Introduction
Prostate cancer is the most common type of cancer
and the leading cause of cancer-related morbidity among men in the
United States of America. According to the assessment of the
American Society of Clinical Oncology, there were 238,590 new cases
of prostate cancer and 29,720 deaths in 2013 (1). In China, the morbidity associated
with prostate cancer is significantly lower, although it has
increased over the last few years. Due to the limitations in health
awareness and financial resources, the majority of patients in
China have middle- and advanced-stage disease at diagnosis.
Although endocrine therapy may control and improve the patients’
condition, the overwhelming majority are likely to develop
hormone-refractory prostate cancer (HRPC) after 18–30 months of
remission. Chemotherapy has been used for the treatment of HRPC for
~30 years, although the chemotherapeutic regimens were reportedly
associated with severe side effects and poor curative effect. In
1996, the American Food and Drug Administration (FDA) authorized
the therapeutic regimen of mitoxantrone combined with prednisone.
The clinical test results revealed that, although compared to
single-agent prednisone this combination treatment achieved a
remission of the clinical symptoms of the patients, it failed to
improve the overall survival (OS) rate (2). The results of the TAX327 phase III
trial revealed that, compared to mitoxantrone, the treatment
regimen combining docetaxel and prednisone improved the quality of
life of the patients and prolonged their mean survival of 2.5
months (3). In 2004, the American
FDA authorized the use of docetaxel-based therapy, which is
currently the first-line standard treatment and a focus of ongoing
investigation. The results of a meta-analysis of docetaxel-based
therapy with or without estramustine, revealed that there were no
statistically significant differences in the incidence of grade 3/4
adverse events or in OS rates; however, there was a statistically
significant difference in the prostate-specific antigen (PSA)
response rate [odds ratio=1.55, 95% confidence interval (CI):
1.10–2.18, P=0.012] (4),
suggesting that docetaxel-based therapy with estramustine may
increase the PSA response rate. There are several RCTs on the
combination of docetaxel with antiangiogenic agents available in
the international literature; however, the results of those studies
have been inconsistent. Therefore, a systematic assessment method
was adopted, with the aim to evaluate the effectiveness and safety
of docetaxel-based therapy with and without antiangiogenic agents
for the treatment of castration-resistant prostate cancer, in order
to provide the basis for clinical decision making.
Materials and methods
Study design and inclusion criteria
The study design was RCT and the record type was not
limited by the blinding method. The study included patients with a
definitive pathological diagnosis of prostate adenocarcinoma,
exhibiting disease relapse following endocrine therapy, without
underlying diseases, such as severe cardiac and/or pulmonary
conditions. The patient selection was not limited by race or
ethnicity.
Treatment
The study compared the combination of
docetaxel-based therapy with antiangiogenic agents with
docetaxel-based therapy alone. The outcome indicators were overall
survival, progression-free survival (PFS), PSA response rate, grade
3/4 toxicity and treatment-related mortality.
Exclusion criteria
The studies were excluded if i) they applied
inappropriate methods; ii) provided incomplete information that
could not be otherwise obtained; iii) in case of duplicate
publications, the one with the more precise methodology report was
selected; and iv) studies with undefined outcome indicators.
Search strategy
Computerized electronic databases, including Embase,
PubMed and The Cochrane Library, were searched. The search time
limit was from the building of the database until July 18, 2013.
The search used a method that combines subject terms [MEDLINE
(Mesh), Embase (EMTREE)] and free terms. The keywords used included
prostate cancer, prostatic carcinoma, carcinoma of the prostate,
chemotherapy, docetaxel and randomized controlled trial, which were
adjusted according to the specific database. The reference lists of
the identified studies were also reviewed. If a study report was
unclear or information was lacking, the author was contacted via
e-mail.
Literature screening and data
extraction
Data were extracted from the studies that conformed
to the inclusion criteria by two independent researchers, filled
into a data extraction table and cross-checked. Any differences
were resolved by consulting a third researcher and the
corresponding author of the study was contacted in case of missing
data.
Quality assessment
An open assessment of the trials was performed using
the method reported by Jadad et al (5), which assessed the trials on whether
they reported i) an appropriate randomization method (score, 0–2);
ii) an appropriate blinding method (score, 0–2); and iii)
withdrawals and dropouts (score, 0–1).
Statistical analysis
RevMan 5.2 and Stata 12.0 software were used to
perform the statistical analyses. The measurement data used
weighted or standard mean difference and 95%CI, while the
enumeration data used risk ratio (RR) and 95%CI as a statistical
magnitude of curative effect analysis. Heterogeneity was assessed
with the Chi-square test. When there was no heterogeneity
(I2<50%, P>0.10), a fixed-effects model was used
for the analysis. In the case of heterogeneity, the possible
sources were investigated. When clinical heterogeneity was
detected, a random-effects model was used for analysis and subgroup
or sensitivity analysis was performed based on the sources. If
heterogeneity was significant, a descriptive analysis was
undertaken. The indicators that could not be merged were used in
the descriptive method.
Results
Characteristics of included studies
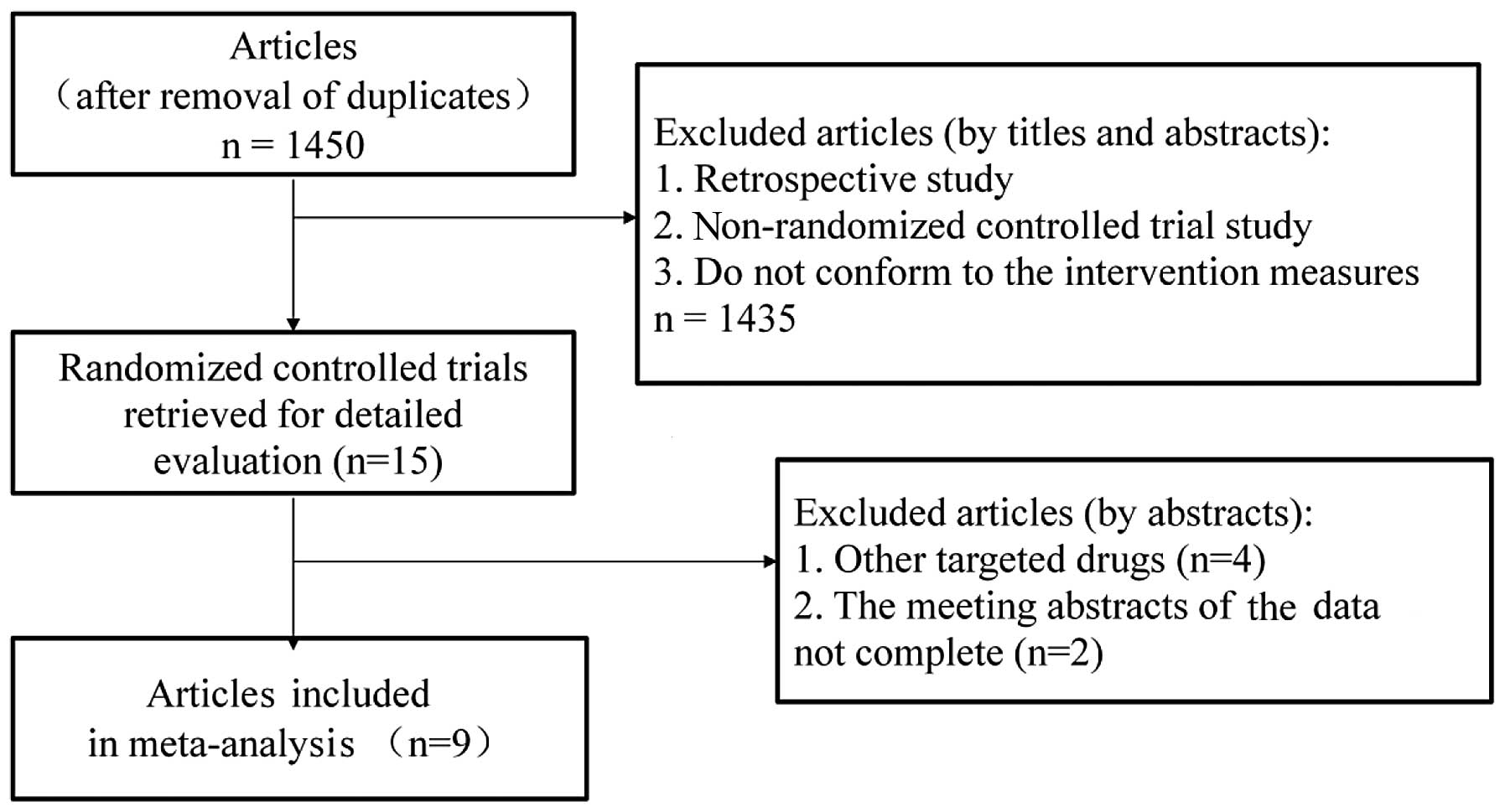
A total of 1,761 relevant articles were identified
through an initial literature search. Duplicate articles were
eliminated using the literature management software, EndNote X6,
leaving a total of 1,450 studies. Subsequently, the titles and
abstracts were read to exclude retrospective studies, non-RCTs and
studies that did not conform to the intervention measures, leading
to the exclusion of a further 1,435 studies that did not conform to
the inclusion criteria. Of the remaining 15 relevant articles
(6–20), 2 meeting abstracts (14,16)
and 4 studies that investigated other targeted drugs (17–20)
were also excluded. The flowchart of the study selection process is
shown in Fig. 1. The baseline
parallel comparison of all the docetaxel-based therapy with and
without antiangiogenic agents groups included in the studies is
presented in Table I.
 | Table IBaseline characteristics of the
studies included in the meta-analysis. |
Table I
Baseline characteristics of the
studies included in the meta-analysis.
| First author
(year) | Inclusion period | Journal | Jadad score | Study design | Treatment
groupsa | Patients no./age,
yrs | ECOG PS 0–1, % | Chemotherapeutic
regimen |
|---|
| Kelly et al
(2007) | 2005–2007 | J Clin Oncol | 4 | Randomized,
double-blind, phase III | A | 524/69 | 96 | Docetaxel 75
mg/m2 i.v. q3w + bevacizumab 15 mg/kg i.v. q3w |
| | | | | B | 526/69 | 95 | Docetaxel 75
mg/m2 i.v. q3w + placebo |
| Tannock et al
(2013) | 2007–2010 | Lancet Oncol | 5 | Randomized,
double-blind, phase III | A | 612/68 | 96 | Docetaxel 75
mg/m2 i.v. q3w + aflibercept 6mg/kg i.v. q3w |
| | | | | B | 612/8 | 96 | Docetaxel 75
mg/m2 i.v. q3w + placebo |
| Mathew et al
(2007) | 2003–2005 | Clin Cancer Res | 3 | Randomized,
double-blind, phase II | A | 57/- | 95 | Docetaxel 30
mg/m2 i.v. qw for 3w + imatinib 600 mg p.o. qd |
| | | | | B | 59/- | 93 | Docetaxel 30
mg/m2 i.v. qw for 3 weeks + placebo |
| Horti et al
(2009) | 2006 | Cancer Biother
Radiopharm | 3 | Randomized,
double-blind, phase II | A | 43/67 | - | Docetaxel 75
mg/m2 i.v. q3w + vandetanib 100 mg p.o. qd |
| | | | | B | 43/67 | - | Docetaxel 75
mg/m2 i.v. q3w + placebo |
| Fizazi et al
(2013) | 2008–2011 | J Clin Oncol | 5 | Randomized,
double-blind, phase III | A | 524/68 | 100 | Docetaxel 75
mg/m2 i.v. q3w + zibotentan 10 mg p.o. qd for 3
weeks |
| | | | | B | 528/68 | 100 | Docetaxel 75
mg/m2 i.v. q3w + placebo |
| Quinn et al
(2013) | 2006–2010 | Lancet Oncol | 3 | Randomized,
double-blind, phase III | A | 498/69 | 93 | Docetaxel 75
mg/m2 i.v. q3w + atrasentan 10 mg p.o. qd for 3
weeks |
| | | | | B | 496/69 | 92 | Docetaxel 75
mg/m2 i.v. q3w + placebo |
| Dreicer et
al (2013) | 2007–2009 | Invest New
Drugs | 3 | Randomized,
double-blind, phase II | A | 48/69 | 100 | Docetaxel 75
mg/m2 i.v. q3w + enzastaurin 500 mg p.o. qd for 3
weeks |
| | | | | B | 46/71 | 100 | Docetaxel 75
mg/m2 i.v. q3w + placebo |
| Pili et al
(2010) | - | Clin Cancer
Res | 2 | Randomized
open-label phase II | A | 33/68 | 100 | Docetaxel 75
mg/m2 i.v. q3w + vadimezan 1200 mg/m2 i.v.
q3w |
| | | | | B | 38/67 | 92 | Docetaxel 75
mg/m2 i.v. q3w |
| Heidenreich et
al (2012) | 2007–2009 | Ann Oncol | 3 | Randomized,
double-blind, phase II | A | 66/68 | 97 | Docetaxel 75
mg/m2 i.v. q3w + intetumumab 10 mg/kg i.v. q3w |
| | | | | B | 65/68 | 97 | Docetaxel 75
mg/m2 i.v. q3w + placebo |
Quality assessment of the included
studies
The baseline condition of the patients was reported
in the 9 RCTs that were included, among which 8 RCTs were
randomized and double-blind and 1 RCT was randomized and
open-label. Of the 9 RCTs, 4 were phase III and 5 were phase II
clinical trials.
Meta analysis results
PSA response rate
In total, 8 studies (6–11,13,14)
(including 4,593 patients) compared the PSA response between the
docetaxel-based therapy with and without antiangiogenic agents
groups. Statistical heterogeneity was observed among the research
results (I2=79%, P<0.0001). Subsequent to using the
random-effects model to perform the meta-analysis, the result
demonstrated that the differences between the two groups were not
statistically significant with respect to the PSA response rate
[RR=0.99, 95%CI: 0.87–1.12)] (Fig.
2).
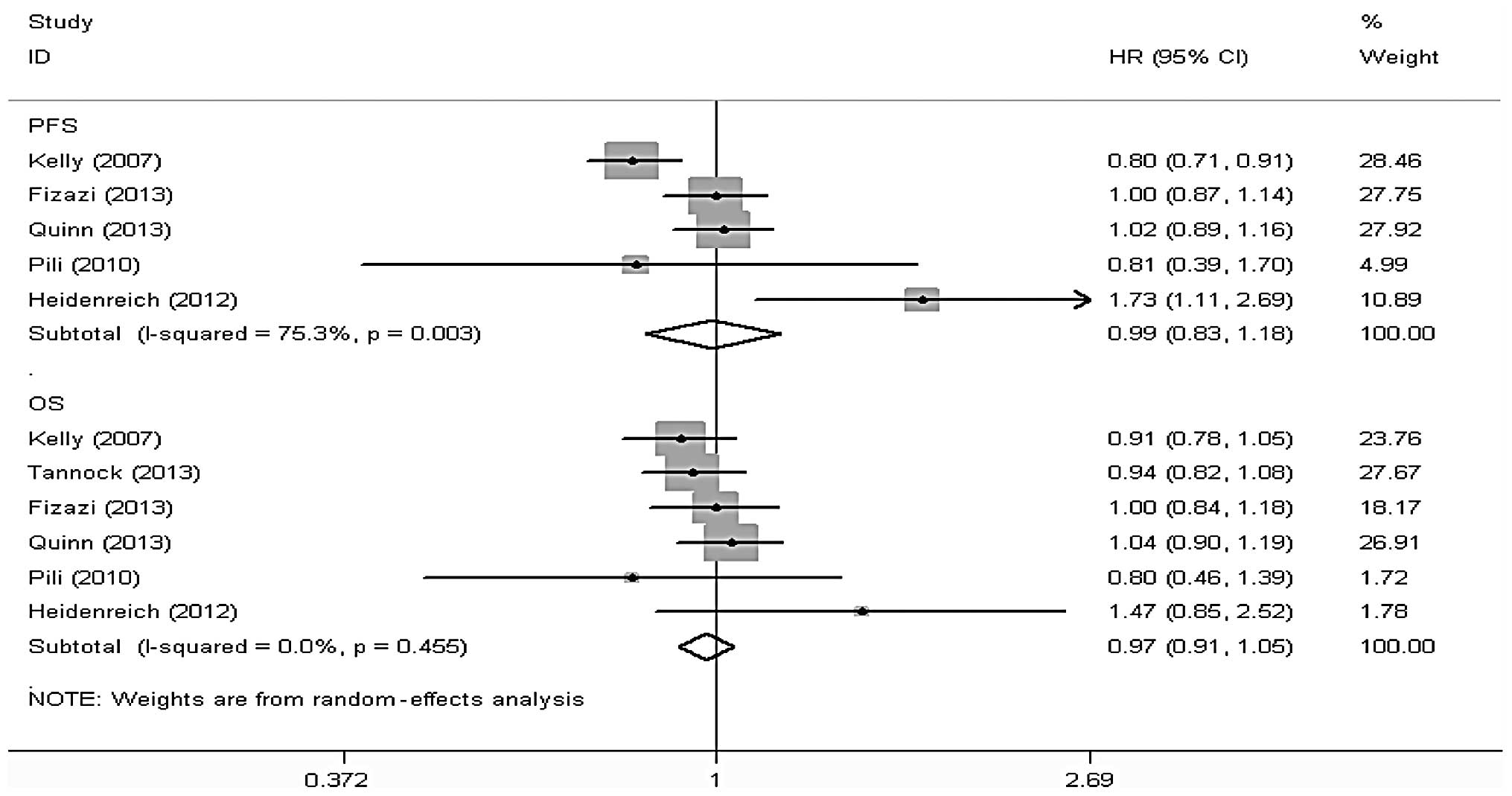
OS
A total of 7 studies (6–11,13,14),
including 4,636 patients, reported data on the OS of the
docetaxel-based therapy with and without antiangiogenic agents
groups, among which 6 studies (6,7,10,11,13,14),
including a total of 4,521 patients, reported the results of the
comparison between the two groups with respect to OS. Stata 12
software was used to perform a meta-analysis according to effect
size and there was no statistical heterogeneity among the results
of the included studies (I2=0.0%, P=0.455). Thus, the
random-effects model was used for the meta-analysis and the results
revealed that the differences in OS between the two groups were not
statistically significant [hazard ratio (HR)=0.97, 95%CI: 091–1.05]
(Fig. 3).
PFS
In total, 8 studies (5–8,10–13),
including 4,722 patients, reported data on the PFS of the
docetaxel-based therapy with and without antiangiogenic agents
groups, among which 5 studies (5,9,10,12,13),
including a total of 3,297 patients, reported the results of the
comparison between the two groups with respect to PFS. Stata 12
software was used to conduct a meta-analysis according to the
effect size and there was statistical heterogeneity among the
results of the included studies (I2=75.3%, P=0.003).
Thus, the random-effects model was used for the meta-analysis and
the results revealed that the differences in PFS between the two
groups were not statistically significant (HR=0.99, 95%CI:
0.83–1.18) (Fig. 3).
Adverse reactions
All the studies reported data on adverse reactions.
As regards grade 3/4 neutropenia, anemia, diarrhea and fatigue, the
results demonstrated that the differences between the two groups
were not statistically significant (Table II), whereas with respect to
thrombus incidence, there was no statistical heterogeneity among
the results of the included studies (I2=0%, P=0.75).
Thus, the fixed-effects model was used for the meta-analysis and
the results revealed that the differences between the two groups
were statistically significant regarding thrombus incidence
(RR=0.57, 95%CI: 0.41–0.80, P=0.001), with a higher incidence in
the docetaxel-based therapy with antiangiogenic agents group
compared to the docetaxel-based therapy alone group (Table II).
 | Table IIOutcome of grade 3/4 toxicity
meta-analysis comparing docetaxel-based therapy with
anti-angiogenesis agents vs. docetaxel-based therapy. |
Table II
Outcome of grade 3/4 toxicity
meta-analysis comparing docetaxel-based therapy with
anti-angiogenesis agents vs. docetaxel-based therapy.
| Grade 3/4
toxicity | Trials | Combination
groupa | DC alone
groupb | Heterogeneity | RR (95% CI) | P-value |
|---|
|
|---|
| P-value | I2,
% |
|---|
| Neutropenia | 7 | 384/1,853 | 319/1831 | 0.009 | 65 | 1.16
(0.91–1.47) | 0.230 |
| Anemia | 5 | 66/1,181 | 53/1192 | 0.600 | 0 | 1.26
(0.89–1.79) | 0.200 |
| Diarrhea | 5 | 59/1,316 | 42/1288 | 0.280 | 21 | 1.36
(0.93–2.00) | 0.120 |
| Fatigue | 5 | 218/1,764 | 131/1734 | 0.030 | 63 | 1.49
(0.99–2.25) | 0.060 |
| Thrombosis | 4 | 50/1,232 | 86/1203 | 0.760 | 0 | 0.57
(0.41–0.80) | 0.001 |
Prostate cancer-related mortality
In total, 7 studies (6–8,10–13),
including 4,531 patients, reported data on prostate cancer-related
mortality in the docetaxel-based therapy with and without
antiangiogenic agents groups. There was no statistical
heterogeneity among the results of the included studies
(I2=14%, P=0.32). Thus, a fixed-effects model was used
for the meta-analysis and the results demonstrated that the
differences in prostate cancer-related mortality between the two
groups were statistically significant (RR=1.95, 95%CI: 1.23–3.11,
P=0.005), with a higher incidence in the docetaxel-based therapy
alone group compared to that in the docetaxel-based therapy with
antiangiogenic agents group (Fig.
4).
Discussion
The results of the present meta-analysis revealed
that, in prostate cancer, docetaxel-based therapy with
antiangiogenic agents was associated with an increased mortality
risk compared to docetaxel-based therapy alone. However, with
respect to thrombosis (RR=0.57, 95%CI: 0.41–0.80, P=0.001),
docetaxel-based therapy with antiangiogenic agents decreased the
risk of thrombosis compared to docetaxel-based therapy alone. There
were no significant differences between the two groups regarding OS
(HR=0.97, 95%CI: 091–1.05), PFS (HR=0.99, 95%CI: 0.83,1.18) and PSA
response rate (RR=0.99, 95%CI: 0.87,1.12). Furthermore, there were
no significant differences between the two groups regarding common
grade 3/4 adverse reactions, including neutropenia (RR=1.16, 95%CI:
0.91–1.47, P=0.23, anemia (RR=1.26, 95%CI: 0.89–1.79, P=0.20),
diarrhea (RR=1.36, 95%CI: 0.93–2.00, P=0.12) and fatigue (RR=1.49,
95%CI: 0.99–2.25, P=0.06).
Patients with malignant tumors commonly exhibit a
hyperfunctional coagulation pathway and are at high risk of
thrombotic events. However, the results of the present
meta-analysis with respect to thrombosis (RR=0.57, 95%CI:
0.41–0.80, P=0.001), revealed that docetaxel-based therapy with
antiangiogenic agents was associated with a decreased the risk of
thrombosis when treating prostate cancer. Not all studies reporting
thrombosis as an adverse reaction analyzed thrombosis-related data;
however, multi-targeted drugs are known to be associated with
adverse reactions such as thrombosis and this conclusion is
supported by numerous basic and high-quality clinical studies.
In the present meta-analysis, there were no distinct
statistical differences between the docetaxel-based therapy with
and without antiangiogenic agents groups with respect to PSA
response rate, OS and PFS. However, as regards treatment cost, the
addition of the targeted drug to the regimen clearly increases the
medical expenses. For example, by combining docetaxel with
bevacizumab (Avastin; Roche Group), the cost per cycle increases
5-fold compared to that of docetaxel-based therapy alone.
In the meta analysis, the dosage of docetaxel in the
docetaxel-based therapy group was in line with that in the
docetaxel-based therapy with antiangiogenic agents group. There was
a statistically significant difference between the two groups
regarding treatment-related mortality; however, whether this is
associated with the drug combination or the drug dosage remains to
be determined by further clinical trials including a larger patient
sample. In one of the RCTs (6),
docetaxel was administered at a dosage of 30 mg/m2
intravenously (i.v.), once per week for 3 weeks, while in other
randomized trials docetaxel was administered at a dosage of 75
mg/m2 i.v. every 3 weeks. The results revealed that,
with the dose of 30 mg/m2, the mean OS was 4.2 months in
both treatment groups, which was significantly lower compared to
the results of other randomized trials. The results of the TAX327
phase III trial (21) revealed
that docetaxel at 75 mg/m2 i.v. every 3 weeks was
superior to 30 mg/m2 docetaxel i.v. weekly for 5 to 6
weeks. However, in another randomized trial (22), 50 mg/m2 docetaxel
administered i.v. on days 1 and 15 of a 4-week cycle was superior
to 75 mg/m2 docetaxel administered i.v. on day 1 of a
3-week cycle, indicating that the administration schedule and
dosage of docetaxel may be correlated with the results.
In the present meta-analysis, all the included
studies were obtained through strict screening. According to the
Jadad scale, 1 study scored 2 points and 8 studies scored ≥3
points. Of the 9 RCTs, 8 were randomized double-blind and 1 RCT was
randomized open-label. Four of the 9 studies were phase III and 5
were phase II clinical trials. Furthermore, a number of trials are
global multicenter randomized clinical trials covering Europe,
Africa and Asia. Of note, 9 antiangiogenic agents in total were
included in the present meta-analysis.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
Statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Tannock IF, Osoba D, Stockler MR, et al:
Chemotherapy with mitoxantrone plus prednisone or prednisone alone
for symptomatic hormone-resistant prostate cancer: a Canadian
randomized trial with palliative end points. J Clin Oncol.
14:1756–1764. 1996.
|
|
3
|
Tannock IF, Wit R, Berry WR, et al:
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi WX, Shen Z and Yao Y: Docetaxel-based
therapy with or without estramustine as first-line chemotherapy for
castration-resistant prostate cancer: a meta-analysis of four
randomized controlled trials. J Cancer Res Clin Oncol.
137:1785–1790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jadad AR, Moore RA, Carroll D, et al:
Assessing the quality of reports of randomized clinical trials: Is
blinding necessary? Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
6
|
Kelly WK, Halabi S, Carducci M, et al:
Randomized, double-blind, placebo-controlled phase III trial
comparing docetaxel and prednisone with or without bevacizumab in
men with metastatic castration-resistant prostate cancer: CALGB
90401. J Clin Oncol. 30:1534–1540. 2012. View Article : Google Scholar
|
|
7
|
Tannock IF, Fizazi K, Ivanov S, et al:
Aflibercept versus placebo in combination with docetaxel and
prednisone for treatment of men with metastatic
castration-resistant prostate cancer (VENICE): a phase 3,
double-blind randomised trial. Lancet Oncol. 14:760–768. 2013.
View Article : Google Scholar
|
|
8
|
Mathew P, Thall PF, Bucana CD, et al:
Platelet-derived growth factor receptor inhibition and chemotherapy
for castration-resistant prostate cancer with bone metastases. Clin
Cancer Res. 13:5816–5824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horti J, Widmark A, Stenzl A, et al: A
randomized, double-blind, placebo-controlled phase II study of
vandetanib plus docetaxel/prednisolone in patients with
hormone-refractory prostate cancer. Cancer Biother Radiopharm.
24:175–180. 2009. View Article : Google Scholar
|
|
10
|
Fizazi KS, Higano CS, Nelson JB, et al:
Phase III, randomized, placebo-controlled study of docetaxel in
combination with zibotentan in patients with metastatic
castration-resistant prostate cancer. J Clin Oncol. 31:1740–1747.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quinn DI, Tangen CM, Hussain M, et al:
Docetaxel and atrasentan versus docetaxel and placebo for men with
advanced castration-resistant prostate cancer (SWOG S0421): a
randomised phase 3 trial. Lancet Oncol. 14:893–900. 2013.
View Article : Google Scholar
|
|
12
|
Dreicer R, Garcia J, Rini B, et al: A
randomized, double-blind, placebo-controlled, phase II study with
and without enzastaurin in combination with docetaxel-based
chemotherapy in patients with castration-resistant metastatic
prostate cancer. Invest New Drugs. 31:1044–1050. 2013. View Article : Google Scholar
|
|
13
|
Pili R, Rosenthal MA, Mainwaring PN, et
al: Phase II study on the addition of ASA404 (vadimezan;
5,6-dimethylxanthenone-4-acetic acid) to docetaxel in CRMPC. Clin
Cancer Res. 16:2906–2914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heidenreich A, Rawal SK, Szkarlat K, et
al: A randomized, double-blind, multicenter, phase 2 study of a
human monoclonal antibody to human αv integrins (intetumumab) in
combination with docetaxel and prednisone for the first-line
treatment of patients with metastatic castration-resistant prostate
cancer. Ann Oncol. 24:329–336. 2013.
|
|
15
|
Heath EI, Mannuel HD, Liu G, et al:
Randomized phase II trial of docetaxel (Doc) and prednisone (Pred)
with or without AZD2171 (cediranib), in chemotherapy-naive,
metastatic castrate-resistant prostate cancer (mCRPC) (NCI 7451). J
Clin Oncol. 31:(Suppl 6; abstr 38). 2013.
|
|
16
|
Wiechno PJ, Chlosta P, Pikiel J, et al:
Randomized phase II study with window-design to evaluate anti-tumor
activity of the survivin antisense oligonucleotide (ASO) ly2181308
in combination with docetaxel for first-line treatment of
castrate-resistant prostate cancer (CRPC). J Clin Oncol. 31:(Suppl;
abstr 5019). 2013.
|
|
17
|
Sternberg CN, Dumez H, Poppel HV, et al:
Docetaxel plus oblimersen sodium (Bcl-2 antisense oligonucleotide):
an EORTC multicenter, randomized phase II study in patients with
castration-resistant prostate cancer. Ann Oncol. 20:1264–1269.
2009. View Article : Google Scholar
|
|
18
|
Chi KN, Hotte SJ, Yu EY, et al: Randomized
phase II study of docetaxel and prednisone with or without OGX-011
in patients with metastatic castration-resistant prostate cancer. J
Clin Oncol. 28:4247–4254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dahut WL, Gulley JL, Arlen PM, et al:
Randomized phase II trial of docetaxel plus thalidomide in
androgen-independent prostate cancer. J Clin Oncol. 22:2532–2539.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sonpavde G, Matveev V, Burke JM, et al:
Randomized phase II trial of docetaxel plus prednisone in
combination with placebo or AT-101, an oral small molecule Bcl-2
family antagonist, as first-line therapy for metastatic
castration-resistant prostate cancer. Ann Oncol. 23:1803–1808.
2012. View Article : Google Scholar
|
|
21
|
Berthold DR, Pond GR, Soban F, Wit R,
Eisenberger M and Tannock IF: Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer: updated
survival in the TAX 327 study. J Clin Oncol. 26:242–245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kellokumpu-Lehtinen PL, Harmenberg U,
Joensuu T, et al: 2-weekly versus 3-weekly docetaxel to treat
castration-resistant advanced prostate cancer: a randomised, phase
3 trial. Lancet Oncol. 14:117–124. 2013. View Article : Google Scholar
|