Introduction
Lung cancer is the leading cause of cancer-related
mortality in numerous industrialized countries, with an incidence
that is increasing worldwide (1).
Platinum-based combination chemotherapy has been shown to improve
the survival and quality of life of patients with advanced
non-small-cell lung cancer (NSCLC), which accounts for ∼80% of all
lung cancers; however, its prognosis remains poor.
Pemetrexed (PEM) is a novel
pyrrolo[2,3-d]pyrimidine-based antifolate. It is transported into
the cells via the reduced folate carrier. Upon cell entry, PEM is
polyglutamylated to the activate pentaglutamate in a reaction
catalyzed by folylpolyglutamate synthase. PEM inhibits multiple
enzymes involved in pyrimidine and purine synthesis, including
thymidylate synthase, dihydrofolate reductase and glycinamide
ribonucleotide formyltransferase (2, 3).
Available data suggest that PEM is exclusively more effective in
non-squamous NSCLC compared to other non-platinum agents (4, 5).
Elevation of alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) levels has been commonly reported
in previous clinical studies of PEM (6–9). In
a randomized study of two different doses of PEM, the incidence of
≥grade 2 AST and ALT elevation was 29.8 and 34.2%, respectively, in
patients who received the standard dose (500 mg/m2) of
PEM (8). Despite the high
incidence of AST and ALT elevation, however, its effect on clinical
outcome has not been investigated. If carcinoma cells exhibit
characteristics similar to those of host cells, the therapeutic
effect may be predictable from the reaction of hepatocytes to
PEM.
In this study, we defined as liver toxicity (LT) any
grade of AST or ALT elevation from baseline and investigated the
association between LT and clinical outcome in patients with
non-squamous NSCLC who were treated with a PEM-containing
regimen.
Materials and methods
Patients
Between June, 2009 and June, 2012, 95 consecutive
patients with advanced NSCLC were treated with PEM or PEM plus a
platinum agent as first-line chemotherapy at the Department of
Respiratory Medicine, Kyoto University Hospital. Patients who had
received PEM-based chemotherapy as perioperative treatment were
excluded from the analysis. Staging was performed according to the
7th edition of TNM classification (10). This study was approved by the
institutional review board of Kyoto University and informed consent
was obtained from all patients prior to enrollment in this
study.
Evaluation of response and
survival
Tumor response was assessed by the response
evaluation criteria in solid tumors (RECIST, version 1.1) (11) every 2 cycles during chemotherapy
and then based on clinical practice. Survival data were obtained
through active follow-up based on the verification of the patients'
vital status up to March 1, 2012. Progression-free survival (PFS)
was defined as the time from the first administration of PEM to
disease progression or death from any cause.
Evaluation and definition of LT
The following biochemical parameters were evaluated:
AST, ALT, alkaline phosphatase (ALP), γ-glutamyl transpeptidase
(γ-GTP), total bilirubin (TBil) and renal function (estimated
creatinine clearance using the modified Cockcroft-Gault formula).
Each toxicity was graded according to the National Cancer Institute
Common Toxicity Criteria, version 4.0. The worst grade was
identified and toxicities were recorded following deterioration by
one or more grades compared to baseline. In this study, LT was
defined as any grade of AST or ALT elevation from baseline.
Statistical analysis
Statistical comparisons were performed using the
Student's t-test when data were normally distributed and
non-parametric analysis using the Wilcoxon signed-rank test
otherwise. The significance of the association between individual
clinical factors was evaluated using the χ2 or Fisher's
exact tests, as appropriate. A multivariate regression analysis was
conducted using the Cox proportional hazards model. The survival
rate was calculated by the Kaplan-Meier method and the statistical
significance of the differences was evaluated using the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using JMP 10.0.0 software (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 95 patients with NSCLC were included in
this analysis. The clinical characteristics of the patients are
summarized in Table I. The
patients comprised 51 men and 44 women, with a median age of 68
years. The performance status (PS) was generally good, with ∼92% of
the patients exhibiting a PS of 0 or 1. In total, 55 patients
(57.9%) were former or current smokers and 27 patients (28.4%) had
a history of habitual alcohol consumption. There were few patients
with positive hepatitis virus B (HBV) surface antigen, positive
hepatitis virus C (HCV) antibody, or fatty liver. The majority of
patients had adenocarcinoma histology and were diagnosed with stage
IV disease. Liver metastasis was recorded in 10 patients.
 | Table I.Patient characteristics (n=95). |
Table I.
Patient characteristics (n=95).
| Variables | Patient no.
(n=95) | % | Patient no. without
LT (n=28) | Patient no. with
LTa (n=67) | P-value |
|---|
| Gender |
|
|
|
|
|
| Male | 51 | 53.7 | 17 | 34 | 0.3728 |
|
Female | 44 | 46.3 | 11 | 33 |
|
| Age, years [median
(range)] | 68 (35–85) | − | 68 (41–85) | 68 (35–82) | 0.6184 |
| BMI, kg/m2
(m ean ± standard deviation) | − | − | 21.0±2.1 | 22.2±3.0 | 0.0540 |
| ECOG PS |
|
|
|
|
|
| 0-1 | 87 | 91.6 | 24 | 63 | 0.2290 |
| 2 | 8 | 8.4 | 4 | 4 |
| Smoking history |
|
|
|
|
|
|
Never | 40 | 42.1 | 10 | 30 | 0.4123 |
| Former +
current | 55 | 57.9 | 18 | 37 |
| History of alcohol
consumption |
|
|
|
|
|
| Yes | 27 | 28.4 | 8 | 19 | 0.9832 |
| No | 68 | 71.6 | 20 | 48 |
| HBV or HCV or
alcoholic hepatitis |
|
|
|
|
|
| Yes | 4 | 4.2 | 1 | 4 | 1.000 |
| No | 91 | 95.8 | 27 | 63 |
| Disease stage at the
beginning of the treatment |
|
|
|
|
|
| IIIA | 2 | 2.1 | 0 | 2 | 0.1789 |
| IIIB | 5 | 5.3 | 3 | 2 |
| IV | 88 | 92.6 | 25 | 63 |
| Liver metastasis |
|
|
|
|
|
| Yes | 10 | 10.5 | 6 | 4 | 0.0332b |
| No | 85 | 89.5 | 22 | 63 |
| Histology |
|
|
|
|
|
Adenocarcinoma | 93 | 97.9 | 27 | 66 | 0.5048 |
|
NOS/other | 2 | 2.1 | 1 | 1 |
| Treatment
regimen |
|
|
|
|
|
|
CBDCA+PEM | 59 | 62.1 | 16 | 43 | 0.8139 |
|
CDDP+PEM | 3 | 3.2 | 1 | 2 |
| PEM | 33 | 34.7 | 11 | 22 |
| Treatment course
[median (range)] | 4 (1–11) | − | 4 (1–6) | 5 (1–11) | − |
| Maintenance |
|
|
|
|
|
| Yes | 21 | 22.1 | 5 | 16 | 0.5125 |
| No | 74 | 77.9 | 23 | 51 |
| PEM dose intensity,
mg/m2/week (mean ± standard deviation) | 153.2±15.6 | − | 158.1±10.1 | 151.2±17.1 | 0.0478b |
| Baseline liver enzyme
elevationa |
|
|
|
|
|
| Yes | 13 | 13.7 | 5 | 8 | 0.5163 |
| No | 82 | | 86.3 | 23 | 59 |
| eCCr (m ean ±
standard deviation) | 78.9±30.9 | − | 74.2±23.9 | 80.9±33.4 | 0.3293 |
Frequency and severity of LT
Compared with the high incidence of AST (63.2%) or
ALT elevation (62.1%), the incidence of cholestatic enzyme
elevation was relatively low (ALP, 16.8%; γ-GTP, 37.9%; and TBil,
6.3%). As shown in Fig. 1, 47 of
the 67 patients who developed LT (70.1%) did so during the first
cycle of chemotherapy. All cases of grade 1 LT improved
spontaneously and there was no effect on subsequent PEM
administration. By contrast, of the 16 patients with ≥ grade 2 LT,
10 patients required a treatment delay or a dose reduction from the
subsequent cycle and PEM discontinuation was required in 1 patient
(Table II).
 | Table II.Characteristics of 16 patients with
grade 2 or higher AST or ALT elevation following chemotherapy with
PEM . |
Table II.
Characteristics of 16 patients with
grade 2 or higher AST or ALT elevation following chemotherapy with
PEM .
| Case no. | Gender | Age (years) | PS | Alcohol
history | Hepatic
comorbidity | Liver
metastasis | Regimen | Best response | Cycle at event | AST peak value
(IU/l) | ALT peak value
(IU/l) | Treatment
interruption due to liver dysfunction |
|---|
| 1 | F | 61 | 0 | No | No | No | CBDCA+PEM | SD | 2 | 101 | 150 | Yesa |
| 2 | F | 69 | 0 | No | HBV | No | CBDCA+PEM | PR | 1 | 73 | 155 | Yesb |
| 3 | F | 60 | 0 | No | HCV | Yes | CBDCA+PEM | PR | 1 | 100 | 102 | Yesb |
| 4 | F | 43 | 1 | No | No | No | CBDCA+PEM | SD | 2 | 75 | 94 | Yesb |
| 5 | M | 63 | 0 | No | No | No | CBDCA+PEM | PR | 1 | 85 | 160 | No |
| 6 | M | 64 | 0 | No | No | No | CBDCA+PEM | PR | 4 | 73 | 155 | Yesa |
| 7 | F | 51 | 0 | No | No | No | CBDCA+PEM | SD | 1 | 141 | 247 | Yesa |
| 8 | F | 74 | 0 | No | No | No | CBDCA+PEM | SD | 4 | 125 | 122 | Yesb |
| 9 | F | 74 | 1 | No | No | No | CBDCA+PEM | PD | 1 | 152 | 83 | Yesb |
| 10 | M | 62 | 0 | Yes | Fatty/liver | Yes | CBDCA+PEM | PR | 1 | 174 | 344 | Yesa |
| 11 | M | 35 | 0 | No | No | No | CBDCA+PEM | SD | 1 | 107 | 234 | Yesa |
| 12 | F | 39 | 0 | Yes | No | No | CBDCA+PEM | PR | 4 | 95 | 97 | No |
| 13 | M | 75 | 1 | Yes | No | No | PEM | PR | 1 | 94 | 130 | No |
| 14 | M | 70 | 1 | Yes | No | No | PEM | SD | 1 | 113 | 190 | Yesc |
| 15 | F | 82 | 0 | No | No | No | PEM | SD | 4 | 129 | 82 | No |
| 16 | F | 75 | 1 | Yes | No | No | PEM | PR | 3 | 223 | 160 | No |
Risk factors for AST and ALT
elevation
We investigated the association between LT and
clinical factors, such as gender, age, body mass index (BMI), PS,
disease stage, liver metastasis, alcohol consumption, hepatic
comorbidity, treatment regimen, estimated creatinine clearance,
dose intensity of PEM and baseline liver function, that may affect
the pharmacokinetics of PEM (Table
I). There was no significant difference between the LT and
non-LT groups, with the exception of liver metastasis and the dose
intensity of PEM: the incidence of liver metastasis and the dose
intensity were higher in the non-LT group (P=0.0332 and 0.0478,
respectively).
Efficacy
There were no recorded cases with complete response
(CR), 35 with partial response (PR), 45 with stable disease (SD)
and 15 with progressive disease (PD). The response rate (RR) and
disease control rate (DCR) were 36.8 and 84.2%, respectively.
The association between clinical characteristics,
including LT, and response to PEM, is shown in Table III. Younger age (<70 years),
good PS (<2) and a doublet regimen were significant positive
predictive factors for disease control (PR+SD) in the univariate
analysis. The incidence of LT was significantly higher among non-PD
patients compared to that in PD patients (P=0.0352). The RR and DCR
were 43.3 and 89.6%, respectively, in patients with LT and 21.4 and
71.4%, respectively, in patients without LT (RR, P=0.0387; DCR,
P=0.0352).
 | Table III.Association between clinical
characteristics and LT (n=95). |
Table III.
Association between clinical
characteristics and LT (n=95).
| Variables | No. of patients
with controlled disease (CR+PR+SD) (n=80) | No. of patients
with progressive disease (PD) (n=15) | P-value |
|---|
| Gender |
|
|
|
|
Male | 42 | 9 | 0.5916 |
|
Female | 38 | 6 |
| Age, years [median
(range)] | 67 (35–85) | 75 (64–84) |
|
<70 | 52 | 3 |
0.0011a |
|
≥70 | 28 | 12 |
| ECOG PS |
|
|
|
|
0-1 | 76 | 11 |
0.0199a |
| 2 | 4 | 4 |
| Smoking
history |
|
|
|
|
Never | 36 | 4 |
0.1773 |
| Former
+ current | 44 | 11 |
| Histology |
|
|
|
|
Adenocarcinoma | 78 | 15 |
1.000 |
|
Other | 2 | 0 |
| EGFR mutation
status |
|
|
|
|
Mutation-positive | 22 | 4 |
1.000 |
|
Wild-type or unknown | 58 | 11 |
| Disease stage |
|
|
|
|
III | 6 | 2 |
0.6080 |
| IV | 74 | 13 |
| Treatment
regimen |
|
|
|
|
Platinum+PEM | 58 | 4 |
0.0008a |
|
PEM | 22 | 11 |
| PEM dose intensity,
mg/m2/week (mean ± standard deviation) | 152.1±16.5 | 160.0±7.6 |
0.1863 |
| Presence of LT |
|
|
|
|
Yes | 60 | 7 |
0.0352a |
| No | 20 | 8 |
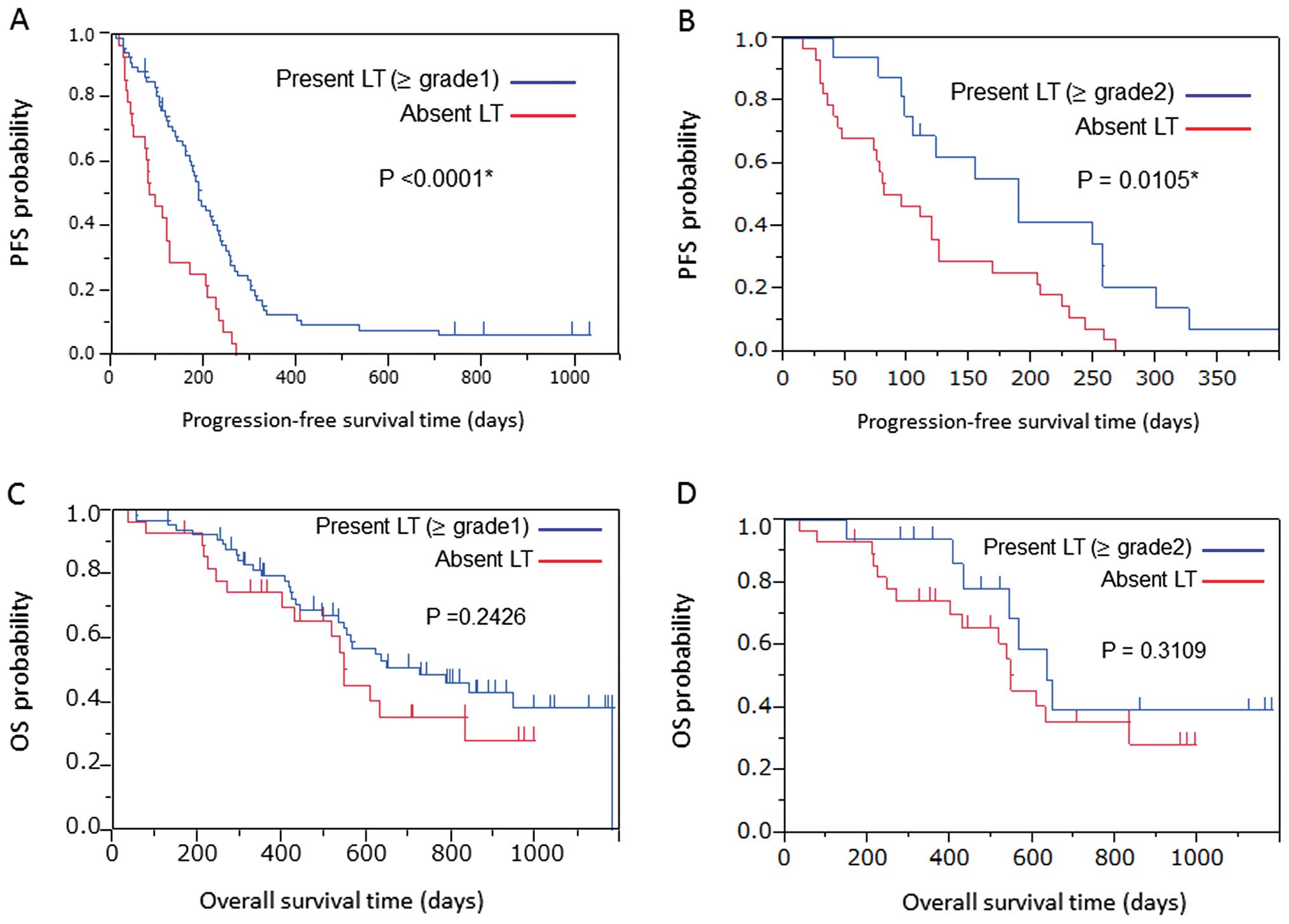
The median PFS of all patients was 5.6 months (95%
CI: 4.2-6.5 months). Patients with LT achieved a significantly
longer PFS compared to those without LT (6.3 vs. 2.9 months,
P<0.0001; Fig. 2A). Similarly,
the 16 patients with grade 2 or worse LT achieved a significantly
longer PFS compared to their counterparts (6.3 and 4.0 months,
respectively; P=0.0105; Fig. 2B).
Furthermore, the median survival time and 1-year survival rate from
the beginning of the treatment were 24.2 months and 79.6%,
respectively, in patients with grade 2 or worse LT vs. 18.3 months
and 74.3%, respectively, in their counterparts. There was no
difference in overall survival (OS) (P=0.2426 vs. P=0.3109;
Fig. 2C and D).
Subsequently, we conducted a Cox regression analysis
to determine the correlation between PFS and clinical factors such
as age (<70 vs. ≥70 years), gender (female vs. male), PS (0–1
vs. 2), epidermal growth factor receptor (EGFR) mutation status
(mutant vs. wild-type/unknown), disease stage (III vs. IV),
first-line therapy regimen (platinum plus PEM vs. PEM) and the
presence of LT (present vs. absent). Among these factors, the
presence of LT [hazard ratio (HR) = 0.389, 95% CI : 0.244-0.633 and
P=0.0002] exerted a significant positive effect on PFS based on the
univariate analysis. The multivariate analysis revealed that
platinum plus PEM therapy (HR = 0.438, 95% CI : 0.210-0.823 and
P=0.0119) and the presence of LT (HR = 0.341, 95% CI : 0.206-0.574
and P<0.0001) exerted a significant positive effect on PFS
(Table IV).
 | Table IV.Cox proportional hazards model
analysis of factors affecting progression-free survival (n=95). |
Table IV.
Cox proportional hazards model
analysis of factors affecting progression-free survival (n=95).
| A, Univariate
analysis |
|---|
| Factors | OR | 95% CI | P-value |
|---|
| Gender (female vs.
male) | 1.109 | 0.727-1.685 | 0.6289 |
| Age (<70 vs. ≥70
years) | 0.768 | 0.504-1.178 | 0.2237 |
| ECOG PS (0–1 vs.
≥2) | 0.800 | 0.396-1.916 | 0.5860 |
| EGFR mutation
status (positive vs. wild-type/unknown) | 1.235 | 0.762-1.942 | 0.3815 |
| Stage (III vs. IV
or postoperative recurrence) | 0.678 | 0.261-1.449 | 0.3402 |
| Chemotherapy
regimen (platinum+PEM vs. PEM alone) | 0.631 | 0.407-0.998 | 0.0492a |
| LT (present vs.
absent) | 0.389 | 0.244-0.633 | 0.0002a |
| B, Multivariate
analysis |
| Factors | OR | 95% CI | P-value |
| Gender (female vs.
male) | 1.295 | 0.824-2.029 | 0.2601 |
| Age (<70 vs. ≥70
years) | 1.189 | 0.675-2.145 | 0.5543 |
| ECOG PS (0–1 vs.
≥2) | 2.200 | 0.839-6.343 | 0.1109 |
| EGFR mutation
status (positive vs. wild-type/unknown) | 1.228 | 0.725-2.027 | 0.4366 |
| stage (III vs. IV
or postoperative recurrence) | 0.508 | 0.188-1.149 | 0.1087 |
| Chemotherapy
regimen (platinum+PEM vs. PEM alone) | 0.416 | 0.210-0.823 | 0.0119a |
| LT (present vs.
absent) | 0.341 | 0.206-0.574 |
<0.0001a |
Discussion
The incidence of LT in this study was 70.5%. This is
almost identical to that reported by previous clinical trials
(6, 8, 9).
Although approximately two-thirds of the patients who developed ≥
grade 2 LT required a treatment delay or a dose reduction from the
subsequent cycle, PEM was successfully continued, except in 1
patient. These results suggest that LT is common among patients
treated with PEM, although it is generally easily manageable. The
most interesting result in this study was that patients who
developed LT achieved a significantly higher RR and PFS compared to
those without LT.
PEM is primarily eliminated in the urine, with
70–90% of the dose recovered as the unchanged parent drug within
the first 24 h. It was hypothesized that PEM undergoes limited
hepatic metabolism and the precise mechanism of PEM-induced liver
injury has not been fully elucidated. The potential determinants of
PEM activity include : the folate receptor carrier, which is the
predominant route by which folates and several anti-folates gain
entry into the cells; γ-glutamyl hydrolase, which removes glutamyl
residues from polyglutamylated substrates, decreasing intracellular
activity and retention of PEM; thymidylate synthase, which is the
main target of this drug; and 5,10-methylenetetrahydrofolate
reductase (MTHFR), which has a major impact on the regulation of
the folic acid pathway (3,
12). These enzymes exist in liver
cells as well as in tumor cells; therefore, PEM may also affect
liver cells, leading to the development of LT.
The response of cancer cells to chemotherapy depends
on a sufficient amount of active drug reaching the target and on
whether that target is sensitive to the effects of the drug. These
factors may also apply to normal cells, such as liver cells. The
availability of the active drug to tumor or normal cells is
affected by the pharmacokinetics of the drug, which may produce a
similar effect in tumor and normal cells. However, no correlation
between the levels of AST or ALT and PEM pharmacokinetic parameters
has been reported thus far (13).
In the present study, we were unable to verify a correlation
between LT and the factors that affect the pharmacokinetics of PEM
(such as, dose intensity and estimated creatinine clearance)
(14).
The sensitivity of tumor and normal cells to
chemotherapeutic agents may also be affected by genetic
predisposition, which may similarly affect the two cell types.
According to a previous randomized phase II study comparing PEM
with PEM plus carboplatin (CBDCA), patients harbouring the C677T
homozygous mutation of MTHFR, a key enzyme involved in folate
metabolism, exhibited a prolonged PFS compared to patients with
wild-type or heterozygous MTHFR mutations (15). This type of mutation has been shown
to be associated with efficacy as well as toxicity in patients
receiving methotrexate (16). In
addition, Taniguchi et al (17) reported that the presence of MTHFR
677T was associated with an increased risk of ALT elevation in
patients with rheumatoid arthritis treated with antirheumatic
drugs. Those observations suggested that the polymorphism of genes
that encode enzymes involved in folate metabolism, transportation
and activation/inactivation may be associated with clinical outcome
and LT in patients with advanced NSCLC receiving PEM-based
therapy.
The present study had several limitations. Firstly,
there was no definite rule for the evaluation of LT and the
follow-up interval was based on clinical practice, due to its
retrospective nature. Secondly, a false correlation between LT and
patient outcome may have occured, since a higher incidence of LT
was expected with the increasing number of chemotherapy cycles and
patients surviving longer have a greater chance of receiving
additional cycles of chemotherapy; however, these effects appear to
be limited, as LT was mostly observed during the first 2 cycles in
this analysis. To address this issue, we applied a novel strategy,
restricting the primary analysis to patients who remained alive 30
days after the initiation of chemotherapy and the results did not
change (data not shown).
To the best of our knowledge, this is the first
study suggesting an association between LT and clinical outcome in
NSCLC patients treated with PEM. Further studies are required to
confirm this hypothesis.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schultz RM, Patel VF, Worzalla JF and Shih
C: Role of thymidylate synthase in the antitumor activity of the
multitargeted antifolate, LY231514. Anticancer Res. 19:437–443.
1999.PubMed/NCBI
|
|
3
|
Shih C, Chen VJ, Gossett LS, et al:
LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits
multiple folate-requiring enzymes. Cancer Res. 57:1116–1123.
1997.PubMed/NCBI
|
|
4
|
Scagliotti G, Hanna N, Fossella F, et al:
The differential efficacy of pemetrexed according to NSCLC
histology: a review of two phase III studies. Oncologist.
14:253–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scagliotti G, Brodowicz T, Shepherd FA, et
al: Treatment-by-histology interaction analyses in three phase III
trials show superiority of pemetrexed in nonsquamous non-small cell
lung cancer. J Thorac Oncol. 6:64–70. 2011.PubMed/NCBI
|
|
6
|
Clarke SJ, Abratt R, Goedhals L, Boyer MJ,
Millward MJ and Ackland SP: Phase II trial of pemetrexed disodium
(ALIMTA, LY231514) in chemotherapy-naive patients with advanced
non-small-cell lung cancer. Ann Oncol. 13:737–741. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manegold C, Gatzemeier U, von Pawel J,
Pirker R, Malayeri R, Blatter J and Krejcy K: Front-line treatment
of advanced non-small-cell lung cancer with MTA (LY231514,
pemetrexed disodium, ALIMTA) and cisplatin: a multicenter phase II
trial. Ann Oncol. 11:435–440. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohe Y, Ichinose Y, Nakagawa K, et al:
Efficacy and safety of two doses of pemetrexed supplemented with
folic acid and vitamin B12 in previously treated patients with
non-small cell lung cancer. Clin Cancer Res. 14:4206–4212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smit EF, Mattson K, von Pawel J, Manegold
C, Clarke S and Postmus PE: ALIMTA (pemetrexed disodium) as
second-line treatment of non-small-cell lung cancer: a phase II
study. Ann Oncol. 14:455–460. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldstraw P, Crowley J, Chansky K, et al
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schneider E and Ryan TJ: Gamma-glutamyl
hydrolase and drug resistance. Clin Chim Acta. 374:25–32. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McDonald AC, Vasey PA, Adams L, et al: A
phase I and pharmacokinetic study of LY231514, the multitargeted
antifolate. Clin Cancer Res. 4:605–610. 1998.PubMed/NCBI
|
|
14
|
Mita AC, Sweeney CJ, Baker SD, et al:
Phase I and pharmacokinetic study of pemetrexed administered every
3 weeks to advanced cancer patients with normal and impaired renal
function. J Clin Oncol. 24:552–562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smit EF, Burgers SA, Biesma B, Smit HJ,
Eppinga P, Dingemans AM, Joerger M, Schellens JH, Vincent A, van
Zandwijk N and Groen HJ: Randomized phase II and pharmacogenetic
study of pemetrexed compared with pemetrexed plus carboplatin in
pretreated patients with advanced non-small-cell lung cancer. J
Clin Oncol. 27:2038–2045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urano W, Taniguchi A, Yamanaka H, Tanaka
E, Nakajima H, Matsuda Y, Akama H, Kitamura Y and Kamatani N:
Polymorphisms in the methylenetetrahydrofolate reductase gene were
associated with both the efficacy and the toxicity of methotrexate
used for the treatment of rheumatoid arthritis, as evidenced by
single locus and haplotype analyses. Pharmacogenetics. 12:183–190.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taniguchi A, Urano W, Tanaka E, Furihata
S, Kamitsuji S, Inoue E, Yamanaka M, Yamanaka H and Kamatani N:
Validation of the associations between single nucleotide
polymorphisms or haplotypes and responses to disease-modifying
antirheumatic drugs in patients with rheumatoid arthritis: a
proposal for prospective pharmacogenomic study in clinical
practice. Pharmacogenet Genomics. 17:383–390. 2007. View Article : Google Scholar : PubMed/NCBI
|