Introduction
Despite advances in surgical techniques, the outcome
of patients with locally advanced gastric cancer following surgery
remains poor (1). Locoregional
recurrence is the main pattern of failure in gastric cancer
patients treated with complete resection (2, 3).
Although the efficacy of postoperative chemoradiotherapy following
radical surgery for locally advanced gastric cancer has been
confirmed in the INT-0116 trial (4), the prognosis remains suboptimal.
Theoretically, a greater radiation dose may provide a higher tumor
control. However, owing to the dose-limiting surrounding tissues in
the planning treatment volume, including small intestine, pancreas,
bile ducts and spinal cord, the higher doses that are necessary for
disease control cannot be safely delivered with conventional
external-beam radiotherapy (EBRT) (5). Intraoperative radiotherapy (IORT)
allows the delivery of a boost of radiation to a localized area in
a single fraction without affecting the surrounding tissues
(6). An IORT boost component has
also been included in the context of surgical resection, adjuvant
EBRT and chemotherapy, with acceptable tolerance and improved
locoregional control (7, 8).
Although the efficacy of IORT for locally advanced
gastric cancer has been previously addressed in several studies and
suggests that the addition of IORT may increase the locoregional
control and thereby may improve the overall survival (OS), the
results from all available studies have been equivocal (9–11).
To determine whether there is a benefit of IORT for resectable
gastric cancer, a meta-analysis was performed of studies that
focused on this topic.
Materials and methods
Search strategy and selection
criteria
A bibliographical study was performed using the
PubMed, Web of knowledge and Embase electronic databases. The
following medical subject headings, keywords and text words were
used: i) Gastric or stomach, and cancer, carcinoma or
adenocarcinoma; and ii) intraoperative radiotherapy or IORT. The
search included the studies that were published between January,
1990 and July, 2013. The computer search was supplemented with a
manual search of the reference lists from all available review
studies, primary studies, meetings abstracts and bibliographies of
books, in order to identify other studies that were not found
during the computer search. When the results of a single study were
reported in more than one publication, only the most recent and
complete data were included in the meta-analysis.
The potentially eligible studies were retrieved and
a full-text analysis was performed. Only the studies that included
OS and/or the locoregional control rate comparison between the
patients with histology-proven cancer of the stomach, assigned to
surgery alone (observation arm) or to surgery plus IORT (study arm)
were included in the review process. EBRT and chemotherapy were
administered to the patients in both arms.
Data extraction
Data were carefully extracted independently by two
investigators (W.W. Yu and Y.M. Guo) according to the preferred
reporting items for systematic reviews and meta-analyses (PRISMA)
statement (12). The following
information was extracted from each study: First author's name,
year of publication, study design, number of patients, rates of
dissections and endpoints reported (OS and locoregional control
rates).
Hazard ratios (HRs) for the OS and locoregional
control rates were extracted directly from the original studies or
were estimated indirectly by reading off survival curves as
suggested by Tierney et al (13). In summary, when the estimated HR
and its standard error were described in the publications, these
values were obtained directly; when these statistical variables
were not provided explicitly in a study they were calculated
directly using two of the following parameters: The confidence
interval (CI) for the HR, the log-rank statistic, the P-value or
the O-E statistic (difference between numbers of observed and
expected events). When those data were not available, the following
were studied: The total number of events, the number of patients at
risk in each group and the log-rank statistic or its P-value,
allowing calculation of an approximation of the HR estimate. When
the only available data were in the form of graphical
representations, they were calculated from Kaplan-Meier survival
curves. The Kaplan-Meier curves were read by two investigators
using the Engauge Digitizer 4.1 version software (Mark Mitchell,
Boston, MA, USA) independently to reduce the inaccuracy in the
extracted survival rates. The HRs for OS were also extracted for
the patient subgroups (including patients with stage III) whenever
possible.
Statistical analysis
The heterogeneity was formally investigated by means
of Cochrane Q statistic and I2 statistic. For the Q
statistic, the heterogeneity when P<0.1 was considered to
indicate a statistically significant difference. The I2
statistic, which is the proportion of the total variation among the
studies that is likely to be explained by between-study
heterogeneity rather than chance (14), is reported. Substantial
heterogeneity exists when I2>50%. When the hypothesis
of homogeneity was not rejected, a fixed-effects model was used.
Otherwise, the random-effects model was used (15). By convention, the impact of IORT on
the OS or locoregional control rates was considered to indicate a
statistical significance if the 95% CI for the overall HR did not
overlap 1. The evidence of publication bias was evaluated by the
funnel plot with the test of Begg and Mazumdar (16) and the linear regression asymmetry
test of Egger et al (17).
For these analyses, P<0.05 was considered to indicate
statistically significant publication bias. All statistical
analyses were performed by the STATA 12.0 software (Stata Corp.,
College Station, TX, USA).
Results
Trial selection and characteristics of
the included studies
A total of 607 relevant studies were collected.
Subsequent to the exclusion of the duplicate references by the
‘find duplicates’ function of EndNote X3, there were 173 unique
studies. Following a review of each title and abstract, 12 studies
meeting the eligibility criteria were identified. A careful
examination of these full studies led to the exclusion of four
studies: The updated results from one study were available in a
separate publication (7) and four
were excluded as they did not meet the inclusion criteria (18–20).
Thus, a total of eight studies were included in the meta-analysis
(8–11, 21–24)
(Fig. 1).
Study characteristics
The details regarding the eight studies included in
the analysis are summarized in Table
I. The OS data for all patients in trials were available for
four studies (8, 9, 11,
24). Three studies provided the
OS data for the subgroup of patients with stage III disease, and
they all tested surgery followed by adjuvant IORT against surgery
alone (21–23). The locoregional control rate was
provided in the four studies (8,
10, 11, 24).
 | Table ISummary of the studies included in the
meta–analysis. |
Table I
Summary of the studies included in the
meta–analysis.
| Author (Refs.) | Year | Years of accrual | Study design | Nodal dissection | N pts | Endpoints
reported |
|---|
| Drognitz et al
(9) | 2008 | February 1991 to July
2001 | S + IORT (6–15 MeV,
15–25 Gy) S | D2 | 122 | OS |
| Zhang et al
(8) | 2012 | March 2003 to October
2005 | S + IORT (9–16 MeV,
12–15 Gy) + EBRT + CT S + EBRT + CT | D2 | 97 | OS Locoregional
control |
| Martinez Monge et
al (11) | 1997 | October 1982 to March
1993 | S + IORT (9–20 MeV,
10–17 Gy) + EBRT S + EBRT | D2 | 62 | OS Locoregional
control |
| Sindelar et al
(24) | 1993 | No reported | S + IORT (11–15 MeV,
20 Gy)+ EBRT S + EBRT | No reported | 41 | OS Locoregional
control |
| Santoro et al
(10) | 1998 | July 1976 to July
1993 | S + IORT (27–30 Gy)
S | D2 | 59 | Locoregional
control |
| Qin et al
(21) | 2006 | 1992 to 1998 | S + IORT (6–16 MeV,
10–30 Gy) S | D2 or D3 | 292 | OS (stage III) |
| Ogata et al
(22) | 1995 | August 1983 to July
1992 | S + IORT (12 MeV,
28–30 Gy) S | D2 | 47 | OS (stage III) |
| Abe et al
(23) | 1995 | No reported | S + IORT (28–35 Gy)
S | No reported | 77 | OS (stage III) |
Meta-analysis findings
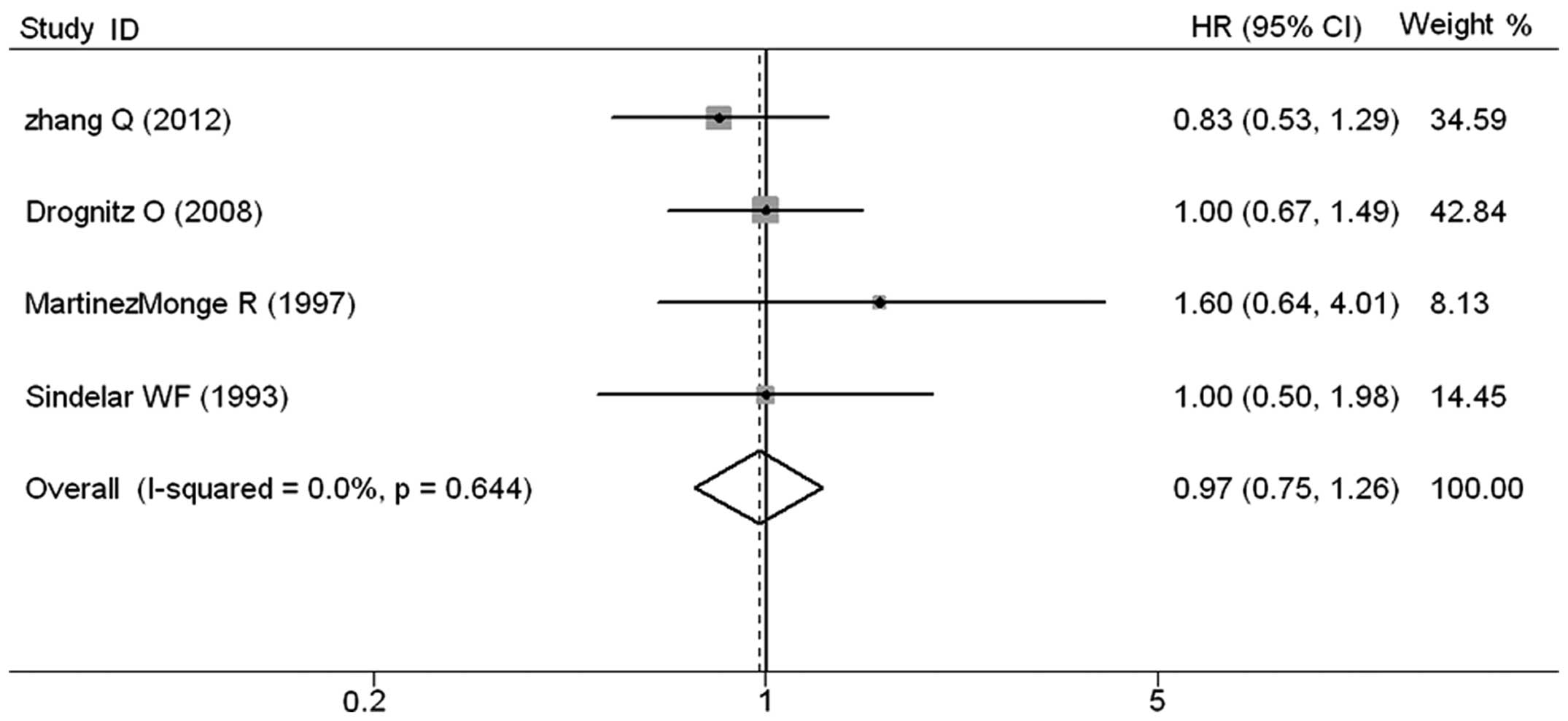
The meta-analysis of the four studies that provided
data on OS revealed that IORT had no significant impact in OS. The
pooled HR was 0.97 (95% CI, 0.75–1.26; Z=0.21; P=0.837), without
any evidence of heterogeneity (P=0.644) (Fig. 2). In the three studies testing the
efficacy of IORT for OS in the subgroup of patients with stage III
disease, there was a significantly improved OS (HR, 0.60; 95% CI;
0.40–0.89; Z=2.53; P=0.011) (Fig.
3). The significant locoregional control improvement was
observed in the four studies that provided locoregional control
data, and the combined HR was 0.40 (95% CI, 0.26–0.62; Z=4.18;
P<0.001), without heterogeneity (P=0.516) (Fig. 4). For all eight studies, there was
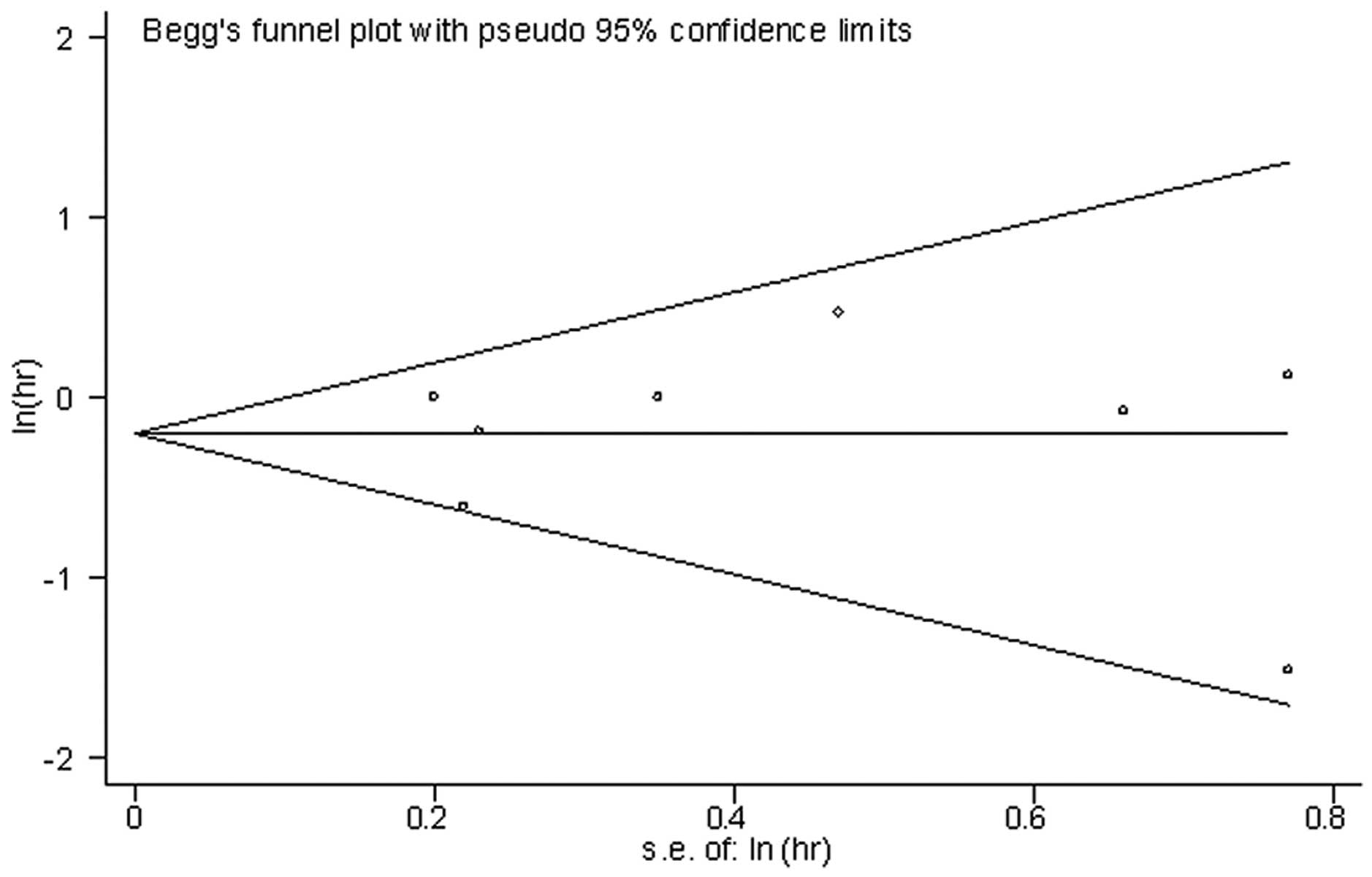
no evidence found of publication bias using the Eggers test
(P=0.969) and Begg's test (P=1.0). The shape of the funnel plot for
the pooled HR appeared to be symmetrical (Fig. 5).
Discussion
To the best of our knowledge, the present study
represents the first specific meta-analysis examining the impact of
IORT for patients with resectable gastric carcinoma. Although
meta-analysis based on individual data is considered to be the gold
standard, a meta-analysis based on the studies was still used in
the present study, as individual patient data were difficult to
access in the various studies published over a 20-year period. By
aggregating data from four eligible studies, it was found that the
use of IORT had no significant affect on OS. Notably, when the
focus was on the subgroup of patients with stage III disease, it
was found that the use of IORT was associated with a clear
reduction in the risk of mortality from any cause. Furthermore, the
combined HR for the four eligible studies that provided
locoregional control data suggested that the use of IORT was
associated with a significant improvement in the locoregional
control.
It is well known that local control by radiation for
subclinical disease is a function of radiation dose (25). Thus, using a greater
biological-radiotherapy dose could further improve the locoregional
control of gastric cancer following complete surgical resection
(26). However, the radiation dose
to the intra-abdominal structures is usually limited to 45 Gy due
to the adjacent dose-limiting structures. However, this dose may
not be sufficient for the eradication of the subclinical residual
disease (27). Significantly
increasing the EBRT dose to the surgical bed and regional nodal
areas is not acceptable when using conventional radiotherapy. IORT
involves the administration of large single doses of radiation
directly to surgically exposed tissues during the surgical
procedures. Thereby, it offers the opportunity to deliver high
doses of radiation to the primary tumor and regional nodal areas,
whilst simultaneously minimizing the possibility of radiation
toxicity for the surrounding normal-radiosensitive tissues. IORT
has been explored in various types of malignancies, including
gastric cancer, with studies focusing on the benefit for the
locoregional control and survival rates (28).
The findings in the present meta-analysis confirmed
that the use of IORT was associated with a notable decrease in
locoregional recurrence. In addition, the impact of IORT on OS
reached statistical significance in the subgroup of patients with
stage III disease, demonstrating that the use of IORT for locally
advanced gastric cancer may yield promising results. However, the
clear improvement of locoregional control did not translate into a
benefit for OS in the entire cohort of patients. This may be due to
the distant metastases offsetting the efficacy of IORT, emphasizing
the requirement for more effective systemic therapies (8, 29,
30). The advantage of IORT in OS
may also be abolished by an increased perioperative mortality rate.
Although the majority of studies did not investigate the impact of
IORT on surgical complications, certain studies found an increase
in the perioperative complications in conjunction with IORT
(10, 20). The surgical and radiotherapy
techniques have improved over the last twenty years, and the higher
mortality rate due to adjuvant IORT-associated toxicities most
probably offset the benefit of IORT.
Particular limitations of the present meta-analysis
should be taken into consideration when interpreting the findings.
First, the meta-analysis was performed using study-level data.
Patient-level data, if available, may provide more reliable
findings. Although there was no statistical evidence of publication
bias detected, this affect cannot be ruled out. Second, numerous
studies that were selected for the meta-analysis were retrospective
studies, which will inevitably have had selection bias. Third, the
analysis was restricted to the published studies that were written
in English, and several studies that met the eligibility criteria
were excluded based on language criteria. Thus, the number of
eligible studies was not sufficiently large for a comprehensive
analysis.
In conclusion, the present meta-analysis suggests
that the use of IORT for patients with resectable gastric cancer
contributed to an increase in the locoregional control rate, but
did not increase the OS rate. Further study is required to optimize
the implementation of adjuvant IORT for gastric cancer with regard
to patient selection and integration with systemic therapy.
Acknowledgements
The present study was funded by a research grant
from the Scientific Research Found Projects of Shanghai Health
Bureau (grant no. 20124246) and a research grant from the National
Nature Science Foundation of China (grant no. 81201883).
References
|
1
|
Hundahl SA, Phillips JL and Menck HR: The
National Cancer Data Base Report on poor survival of U.S. gastric
carcinoma patients treated with gastrectomy: Fifth Edition American
Joint Committee on Cancer staging, proximal disease, and the
‘different disease’ hypothesis. Cancer. 88:921–932. 2000.PubMed/NCBI
|
|
2
|
Gunderson LL: Gastric cancer - patterns of
relapse after surgical resection. Semin Radiat Oncol. 12:150–161.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oehler C and Ciernik IF: Radiation therapy
and combined modality treatment of gastrointestinal carcinomas.
Cancer Treat Rev. 32:119–138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
J Med. 345:725–730. 2001. View Article : Google Scholar
|
|
5
|
Scaife CL, Calvo FA and Noyes RD:
Intraoperative radiotherapy in the multimodality approach to
gastric cancer. Surg Oncol Clin North Am. 12:955–964. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calvo FA, Sole CV, Obregón R, et al:
Intraoperative radiotherapy for the treatment of resectable locally
advanced gastric adenocarcinoma: topography of locoregional
recurrences and long-term outcomes. Clin Transl Oncol. 15:443–449.
2013. View Article : Google Scholar
|
|
7
|
Fu S, Lu JJ, Zhang Q, Yang Z, Peng L and
Xiong F: Intraoperative radiotherapy combined with adjuvant
chemoradiotherapy for locally advanced gastric adenocarcinoma. Int
J Radiat Oncol Biol Phys. 72:1488–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Q, Tey J, Peng LH, et al: Adjuvant
chemoradiotherapy with or without intraoperative radiotherapy for
the treatment of resectable locally advanced gastric
adenocarcinoma. Radiother Oncol. 102:51–55. 2012. View Article : Google Scholar
|
|
9
|
Drognitz O, Henne K, Weissenberger C, et
al: Long-term results after intraoperative radiation therapy for
gastric cancer. Int J Radiat Oncol Biol Phys. 70:715–721. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Santoro E, Carlini M, Garofalo A, Carboni
F, Santoro R and Castelli M: Gastric cancer. Clinico-biological
updating and analysis of 400 operated cases. J Exp Clin Cancer Res.
17:175–185. 1998.PubMed/NCBI
|
|
11
|
Martínez-Monge R, Calvo FA, Azinovic I, et
al: Patterns of failure and long-term results in high-risk resected
gastric cancer treated with postoperative radiotherapy with or
without intraoperative electron boost. J Surg Oncol. 66:24–29.
1997.PubMed/NCBI
|
|
12
|
Moher D, Liberati A, Tetzlaff J and Altman
DGPRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: the PRISMA statement. BMJ. 339:b25352009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Henning GT, Schild SE, Stafford SL, et al:
Results of irradiation or chemoirradiation for primary
unresectable, locally recurrent, or grossly incomplete resection of
gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 46:109–118.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coquard R, Ayzac L, Gilly FN, et al:
Intraoperative radiation therapy combined with limited lymph node
resection in gastric cancer: an alternative to extended dissection?
Int J Radiat Oncol Biol Phys. 39:1093–1098. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Skoropad VY, Berdov BA, Mardynski YS and
Titova LN: A prospective, randomized trial of pre-operative and
intraoperative radiotherapy versus surgery alone in resectable
gastric cancer. Eur J Surg Oncol. 26:773–779. 2000. View Article : Google Scholar
|
|
21
|
Qin HL, Lin CH and Zhang XL: Evaluation of
intraoperative radiotherapy for gastric carcinoma with D2 and D3
surgical resection. World J Gastroenterol. 12:7033–7037.
2006.PubMed/NCBI
|
|
22
|
Ogata T, Araki K, Matsuura K, et al: A
10-year experience of intraoperative radiotherapy for gastric
carcinoma and a new surgical method of creating a wider irradiation
field for cases of total gastrectomy patients. Int J Radiat Oncol
Biol Phys. 32:341–347. 1995.
|
|
23
|
Abe M, Nishimura Y and Shibamoto Y:
Intraoperative radiation therapy for gastric cancer. World J Surg.
19:544–547. 1995.PubMed/NCBI
|
|
24
|
Sindelar WF, Kinsella TJ, Tepper JE, et
al: Randomized trial of intraoperative radiotherapy in carcinoma of
the stomach. Am J Surg. 165:178–187. 1993. View Article : Google Scholar
|
|
25
|
Fletcher GH: Clinical dose-response curves
of human malignant epithelial tumours. Br J Radiol. 46:1–12. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abe M, Takahashi M, Ono K, Tobe T and
Inamoto T: Japan gastric trials in intraoperative radiation
therapy. Int J Radiat Oncol Biol Phys. 15:1431–1433. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Withers HR, Peters LJ and Taylor JM:
Dose-response relationship for radiation therapy of subclinical
disease. Int J Radiat Oncol Biol Phys. 31:353–359. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Skandarajah AR, Lynch AC, Mackay JR, Ngan
S and Heriot AG: The role of intraoperative radiotherapy in solid
tumors. Ann Surg Oncol. 16:735–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miller RC, Haddock MG, Gunderson LL, et
al: Intraoperative radiotherapy for treatment of locally advanced
and recurrent esophageal and gastric adenocarcinomas. Dis
Esophagus. 19:487–495. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Calvo FA, Aristu JJ, Azinovic I, et al:
Intraoperative and external radiotherapy in resected gastric
cancer: updated report of a phase II trial. Int J Radiat Oncol Biol
Phys. 24:729–736. 1992. View Article : Google Scholar : PubMed/NCBI
|