Introduction
Non-Hodgkin lymphoma (NHL) accounts for 8–10% of all
childhood malignancies (1). NHL is
a heterogeneous group of diseases, as malignant clonal
proliferation may occur at any stage during lymphocyte
proliferation. Four NHL subtypes comprise 90% of the NHL cases in
children, namely Burkitt's lymphoma (BL), diffuse large B-cell
lymphoma (DLBCL), lymphoblastic lymphoma (precursor T- and
precursor B-cell lymphoma) and anaplastic large-cell lymphoma
(ALCL) (2–5). The remaining 10% include marginal
zone, cutaneous, follicular and peripheral T-cell lymphomas, which
are the NHL subtypes common in the adult population (6–8).
There has been a significant improvement in the overall survival
(OS) of children and adolescents with NHL. The reported
disease-free survival for pediatric NHL, independent of subtype, is
currently ~80% (3). The most
significant prognostic determinants are type of treatment and the
extent of the disease, as determined by pretreatment staging
(9,10). There is geographic variation in the
incidence of NHL, particularly regarding BL, which is endemic in
Equatorial Africa and accounts for 50% of all childhood cancers,
affecting 50 children per million diagnoses (11). However, in other areas of the
world, BL occurs sporadically and is less common, affecting ~2 per
million children (1). Depending on
the geographic region investigated, there are major differences in
terms of the clinical and epidemiological characteristics of NHLs
diagnosed during childhood (1,12).
The scarcity of data available in the literature on the behavior of
NHL among children in our region prompted us to conduct this study,
with the aim of describing the clinical and epidemiological
characteristics of NHL patients, assessing their survival and
identifying possible prognostic associations with the variables
under analysis.
Patients and methods
Patients and inclusion criteria
We retrospectively reviewed the records of children
and adolescents with NHL treated consecutively between February,
2004 and February, 2012 at the Pediatric Oncology Unit of Zagazig
University Hospital and the Benha Specialized Pediatric Hospital.
The study was approved by the Research and Ethics Committees of the
contributing hospitals. Patient records from the Pediatric Oncology
Department containing oncological details on diagnosis, treatment
course and follow-up were analyzed for this study. The inclusion
criteria were as follows: i) children and adolescents aged ≤18
years with pathologically confirmed NHL; ii) the diagnosis of NHL
was established in all the cases by pathology in conjunction with
immunophenotype studies and subclassified according to World Health
Organization (WHO) criteria (13);
and iii) all the anticancer treatments and follow-up examinations
were completed at the abovementioned two centers. Disease staging
was performed according to the St. Jude staging system (14).
Diagnosis and staging
Diagnostic investigations included biopsy of a
clinically involved lymph node or mass, computed tomography (CT)
scan of the chest, abdominopelvic ultrasonography and CT scan, bone
marrow aspirates and biopsies and bone scintigraphy in selected
cases. Diagnosis, staging and treatment planning for all the
patients were performed by the center's multidisciplinary Pediatric
Oncology Group. Laboratory tests included a complete blood count,
liver and renal function tests, serum electrolyte, uric acid and
lactate dehydrogenase levels and cerebrospinal fluid analysis. The
clinical and laboratory findings evaluated for prognostic
significance on event-free survival (EFS) and OS were age, gender,
stage (I/II vs. III/IV) and pathology.
Treatment protocols
The therapeutic regimen was prescribed depending on
histological type and immunophenotype of the lymphoma. BL and large
B-cell lymphomas were treated with a chemotherapy protocol based on
LMB-96. A therapeutic protocol based on modified CCG-1991 and
CCG-1961 high-risk protocols was used for lymphoblastic lymphoma. A
therapeutic protocol based on BFM-NHL 90 was used for ALCL. An
ifosamide, caboblatin, etoposide (ICE) protocol was used for
relapsed cases.
Statistical analysis
Data were statistically described as means ±
standard deviation (SD) and range, or frequencies (number of cases)
and percentages where appropriate. A survival analysis was
performed for the different outcome measures using Kaplan-Meier
statistics, calculating the mean and median survival time for each
group with their 95% confidence intervals and corresponding
survival graphs. P<0.05 was considered to indicate a
statistically significant difference. All the statistical
calculations were performed using the Statistical Package for the
Social Sciences software, version 15 (SPSS, Inc., Chicago, IL, USA)
for Microsoft Windows.
Results
Patients
Between February, 2004 and February, 2012, 142
patients with NHL were admitted to our Pediatric Oncology Units.
The age at presentation ranged between 2 and 15 years, with a mean
± SD of 6.1±2.8 years. The majority of the cases (59.2%) of BL were
diagnosed in children aged 2–5 years, followed by children aged
5–10 years (32.6%); only 8.2% of the BL cases occurred in children
aged >10 years. The majority of the patients with lymphoblastic
lymphoma (78.6%) were aged 5–10 years and only 14.2% were aged
<5 years. However, all the cases of large-cell lymphoma were
diagnosed at an age of >5 years, with 72.2% aged 5–10 and 27.7%
>10 years. The NHL patient population included 90 male and 52
female patients (male:female ratio, 1.7:1). A total of 89 patients
resided in a rural and 53 in an urban area. A total of 33 patients
(23.2%) were born to consanguineous parents (Table I).
 | Table IClinical and demographic
characteristics of patients. |
Table I
Clinical and demographic
characteristics of patients.
| Characteristics | Patient no. (%)
(n=142) |
|---|
| Age (years) | | |
|
<5 | 58 | (40.8) |
| 5–10 | 65 | (45.8) |
|
>10 | 19 | (13.4) |
| Gender | | |
|
Female | 52 | (36.6) |
| Male | 90 | (63.4) |
| Residence | | |
|
Rural | 89 | (62.7) |
|
Urban | 53 | (37.3) |
| Consanguinity | | |
|
Positive | 33 | (23.2) |
|
Negative | 109 | (76.8) |
|
Histological type | | |
| Burkitt's
lymphoma | 98 | (69.0) |
|
Lymphoblastic lymphoma | 26 | (18.3) |
|
Large-cell lymphoma | 15 | (10.6) |
|
Anaplastic large-cell
lymphoma | 3 | (2.1) |
| Primary site | | |
|
Abdomen | 104 | (73.2) |
|
Mediastinum | 23 | (16.2) |
|
Peripheral lymph nodes | 9 | (6.4) |
|
Other | 6 | (4.2) |
| Stage | | |
| I | 2 | (1.4) |
| II | 14 | (9.9) |
| III | 95 | (66.9) |
| IV | 31 | (21.8) |
| LDH (IU/dl) | | |
|
≤500 | 41 | (28.9) |
|
>500 | 101 | (71.1) |
| Protocol | | |
|
LMB-96 | 113 | (79.6) |
|
CCG-1961 | 26 | (18.3) |
| BFM-NHL
90 | 3 | (2.1) |
| Outcome | | |
| Alive
in CR | 120 | (84.5) |
| Alive
following relapse | 6 | (4.2) |
|
Deceased | 16 | (11.3) |
Types of NHL
Based on the WHO 2008 classification for
hematolymphoid neoplasms (13),
NHL was classified into two main groups, namely precursor and
mature B- and T-cell hematolymphoid neoplasms.
Mature-cell NHL corresponded to 116 (81.7%) of all
NHL cases, including 113 (79.6%) B- and 3 (2.1%) T-cell lymphomas.
BL included 98 cases (84.5% of all mature lymphomas and 86.7% of
all mature B-cell lymphomas). BL is the most common NHL subtype,
affecting 98 (69%) of all NHL patients, followed by lymphoblastic
lymphoma with 26 (18.3%), DLBCL with 15 (10.6%) and ALCL with 3
(2.1%) patients (Table I).
Disease localization and staging
Primary abdominal tumors were the most common,
affecting 104 (73.2%) of our patients and bone marrow infiltration
was the most common site of metastasis, affecting 31 (21.8%)
patients, followed by the central nervous system in 5 (3.5%) and
bone infiltration in 2 (1.4%) patients.
Only 16 (11.3%) patients had localized disease
(stage I/II) and 126 (88.7%) presented with advanced disease (stage
III/IV). The majority of advanced-stage patients (95/126; 66.9%),
had stage III lymphoma. LMB-96 was the most used protocol, as BL
and large-cell lymphoma predominated in our patients, accounting
for 113 (79.6%) of the cases (Table
I).
Survival outcomes
The mean follow-up period ± SD was 34.64±25.14
months (range, 3–84 months). A total of 126 patients achieved
remission and 16 (11.3%) patients succumbed to the disease. Death
was attributed to tumor lysis syndrome in 2 and infection in 14
patients. Of the patients who achieved remission, 6 (4.2%)
developed a relapse, were treated and survived. The 5-year OS and
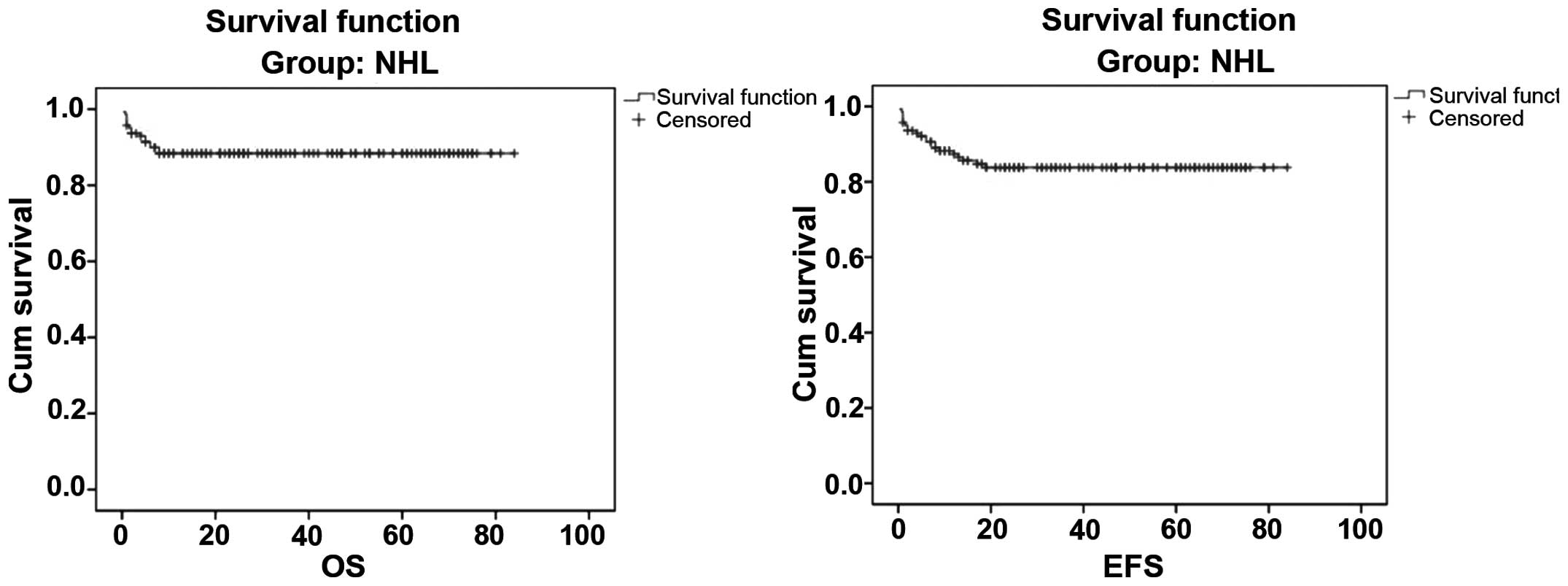
EFS for all NHL cases were 88.7 and 85.1%, respectively (Fig. 1). The 5-year OS and EFS for stage
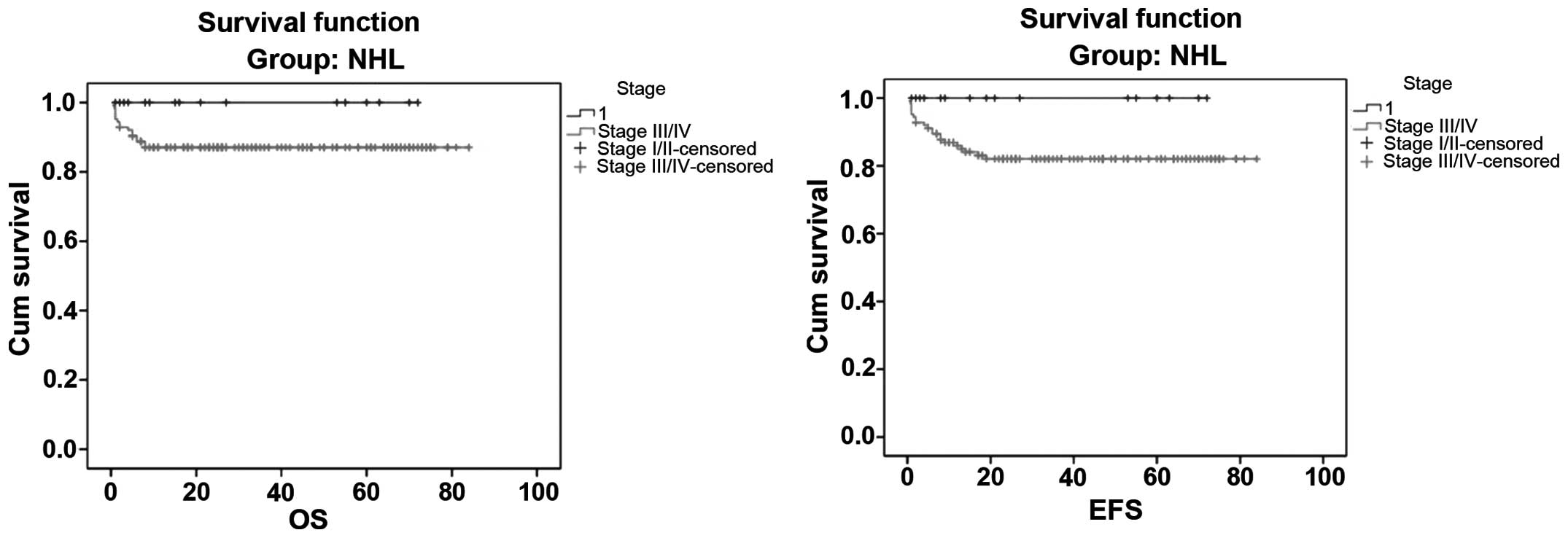
I/II cases were both 100%, whereas for stage III/IV cases they were
87.3 and 83.2%, respectively (Fig.
2). There was no statistically significant difference in the OS
and EFS rates between the four stages (log-rank test, P>0.5;
Table II). The 5-year OS and EFS
for BL were 90.8 and 87.8%, respectively, whereas for lymphoblastic
lymphoma they were 80.7 and 80%, respectively (Fig. 3). None of the clinical,
epidemiological or pathological variables exhibited a statistically
significant association with global survival or EFS, i.e., age,
gender, stage and pathology were not significantly associated with
patient outcome (P>0.05) (Table
II).
 | Table IIResults of statistical tests for
associations between overall survival and event-free survival and
the study variables in non-Hodgkin lymphoma patients. |
Table II
Results of statistical tests for
associations between overall survival and event-free survival and
the study variables in non-Hodgkin lymphoma patients.
| Overall
survival | Event-free
survival |
|---|
|
|
|
|---|
| Variables | Log-rank | P-value | Log-rank | P-value |
|---|
| Age |
0.71 |
0.70 |
0.45 |
0.80 |
| Gender |
0.30 |
0.59 |
1.78 |
0.18 |
| Pathology |
2.43 |
0.49 |
3.02 |
0.39 |
| Stage |
1.94 |
0.16 |
2.50 |
0.11 |
Discussion
Lymphoma in children and adolescents comprises a
heterogeneous group of malignant diseases of lymphoid tissues. The
various lymphoma diagnoses present with distinctive biological and
epidemiological characteristics. There are significant variations
worldwide in the incidence of childhood malignancies, among which
lymphomas are the dominant type (15). The present study describes the
epidemiological and clinical characteristics of 142 Egyptian
pediatric NHL patients admitted to two Pediatric Oncology Units.
Patients aged <10 years consituted >88.5% of our study sample
and there was a trend toward a decrease of the relative incidence
with increasing age, with 88.5 and 11.5% for the age groups of 0–10
and >10 years, respectively, which was consistent with
previously reported findings (16–18).
Sandlund et al (1) and
Manipadam et al (19)
reported that the incidence of NHL increases uniformly with age and
is rarely diagnosed before 2 years of age. The possibility of an
infectious etiology, principally associated with Epstein-Barr virus
(EBV) (12), being involved in the
pathogenesis of NHL may be a partial explanation of the emergence
of this disease in younger patients in our region, as populations
of lower socioeconomic status are presumably exposed to infectious
agents earlier in life. The incidence of childhood NHL was
generally higher in male (63.4%) compared to female (36.6%)
patients and this male predominance of childhood lymphoma mirrors
the reported general pattern of male predominance exhibited by
lymphoid malignancies, but not by all types of cancer (16,18).
Although the majority of lymphomas in adults are of low or
intermediate grade, >90% of childhood NHLs are of high grade and
may be classified into one of four main categories (20).
BL was the most common NHL subtype (69%) in our
series. Furthermore, BL accounted for 78.2, 77.2, 68, 48.2, 42.2
and 39% of the cases in studies conducted in Brazil, Kuwait, Saudi
Arabia, Germany, UK and Egypt, respectively (16,21–25).
By contrast, BL was encountered at a surprisingly low frequency
(9–12%) in India, Pakistan and Shanghai (21,26).
There is evidence that the incidence of BL varies
markedly worldwide, being particularly high in Equatorial Africa,
which is likely due to early infection by EBV (almost all cases in
Africa are EBV-related) and chronic exposure to malaria. As early
exposure to EBV is associated with a lower socioeconomic status and
BL is commonly associated with EBV worldwide, the variability in
the incidence of BL is likely to be, at least in large part, a
function of the epidemiology of EBV (27).
Our analysis revealed that the proportion of
precursor T-cell lymphoblastic lymphoma (TLL) was 18.3% of all
NHLs, which is lower among children in Egypt compared to other
Asian countries. The proportion of T-cell NHL is significantly
higher in the Korean pediatric and young adult population (28), Japan (29) and India (19,26),
while the frequency of TLL was found to be low in Saudi Arabia
(8%), Pakistan (8%) and nearly the same results were obtained from
the UK (19%) and Germany (16.1%) (23,24).
Overall, DLBCL accounted for 10.6% of all pediatric
NHLs. This finding was similar to that reported in the English
literature. Burkhardt et al (24) found that DLBCL constituted 13% of
pediatric NHLs. Wright et al (23) reported that B-cell NHLs other than
lymphoblastic lymphoma and BL constituted 7.8% of the cases and
comprised centroblastic lymphoma, high-grade B-cell NHL not
otherwise specified, T-cell-rich B-cell lymphoma and primary
mediastinal large B-cell lymphoma. The frequency of DLBCL was
higher in Korea (28) and Japan
(29). Pediatric DLBCL is
considered to be biologically different from its adult counterpart
and is associated with an excellent prognosis. In this study, ALCL
was the fourth most common NHL subtype and constituted only 2.1% of
all pediatric NHLs. In previous studies, ALCLs accounted for 15% of
pediatric NHLs in the UK (23),
11.5% in India (19), 10.3% in
Germany (24) and 20.5% in Korea
(28). However, the possible
reasons of geographic differences in the spectrum of lymphoma
remain unknown, mainly due to the etiology of lymphoma being
largely unknown, although certain risk factors were recently
documented, including genetic factors, immune abnormalities,
individual susceptibility, lifestyle, environmental exposures, as
well as various infections caused by bacteria, viruses, mycoplasma
and chlamydia (30). Epidemiologic
studies suggested that the distribution of lymphoma subtypes
exhibits significant geographic variation (31). However, research regarding such
information in Egypt is limited.
NHL in children is generally considered to be widely
disseminated from the outset, even when apparently localized
(32). In the present study, 88.7%
of NHL patients had Murphy stage III/IV disease and only 11.3% had
stage I/II disease; similar results were reported by several
previous studies (1,14,29,33).
The high prevalence rates of advanced tumor stage in our series was
possibly associated with, among other factors, the large number of
patients with voluminous abdominal lymphomas, late diagnosis and
the predominance of primary mediastinal tumors within the
lymphoblastic histological type, and with the low frequency of NHL
with more superficial primary sites, such as tumors of the head
neck and primary nodal disease, making it difficult to detect the
disease at an earlier stage.
Tumors in pediatric NHL cases are frequently found
extranodally and are difficult to diagnose, clinically as well as
histopathologically (14,34,35).
In our analysis of 142 patients with NHL, abdominal involvement was
the most common presentation (73.2%), followed by mediastinal
involvement, while bone marrow infiltration was the most common
site of metastasis; similar results was reported by many authors
(21,36). In Turkey, abdominal involvement was
the most common presentation (>75%), followed by the jaw and the
central nervous system (21). As
regards identifying prognostic factors, no statistically
significant associations were observed between age, gender,
histopathology and staging and either OS or EFS in our study. The
5-year OS and EFS in the present study were 88.7 and 85.1%,
respectively (Figs. 1 and 2). The survival of our patients is
consistent with the results presented by the major collaborative
childhood cancer treatment groups, reporting a survival rate of
~80–90% for patients with NHL (37–41).
In conclusion, NHL occurs at younger age among
pediatric patients in Egypt, with a higher incidence of BL and
advanced-stage disease. The outcome of NHL in our two centers was
satisfactory, approaching the international rates.
References
|
1
|
Sandlund JT, Downing JR and Crist WM:
Non-Hodgkin's lymphoma in childhood. N Engl J Med. 334:1238–1248.
1996.
|
|
2
|
Cairo MS, Raetz E, Lim MS, et al:
Childhood and adolescent non-Hodgkin lymphoma: new insights in
biology and critical challenges for the future. Pediatr Blood
Cancer. 45:753–769. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gross TG and Termuhlen AM: Pediatric
non-Hodgkin's lymphoma. Curr Oncol Rep. 9:459–465. 2007.
|
|
4
|
Hochberg J, Waxman IM, Kelly KM, et al:
Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the
science. Br J Haematol. 144:24–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brousse N, Vasiliu V, Michon J, et al:
Pediatric non-Hodgkin lymphomas. Ann Pathol. 24:574–586. 2004.(In
French).
|
|
6
|
Setty BA and Termuhlen AM: Rare pediatric
non-Hodgkin lymphoma. Curr Hematol Malig Rep. 5:163–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharon V, Mecca PS, Steinherz PG, et al:
Two pediatric cases of primary cutaneous B-cell lymphoma and review
of the literature. Pediatr Dermatol. 26:34–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Swerdlow SH: Pediatri. follicular
lymphomas, marginal zone lymphomas, and marginal zone hyperplasia.
Am J Clin Pathol. 122 Suppl:S98–S109. 2004.PubMed/NCBI
|
|
9
|
Lones MA, Perkins SL, Sposto R, et al:
Non-Hodgkin lymphoma arising in bone in children and adolescents is
associated with an excellent outcome: a Children's Cancer Group
Report. J Clin Oncol. 20:2293–2301. 2002.PubMed/NCBI
|
|
10
|
Gatta G, Corazziari I, Magnani C,
Peris-Bonet R, Roazzi P and Stiller CEUROCARE Working Group:
Childhood cancer survival in Europe. Ann Oncol. 14 (Suppl
5):v119–v127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gross TG and Perkins SL: Malignant
non-Hodgkin's lymphoma in children. Principles and Practice of
Pediatric Oncology. Pizzo PA and Poplack DG: 6th. Lippincott
Williams: Philadelphia: pp. 663–682. 2011
|
|
12
|
Gutierrez MI, Bhatia K, Barriga F, et al:
Molecular epidemiology of Burkitt's lymphoma from South America:
differences in breakpoint location and Epstein-Barr virus
association from tumors in other world regions. Blood.
79:3261–3266. 1992.
|
|
13
|
Jaffe ES, Harris NL, Stein H, et al:
Introduction and overview of the classification of the lymphoid
neoplasms. WHO Classification of Tumours of Haematopoietic and
Lymphoid Tissues. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri
SA, Stein H, Thiele J and Vardiman JW: 4th. IARC Press; Lyon: pp.
157–166. 2008
|
|
14
|
Murphy SB: Classification, staging and end
results of treatment of childhood non-Hodgkin's lymphomas:
dissimilarities from lymphomas in adults. Semin Oncol. 7:332–339.
1980.PubMed/NCBI
|
|
15
|
Ries LAG, Smith MA, Gurney JG, Linet M,
Tamra T, Young JL and Bruin GR: Cance. Incidence and Survival Among
Children and Adolescents: United States SEER Program 1975–1995.
National Cancer Institute, SEER Program; : Bethesda, MD: 1999
|
|
16
|
Pedrosa MF, Pedrosa F, Lins MM, et al:
Non-Hodgkin's lymphoma in childhood: clinical and epidemiological
characteristics and survival analysis at a single center in
Northeast Brazil. J Pediatr (Rio J). 83:547–554. 2007.
|
|
17
|
Sandlund JT, Fonseca T, Leimig T, et al:
Predominance and characteristics of Burkitt lymphoma among children
with non-Hodgkin lymphoma in northeastern Brazil. Leukemia.
11:743–756. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klumb CE, Schramm MT, De Resende LM, et
al: Treatment of children with B-cell non-Hodgkin's lymphoma in
developing countries: the experience of a single center in Brazil.
J Pediatr Hematol Oncol. 26:462–468. 2004.
|
|
19
|
Manipadam MT, Nair S, Viswabandya A, et
al: Non-Hodgkin lymphoma in childhood and adolescence: frequency
and distribution of immunomorphological types from a tertiary care
center in South India. World J Pediatr. 7:318–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harris NL, Jaffe ES, Diebold J, et al:
Word Health Organization classification of neoplastic disease of
the hematopoietic and lymphoid tissues: report of the Clinical
Advisory Committee meeting-Airlie House, Virginia, November 1997. J
Clin Oncol. 17:3835–3849. 1999.
|
|
21
|
Naresh KN, Agarwal B, Nathwani BN, et al:
Use of the World Health Organization (WHO) classification of
non-Hodgkin's lymphoma in Mumbai, India: a review of 200
consecutive cases by a panel of five expert hematopathologists.
Leuk Lymphoma. 45:1569–1577. 2004.
|
|
22
|
Temmim L, Baker H, Amanguno H, et al:
Clinicopathological features of extranodal lymphomas: Kuwait
experience. Oncology. 67:382–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wright D, McKeever P and Carter R:
Childhood non-Hodgkin lymphomas in the United Kingdom: findings
from the UK Children's Cancer Study Group. J Clin Pathol.
50:128–134. 1997.
|
|
24
|
Burkhardt B, Zimmermann M, Oschlies I, et
al BFM Group: The impact of age and gender on biology, clinical
features and treatment outcome of non-Hodgkin lymphoma in childhood
and adolescence. Br J Haematol. 131:39–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mokhtar N and Khaled H: Lymphoma. 1st.
Aventis Oncol; Cairo: pp. 47–63. pp. 123–134. 2002
|
|
26
|
Srinivas V, Soman CS and Naresh KN: Study
of the distribution of 289 non-Hodgkin lymphomas using the WHO
classification among children and adolescents in India. Med Pediatr
Oncol. 39:40–43. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Magrath IT: African Burkitt's lymphoma.
History, biology, clinical features, and treatment. Am J Pediatr
Hematol Oncol. 13:222–246. 1991.
|
|
28
|
Hwang IG, Yoo KH, Lee SH, et al:
Clinicopathologic features and treatment outcomes in malignant
lymphoma of pediatric and young adult patients in Korea: comparison
of Korean all-ages group and Western younger age group. Clin
Lymphoma Myeloma. 7:580–586. 2007. View Article : Google Scholar
|
|
29
|
Nakagawa A, Nakamura S, Nakamine H, et al:
Pathology review for paediatric non-Hodgkin's lymphoma patients in
Japan; a report from the Japan association of childhood leukemia
study (JACLS). Eur J Cancer. 40:725–733. 2004.
|
|
30
|
Jaffe ES, Harris NL, Stein H and Vardiman
JW: Patholog. and genetics of tumours of haematopoietic and
lymphoid tissues. World Health Organization Classification of
Tumours. 3rd. IARC Press; Lyon: pp. 227–230. 2001;
|
|
31
|
Anderson JR, Armitage JO and Weisenburger
DD: Epidemiology of the non-Hodgkin's lymphomas: distributions of
the major subtypes differ by geographic locations. Non-Hodgkin's
Lymphoma Classification Project. Ann Oncol. 9:717–720. 1998.
|
|
32
|
Patte C: Non-Hodgkin's lymphoma. Eur J
Cancer. 34:359–363. 1998.
|
|
33
|
Boerma EG, van Imhoff GW, Appel IM, et al:
Gender and age-related difference in Burkitt lymphoma -
epidemiological and clinical data from The Netherlands. Eur J
Cancer. 40:2781–2787. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pinkerton CR: The continuing challenge of
treatment for non-Hodgkin's lymphoma in children. Br J Haematol.
107:220–234. 1999.
|
|
35
|
Perkins SL: Work-up and diagnosis of
pediatric non-Hodkin's lymphomas. Pediatr Dev Pathol. 3:374–390.
2000.
|
|
36
|
Temmim L, Baker H, Al-Jarallah M, et al:
Clinical characteristics and pathological classification of
non-Hodgkin's lymphoma in Kuwait. Results of a collaborative study
with the International Lymphoma Study Group (ILSG). Leuk Lymphoma.
45:1865–1871. 2004.
|
|
37
|
Reiter A, Schrappe M, Parwaresch R, et al:
Non-Hodgkin's lymphomas of childhood and adolescence: results of a
treatment stratified for biologic subtypes and stage - a report of
Berlin-Frankfurt-Münster Group. J Clin Oncol. 13:359–372. 1995.
|
|
38
|
Reiter A, Schrappe M, Ludwig WD, et al:
Intensive ALL-type therapy without local radiotherapy provides a
90% event-free survival for children with T-cell lymphoblastic
lymphoma: a BFM group report. Blood. 95:416–421. 2000.PubMed/NCBI
|
|
39
|
Link MP, Shuster JJ, Donaldson SS, et al:
Treatment of children and young adults with early-stage
non-Hodgkin's lymphoma. N Engl J Med. 337:1259–1266.
1997.PubMed/NCBI
|
|
40
|
Seidemann K, Tiemann M, Schrappe M, et al:
Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious
treatment for pediatric anaplastic large cell lymphoma: a report of
the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood.
97:3699–3706. 2001. View Article : Google Scholar
|
|
41
|
Laver JH, Kraveka JM, Hutchison RE, et al:
Advanced-stage large-cell lymphoma in children and adolescents:
results of a randomized trial incorporating intermediate-dose
methotrexate and high-dose cytarabine in the maintenance phase of
the APO regimen: a Pediatric Oncology Group phase III trial. J Clin
Oncol. 23:541–547. 2005. View Article : Google Scholar
|