Introduction
The immune cell function assay (ImmuKnow; Cylex,
Inc., Columbia, MD, USA), which enables the measurement of the
CD4+ T-cell activity, was approved for use by the US
Food and Drug Administration in 2002 (1). To date, a number of studies (2–5) have
investigated the correlation between the immune response and
rejection in transplant recipients since Hooper et al
(5) established immune response
zones using the ImmuKnow assay in 2005. Those studies demonstrated
that low adenosine triphosphate (ATP) levels in CD4+ T
cells are associated with increased risk of infection. In addition,
it was suggested that monitoring using the ImmuKnow assay may
reflect the dynamics of the immune status (4).
In contrast to transplantation, surgical stress
induces postoperative immunosuppression, which has been evaluated
by measuring interleukin (IL), cortisol and C-reactive protein
(CRP) levels and/or the CD4+ T-cell count (6, 7). By
contrast, Wu et al (8)
reported the reference of CRP, IL-6, IL-8 and HLA-DR levels, but
not the CD4/CD8 ratio between laparoscopic and conventional surgery
for colon cancer and concluded that the extent of surgical trauma
may affect those results. However, these parameters have not been
proven sufficient to evaluate the postoperative immune status.
Therefore, it is necessary to identify a novel parameter that may
be used to accurately evaluate the immune status in postoperative
patients.
To the best of our knowledge, there are currently no
available studies evaluating the extent of surgical stress by
assessing the ATP levels of CD4+ T cells. The aim of
this study was to measure ATP levels in CD4+ T cells as
a marker of T-cell activity in the acute phase following colorectal
surgery using the ImmuKnow assay. We investigated the dynamics of
postoperative ATP levels and compared these levels between
laparoscopic and conventional open abdominal surgery as an endpoint
in this study.
Patients and methods
Patients
A total of 16 consecutive patients with colorectal
cancer who underwent surgery at the Department of
Gastroenterological Surgery at Fukuoka University Hospital between
August and December, 2012 were included in the present study.
Patients who had received immunosuppressive drugs or steroids and
underwent organ transplantation were excluded. Our sample included
13 men (81.2%) and 3 women (18.8%), with a mean age of 62.4 years
(range, 41–84 years). The diagnosis was based on radiological
examinations and histological confirmation for staging information.
The clinicopathological characteristics of the patients are
summarized in Table I.
Pathological staging was performed based on the TNM classification
of malignant tumors, 7th edition, published by the Union for
International Cancer Control (9).
 | Table IClinical characteristics of the
patients. |
Table I
Clinical characteristics of the
patients.
| Patient no. |
|---|
| Characteristics | (n=16) |
|---|
| Age, years | |
| Mean
(range) | 62.4 (41–84) |
| Gender | |
| Male | 13 |
|
Female | 3 |
| Cancer location | |
| Colon
(colectomy) | 12 |
| Rectum
(anterior resection) | 4 |
| Surgery | |
| Open
abdominal | 9 |
|
Laparoscopic | 7 |
| pStage | |
| I | 7 |
| II | 5 |
| IIIa | 0 |
| IIIb | 2 |
| IV | 2 |
The present study was approved by the Medical Ethics
Board of Fukuoka University, Faculty of Medicine (no. 12-6-15) and
all the patients provided written informed consent prior to
enrolment.
ImmuKnow assay
Whole-blood samples from the colon cancer patients
were collected using sodium heparin anticoagulant tubes. The
ImmuKnow assay was performed according to the manufacturer's
protocol. In brief, the blood samples were diluted using sample
diluents and incubated for 15–18 h with
phytochemagglutinin-L-containing cell lysate at 37°C, in a 5%
CO2 incubator. Following incubation, the CD4+
T cells were collected using magnetic particles coated with
anti-human CD4 monoclonal antibodies. Following rinsing, the
intracellular ATP released by cell lysis was measured using
luciferin/luciferase and a luminometer (Tristar LB941; Berthold
Technologies GmbH, Bad Wildbad, Germany). The ATP level was
calculated in nanograms per milliliter. The ImmuKnow assay was
performed in quadruplicates preoperatively and on postoperative
days 1, 4 and 8.
Statistical analysis
The correlations between the ImmuKnow assay data and
the clinicopathological parameters were statistically analyzed. In
addition, the blood laboratory data were examined on the same days
as the ImmuKnow assay and these values were investigated for a
correlation with the ImmuKnow data. The statistical analysis was
performed by paired t-test using JMP Statistical Discovery software
(SAS Institute Inc., Cary, NC, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
TNM staging
According to the TNM classification, 7th edition, 7
patients had stage I, 5 had stage II, 2 had stage IIIb and the
remaining 2 had stage IV disease (Table I).
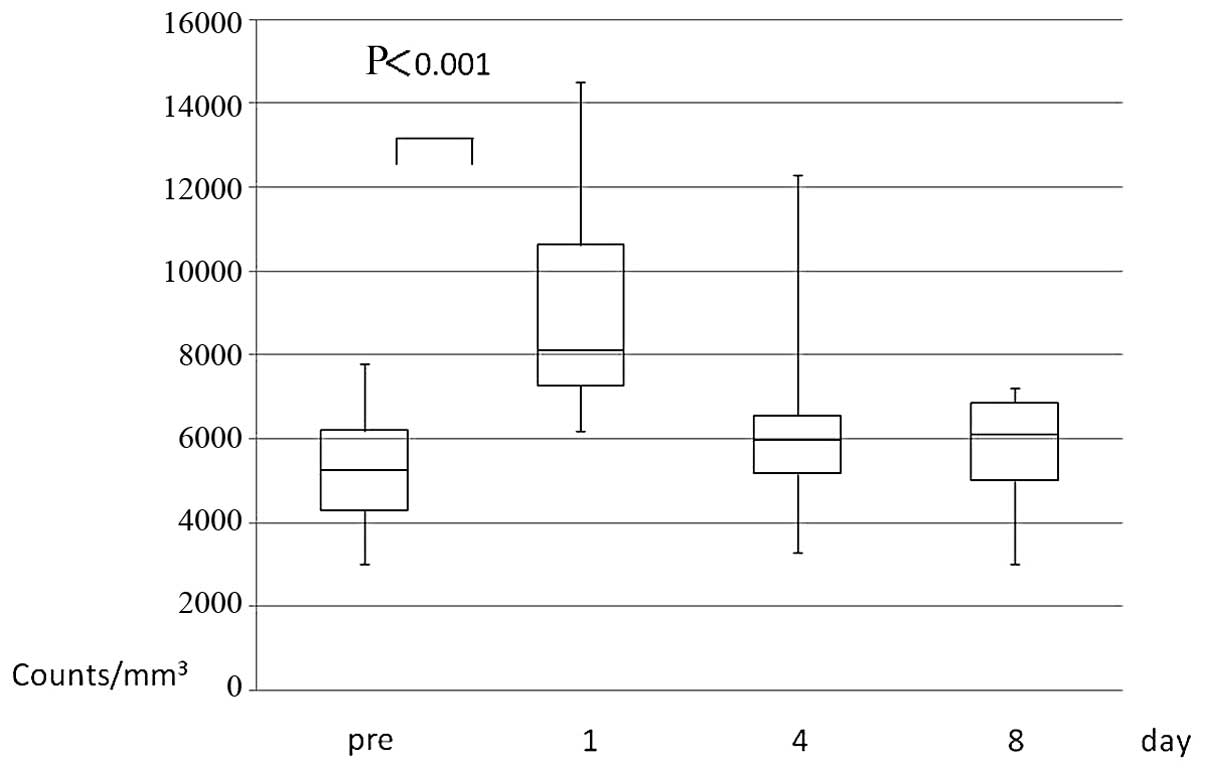
White blood cell (WBC) count
Open abdominal surgery and laparoscopic resection
were performed in 9 and 7 patients, respectively (Table I). The WBC count was significantly
increased on the 1st postoperative day [mean preoperative vs. 1st
postoperative day level ± standard deviation (SD): 5,318.8±1,453.8
vs. 9,087.5±2,5878.6, respectively; P<0.001], followed by a
gradual return to the baseline preoperative levels (Fig. 1).
CRP
The CRP level (mg/dl) was aberrantly high on the 1st
postoperative day (mean preoperative vs. 1st postoperative day
level ± SD: 0.27±0.55 vs. 5.91±2.95, respectively; P<0.0001) and
the 4th postoperative day, but decreased to normal by the 8th day
(Fig. 2).
Lymphocyte count
The lymphocyte count decreased significantly on the
1st postoperative day (mean preoperative vs. 1st postoperative day
level ± SD: 1,740.7±758.7 vs. 1,095.1±515.8, respectively;
P<0.001), but returned to normal on the 8th day (Fig. 3).
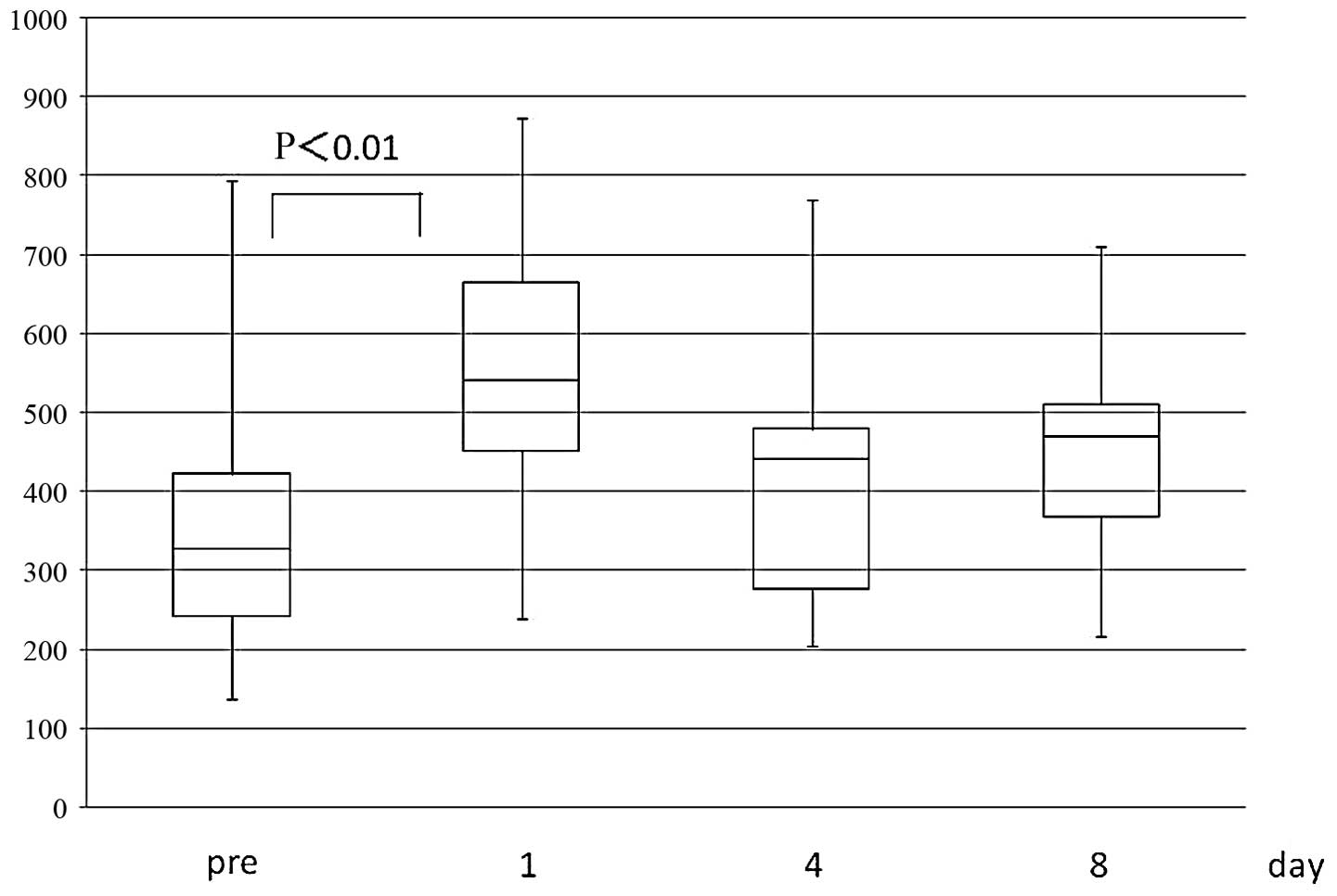
ImmuKnow value
The ImmuKnow value increased significantly on the
postoperative 1st day (mean preoperative vs. 1st postoperative day
level ± SD: 378.5±193.7 vs. 553.3±172.7, respectively;P<0.01),
followed by a gradual return to the preoperative level by the 8th
day (Fig. 4).
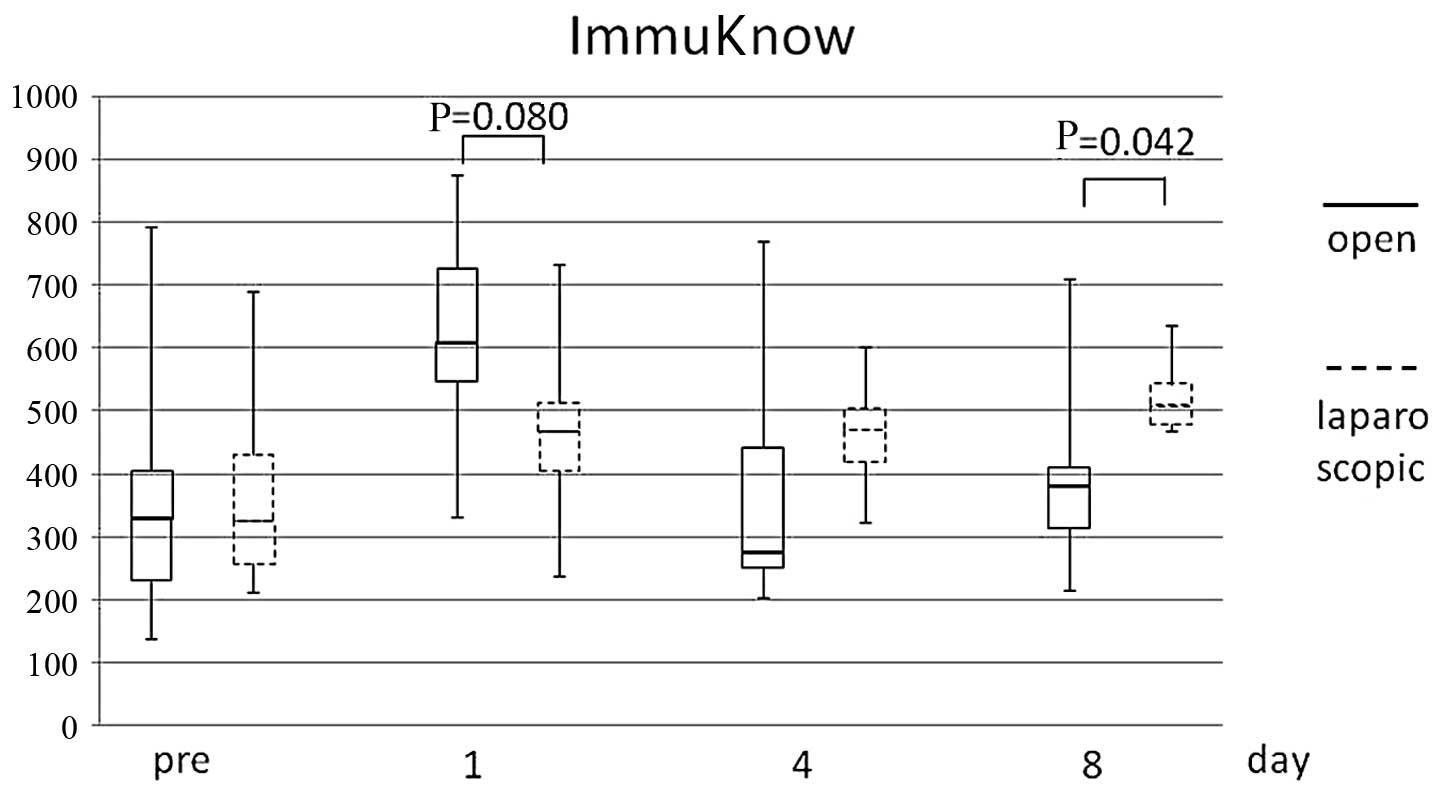
Comparison between surgical
approaches
In a comparison between laparoscopic and open
abdominal surgery, there were no significant differences in the WBC
count, lymphocyte count or CRP value. However, the ImmuKnow assay
demonstrated a tendency for the laparoscopic group to exhibit a
lower response on the 1st postoperative day (open vs. laparoscopic
surgery ± SD: 619.8±166.1 vs. 467.9±150.5, respectively; P=0.080)
and a higher response on the 8th postoperative day (open vs.
laparoscopic surgery ± SD: 394.0±141.0 vs. 522.1±60.6,
respectively; P=0.042) (Fig.
5).
Discussion
Surgical stress induces postoperative
immunosuppression. Ogawa et al (6) reported that the peripheral blood
lymphocyte count and function were suppressed for ≥2 weeks
postoperatively in 20 patients with stage I gastrointestinal cancer
who underwent surgical resection. In particular, the CD4/CD8 T-cell
ratio was decreased during the postoperative acute phase.
Similarly, it was reported that the number of CD4+ T
cells was decreased on the 1st day post-cholecystectomy and
returned to normal at 5–7 days postoperatively (10).
In contrast to those reports, in our study, the
CD4+ T-cell activity was significantly increased on the
1st postoperative day and was gradually reduced by day 4–7
postoperatively. This result may suggest that the CD4+
T-cell activity was stimulated in order to compensate for the
decrease in CD4+ T cells due to the postoperative
immunosuppression. Therefore, the ImmuKnow assay, which monitors
the CD4+ T-cell activity, may be clinically applicable
for the monitoring of the postoperative immune status.
Furthermore, when the effects of laparoscopic and
open abdominal surgery on the CD4+ T-cell activity were
compared, there was a tendency for the laparoscopic approach to
exert a more limited effect compared to open surgery, although the
difference did not reach statistical significance. Wu et al
(8) reported that serum IL-6 and
IL-8 levels were higher in the early phase following surgery in the
open surgery compared to the laparoscopic surgery group in patients
with colorectal cancer. The results of that study were consistent
with ours, which demonstrated that ImmuKnow value correlated with
acute phase response following colorectal surgery, similar to
proinflammatory cytokines. Brune et al (11) also evaluated the immune defense
following laparoscopic and open cholecystectomy and reported a more
prominent immune suppression in the open surgery group. In
addition, Bessler et al (12) reported that the immune activity
associated with T-cell function was maintained closer to normal
following laparoscopic colorectal surgery compared to open surgery.
Thus, ImmuKnow value, as well as cytokine levels, suggest that
laparoscopic colorectal surgery was less traumatic compared to open
surgery, from the view point of acute phase response.
In addition, the CD4+ T-cell activity
revealed a different type of reaction in patients subjected to
surgical stress compared to those receiving immunosuppressive
drugs. The ImmuKnow activity was found to be low in patients
receiving immunosuppressive drugs following transplantation and its
level may be used for risk monitoring of the patients to prevent
transplant rejection and/or infection. Surgical stress is a
well-known immunosuppressive factor following surgery, although the
ImmuKnow activity was actually elevated on the 1st postoperative
day, when the immunosuppression was considered to be at its peak.
The ImmuKnow activity, therefore, may reflect the different
mechanisms underlying the suppression of the immune system by
surgical stress and pharmacotherapy (2–5). To
elucidate the reasons for these differences, a subpopulation
analysis of CD4+ T cells may be undertaken in the
future. A limitation of the present study was that ImmuKnow is a
qualitative assay, but does not directly reflect the level of
immunosuppression, as described in the manufacturer's manual.
In conclusion, the immune activity during the acute
phase following colorectal surgery may be monitored using the
ImmuKnow assay. In addition, the ImmuKnow value may demonstrate the
significant difference of the acute phase response between
laparoscopic and open colorectal surgery. Futher clinical
applications using ImmuKnow assay are expected in the future.
References
|
1
|
Kowalski R, Post D, Schneider MC, et al:
Immune cell function testing: an adjunct to therapeutic drug
monitoring in transplant patient management. Clin Transplant.
17:77–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kowalski RJ, Post DR, Mannon RB, et al:
Assessing relative risks of infection and rejection: a
meta-analysis using an immune function assay. Transplantation.
82:663–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Husain S, Raza K, Pilewski JM, et al:
Experience with immune monitoring in lung transplant recipients:
correlation of low immune function with infection. Transplantation.
87:1852–1857. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xue F, Zhang J, Han L, Li Q, Xu N, Zhou T,
Xi Z, Wu Y and Xia Q: Immune cell functional assay in monitoring of
adult liver transplantation recipients with infection.
Transplantation. 89:620–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hooper E, Hawkins DM, Kowalski RJ, Post
DR, Britz JA, Brooks KC and Turman MA: Establishing pediatric
immune response zones using the Cylex ImmuKnow assay. Clin
Transplant. 19:834–839. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogawa K, Hirai M, Katsube T, Murayama M,
Hamaguchi K, Shimakawa T, Naritake Y, Hosokawa T and Kajiwara T:
Suppression of cellular immunity by surgical stress. Surgery.
127:329–336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slade MS, Simmons RL, Yunis E and
Greenberg LJ: Immunodepression after major surgery in normal
patients. Surgery. 78:363–372. 1975.PubMed/NCBI
|
|
8
|
Wu FP, Sietses C, von Blomberg BM, van
Leeuwen PA, Meijer S and Cuesta MA: Systemic and peritoneal
inflammatory response after laparoscopic or conventional colon
resection in cancer patients: a prospective, randomized trial. Dis
Colon Rectum. 46:147–155. 2003. View Article : Google Scholar
|
|
9
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th. John Wiley &
Sons; West Sussex: pp. 100–105. 2009
|
|
10
|
Vallina VL and Velasco JM: The influence
of laparoscopy on lymphocyte subpopulations in the surgical
patient. Surg Endosc. 10:481–484. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brune IB, Wilke W, Hensler T, Feussner H,
Holzmann B and Siewert JR: Normal T lymphocyte and monocyte
function after minimally invasive surgery. Surg Endosc.
12:1020–1024. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bessler M, Whelan RL, Halverson A, Treat
MR and Nowygrod R: Is immune function better preserved after
laparoscopic versus open colon resection? Surg Endosc. 8:881–883.
1994. View Article : Google Scholar : PubMed/NCBI
|