arch
Surg. 137:653–658. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Myung SJ, Yoon JH, Kim KM, et al: Diffuse
infiltrative hepatocellular carcinomas in a hepatitis B-endemic
area: diagnostic and therapeutic impediments.
Hepatogastroenterology. 53:266–270. 2006.PubMed/NCBI
|
|
21
|
Shi M, Chen JA, Lin XJ, et al:
Transarterial chemoembolization as initial treatment for
unresectable hepatocellular carcinoma in southern China. World J
Gastroenterol. 16:264–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
National Cancer Institute. Common
terminology criteria for adverse events (CTCAE), version 4.0.
http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Accessed June 14, 2010
|
|
24
|
Shi M, Guo RP, Lin XJ, et al: Partial
hepatectomy with wide versus narrow resection margin for solitary
hepatocellular carcinoma: a prospective randomized trial. Ann Surg.
245:36–43. 2007. View Article : Google Scholar
|
|
25
|
Chen MS, Li JQ, Zheng Y, et al: A
prospective randomized trial comparing percutaneous local ablative
therapy and partial hepatectomy for small hepatocellular carcinoma.
Ann Surg. 243:321–328. 2006. View Article : Google Scholar
|
|
26
|
Lau WY, Leung TW, Lai BS, et al:
Preoperative systemic chemoimmunotherapy and sequential resection
for unresectable hepatocellular carcinoma. Ann Surg. 233:236–241.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lau WY, Ho SK, Yu SC, Lai EC, Liew CT and
Leung TW: Salvage surgery following downstaging of unresectable
hepatocellular carcinoma. Ann Surg. 240:299–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lau WY and Lai EC: Salvage surgery
following downstaging of unresectable hepatocellular carcinoma - a
strategy to increase resectability. Ann Surg Oncol. 14:3301–3309.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lau WY, Yu SC, Lai EC and Leung TW:
Transarterial chemoembolization for hepatocellular carcinoma. J Am
Coll Surg. 202:155–168. 2006. View Article : Google Scholar : PubMed/NCBI
|