Introduction
Primary germ cell tumors (GCTs) of the central
nervous system (CNS) in children are common in Asia, where they
account for 15–18% of all CNS tumors of childhood, compared to 3–5%
in the United States and Europe. The peak incidence for CNS GCTs is
at 10–12 years of age, although it varies by tumor histology and
differentiation, with non-germinomatous GCTs (NGGCTs) being more
common in younger children and pure germinomas being more common
among older patients. The majority of the CNS GCTs arise from
primordial germ cells in structures surrounding the third
ventricle. The majority of these tumors (94%) develop along the
midline, most often from the pineal gland (which produces primarily
NGGCTs), followed by tumors arising in the suprasellar cisterns
(which are most often germinomas) (1–3).
NGGCTs and mixed GCTs often consist of one or more
histopathological subtypes, such as mature teratoma, immature
teratoma, teratoma with malignant transformation, embryonal
carcinoma, yolk sac tumor and choriocarcinoma. Cranial NGGCTs are
generally associated with worse outcomes compared to cranial
germinomas (4). Surgery followed
by radiochemotherapy has achieved excellent survival rates for
patients with intracranial germinomas (5–10).
Suprasellar germinomas commonly present as diabetes insipidus,
visual field defects and hypothalamic-pituitary failure (11, 12). The pathological diagnosis is
performed surgically and complete or partial resection is often
possible with advanced neurosurgical techniques.
Reports on the treatment and long-term outcomes for
patients with suprasellar germinomas, in particular, are rare. The
optimal treatment for suprasellar germinomas and NGGCTs remains an
open question and studies on survival or factors that may predict
better or worse outcomes for patients with these relatively rare
tumors are sparse. In this study, we aimed to retrospectively
review cases of pathologically confirmed suprasellar GCTs treated
with surgery followed by radiotherapy, with or without
chemotherapy, at a single institution. Our goal was to identify
factors predictive of overall survival (OS) and progression-free
survival (PFS) in such cases.
Materials and methods
Clinical data
We retrospectively identified 23 consecutive
patients with suprasellar GCT treated at a single institution
between April, 1987 and October, 2008. All the patients had
undergone exploratory craniotomy with tumor resection and the
diagnosis of GCT was pathologically confirmed according to the
criteria of the World Health Organization (13). Computed tomography (CT) and
magnetic resonance imaging (MRI) were used prior to
radiochemotherapy to confirm the absence of lesions outside the
suprasellar region. The tumors of 5 patients had NGGCT components
(2 with immature teratoma and embryonal carcinoma, 1 with embryonal
carcinoma, 1 with mature teratoma and 1 with immature teratoma).
The median age of the 23 patients (9 male and 14 female) was 20
years (range, 9–34 years). The patient characteristics are
summarized in Table I.
 | Table I.Patient characteristics and hazard
ratios from univariate analyses for overall and progression-free
survival. |
Table I.
Patient characteristics and hazard
ratios from univariate analyses for overall and progression-free
survival.
| | Univariate
analysis |
|---|
| |
|
|---|
| Variables | No. of patients | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Gender | | | | |
| Male | 9 |
0.470 | (0.105-2.106) |
0.324 |
|
Female | 14 | | | |
| Age (years) | | | | |
|
<10 | 1 |
0.964 | (0.848-1.095) |
0.570 |
|
10-20 | 12 | | | |
|
21-30 | 9 | | | |
|
>30 | 1 | | | |
| Lesion size (cm) | | | | |
| 2-4 | 17 |
12.183 | (2.230-66.571) |
0.004 |
|
>4 | 6 | | | |
| Extent of surgical
resection | | | | |
| Total or
subtotal resection | 6 |
2.422 | (0.291-20.161) |
0.413 |
| Partial
resection or biopsy | 17 | | | |
| Pathological
classification | | | | |
| Pure
germinoma | 18 |
8.500 | (1.848-39.099) |
0.006 |
| Mixed
germ cell tumor | 5 | | | |
| Radiotherapy
technique | | | | |
|
Whole-brain + boost | 6 |
0.827 | (0.159-4.295) |
0.821 |
|
Craniospinal + boost | 17 | | | |
| Radiation dose
(Gy) | | | | |
|
<50 | 6 |
0.316 | (0.094-1.058) |
0.062 |
|
50-55 | 12 | | | |
|
>55 | 5 | | | |
| Combined modality
therapy | | | | |
|
Radiochemotherapy | 9 |
0.966 | (0.216-4.334) |
0.966 |
|
Radiotherapy | 14 | | | |
| Response to
therapy | | | | |
|
Complete | 15 |
3.392 | (0.752-15.301) |
0.112 |
|
Partial | 8 | | | |
Radiotherapy
All 23 patients underwent postoperative
radiotherapy; 17 patients (13 with pure germinoma and 4 with mixed
GCT) received craniospinal irradiation (CSI) and 6 patients
received whole-brain radiotherapy (WBRT), followed by a boost dose
to the primary tumor bed. Prior to 1995, radiation was delivered
from a cobalt-60 source, whereas linear accelerators were used from
1995 onwards [Varian 2100CD (1995–2008) and Siemens PRIMUS
accelerator (2004–2008)]. The dose range delivered via WBRT was
28–34 Gy (median, 32 Gy) and that delivered by CSI was 17.6-34.2 Gy
(median, 27.6 Gy); all radiation doses were delivered in 1.6- to
2.0-Gy fractions, 5 fractions per week. A total of 3 patients
received three-dimensional conformal radiotherapy (3D-CRT) or
intensity-modulated radiation therapy (IMRT) as a boost to the
tumor bed and 20 patients received a local boost by conventional
radiotherapy (boost dose to the tumor bed of 10–34 Gy, in 2-Gy
fractions; median dose, 23.3 Gy). The range of total doses to the
tumor bed was 39.6-56.8 Gy and the median dose was 51.9 Gy.
Chemotherapy
A total of 9 patients (7 with pure germinoma and 2
with mixed GCTs) received chemotherapy (range, 2–6 cycles); 6
patients received CSI and chemotherapy. All the patients received
intravenous cisplatin 30 mg/m2/day for 3 days every 3–4
weeks; 6 patients also received nimustine 2–3 mg/kg/day for 1 day
every 6 weeks and 3 received cisplatin plus the podophyllotoxin
derivative teniposide 100 mg/day for 3 days every 3–4 weeks.
Assessment of treatment
The extent of surgical resection was estimated from
enhanced CT or MRI scans obtained prior to and again typically 3
days after neurosurgery (the standard protocol at the study
institution). The patients were allocated total, subtotal and
partial resection or biopsy procedures. Total resection was defined
as complete removal of the visible tumor, subtotal resection
indicated a >90% volume reduction, partial resection involved a
50–90% volume reduction and biopsy indicated a <50% volume
resection. The responses to therapy were assessed according to the
Response Evaluation Criteria in Solid Tumors (RECIST) guidelines,
version 1.1 (14) as follows:
complete response, disappearance of all target lesions; partial
response (PR), ≥ 30% decrease in the sum of diameters of target
lesions; progressive disease (PD), ≥ 20% increase in the sum of
diameters of target lesions; and stable disease, neither sufficient
shrinkage to qualify for PR nor sufficient increase to qualify for
PD. Follow-up examinations following completion of radiotherapy
and/or chemotherapy were performed by CT or MRI every 3 months in
the first year, once every 6 months from the second to the third
year following completion of treatment and at 1-year intervals
thereafter.
Statistical analyses
Survival analyses were performed with the S tata
v10.0 software package (StataCorp, College Station, TX, USA). A Cox
proportional hazards model was used for univariate analyses to
identify factors predictive of survival; the variables entered in
the univariate analysis were patient gender, age, lesion size,
extent of surgical resection, pathological classification (pure
germinoma or mixed GCT), radiotherapy technique used, radiation
dose received, chemotherapy administration and response to therapy.
Forward selection was used to introduce variables into the Cox
proportional hazards model. P<0.05 was considered to indicate
statistically significant differences. The results of the Cox
proportional hazard analyses were used to assign patients into
good-prognosis or poor-prognosis groups. The Kaplan-Meier method
was used to estimate OS and PFS rates at 5 and 10 years for each
prognostic group and log-rank tests were used to identify
differences between the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient outcome
The median follow-up time was 12.3 years (range,
1.1-20.0 years). One patient was lost to follow-up after 12.5
years. The OS rates for all 23 patients were 82.6% at 5 and 72.9%
at 10 years. A total of 4 patients succumbed to recurrent disease
as follows: 1 patient developed recurrence at the primary site 1.2
years after radiochemotherapy; re-irradiation with local-field
radiotherapy at that time achieved a PR and the patient eventually
succumbed to the disease 3.1 years after the re-irradiation. The
second patient developed recurrent disease in the left basal
ganglia and spinal cord after radiotherapy, which was treated with
salvage chemotherapy; the patient died during chemotherapy 1.1
years after the radiotherapy. The third patient experienced
metastasis in the left orbit 3.5 years after WBRT plus partial
brain irradiation; at that time, the left orbit was treated with 30
Gy and the patient succumbed to the disease 2 years after the
re-irradiation. The fourth patient experienced multiple metastases
in the pineal region, right temporal lobe and lateral ventricle,
declined further treatment and succumbed to disseminated disease
9.7 years after the radiochemotherapy.
Survival analysis
The univariate analysis of Cox proportional hazards
model revealed that two factors affected survival rates, namely
lesion size (2–4 vs. >4 cm) and pathological classification
(pure germinoma vs. mixed GCT) (Table
I). Based on the results of this analysis, all the patients
were assigned to either a good-prognosis group (n=14, lesions 2–4
cm and pure germinomas) or a poor-prognosis group (n=9, lesions
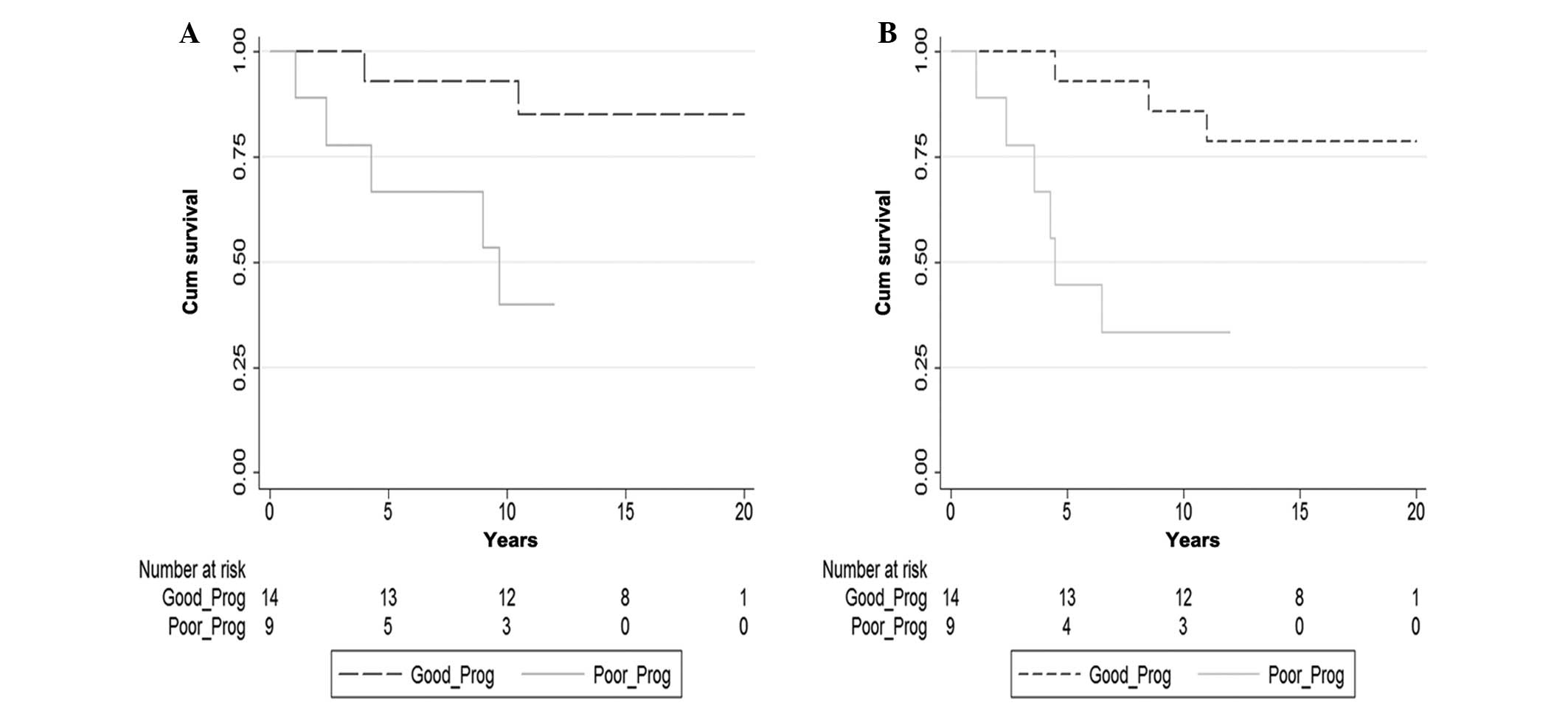
>4 cm and mixed GCTs). The OS rates at 5 and 10 years were both
92.9% in the good-prognosis group; the corresponding OS rates for
the poor-prognosis group were 66.7 and 40.0% (P=0.020) (Fig. 1A). The PFS rates at 5 and 10 years
in the good-prognosis group were 92.9 and 85.7%; the corresponding
PFS rates in the poor-prognosis group were 44.4 and 33.3% (P=0.007)
(Fig. 1B)
Discussion
The key findings from our study may be summarized as
follows: Tumor size and pathological subtype (pure germinoma vs.
mixed GCT) were the only factors that distinguished patients with
good from those with poor survival outcomes following adjuvant
radiotherapy or radiochemotherapy for suprasellar GCTs. These
factors were significant for both OS and PFS.
Previous reports on the survival rates for patients
with suprasellar germinoma have been somewhat sparse and the
majority involved small numbers of subjects and tumors of different
histologies treated with different types of therapy. A 1978 study
of 16 patients with biopsy-confirmed suprasellar germinoma treated
with surgical decompression and relatively high-dose radiotherapy
to the primary site reported a 5-year survival rate of 77%
(15). Our 5-year OS rate of 82.6%
for all patients was higher compared to that value, but lower
compared to the results of other studies, which may reflect the
inclusion of 5 patients with mixed GCTs in our study, who typically
have a worse prognosis than germinomas. Indeed, Jaing et al
(4) reported projected 5-year OS
and event-free rates of 92.6 and 92.6%, respectively, for 27
patients with germinomas vs. 47.3 and 42.1%, respectively, for 17
patients with NGGCTs; in addition, Hoffman et al (12) reported 5-year OS rates of 85.1% for
25 patients with germinomas and 45.5% for 13 patients with NGGCTs.
Other groups, when attempting to rank subtypes of NGGCTs in terms
of good, intermediate (immature teratoma, teratoma with malignant
transformation and mixed tumors with a main component of germinoma
or teratoma) and poor prognosis (other highly malignant tumors),
observed that survival rates were worse in the poor-prognosis group
compared to those in the other two groups (16, 17). These findings were similar to
ours.
Several groups have reported that only tumor
histology predicts outcome for tumors in the pineal region (i.e.,
NGGCTs), whereas patient age, gender, type of surgical procedure,
radiotherapy field and tumor dose do not. The same groups have also
reported that definitive radiotherapy may control germinomas, but
that a more aggressive approach is required for local control of
NGGCTs; they further recommend that WBRT or CSI be considered for
locally advanced tumors (4,
18, 19). In one review of 32 patients with
NGGCT, tumor histology and the use of CSI predicted relapse-free
survival and CSI was found to be significantly associated with OS.
The authors of that review concluded that combined-modality therapy
including surgery, radiotherapy and chemotherapy was effective for
treating NGGCTs and that CSI should be considered for patients with
highly malignant non-teratoma NGGCTs, as most treatment failures in
that group occurred in the cerebrospinal fluid (17). Indeed, another study reported that
the administration of salvage CSI was the only significant factor
in the multivariate analysis for predicting survival following
recurrence of intracranial germinomas (P=0.03), concluding that
CSI, with or without chemotherapy, may be an effective salvage
strategy for disease that recurs following reduced-volume
radiotherapy (20). Another study
reported that large (≥4 cm) or multifocal intracranial disease were
independent risk factors for spinal recurrence in intracranial
germinoma, but that radiation fields, dose and use of chemotherapy
did not predict spinal recurrence (21). Another univariate analysis of
treatment outcomes in 84 patients with intracranial germinoma
demonstrated that tumors sized <3 cm were associated with better
prognosis, but that patient age, gender, tumor location, treatment
volume, radiation dose to both the primary tumor and the spinal
cord and the extent of surgical resection were not (22). Thus, there exists some controversy
as to which factors predict survival for patients with intracranial
germinoma. Our univariate analyses indicated that the only factors
affecting OS and PFS were lesion size and pathological
classification (P<0.05), findings that are consistent with most
of the literature published to date. With the advent of 3D-CRT and
IMRT, whole-ventricular irradiation (WVI) is increasingly used to
treat intracranial germinomas. WVI administered as IMRT may spare
significant amounts of normal CNS tissue compared to WVI
administered as 3D-CRT or with WBRT for the treatment of CNS GCTs
(23). Chen et al (24) reported that WVI with a primary
boost without chemotherapy was sufficient for the treatment of
non-disseminated intracranial germinomas, even with a lower primary
radiation dose (<36 Gy). However, Khatua et al (25) and Paximadis et al (26) reported that neoadjuvant
chemotherapy followed by reduced-dose WVI and local boost
irradiation appeared to be effective for localized pure germinoma
of the CNS.
In conclusion, as the outcomes for patients with
NGGCTs in the suprasellar region are less favorable compared to
those for patients with pure germinomas, we recommend that
pathological confirmation be obtained for all suprasellar tumors,
so that treatment selection may be guided by the pathological
subtype. Indeed, several reports have indicated that the survival
of patients with biopsy-confirmed intracranial germinomas was
superior to that of unbiopsied patients with a presumptive
diagnosis of germinoma (18,
27). No standard treatment has
been established for NGGCTs. Histological confirmation may help
select the most effective treatment strategy and OS rates may be
stratified by histological subtypes (28). As NGGCTs are refractory to
conventional irradiation, radiotherapy alone is not recommended for
NGGCTs. Chemotherapy in conjunction with postoperative radiotherapy
may achieve better outcomes compared to radiation alone (12, 29,
30).
Ackowledgements
This study was supported by the Hunan Province
Development and Reform Committee Science Research Fund (grant no.
2010-1060), the Hunan Province Science and Technology Program
(grant no. 2011SK3223), the Hunan Provincial Natural Science
Foundation of China (grant no. 2012JJ5043) and The Project of New
Clinic Techniques of Central South University, China. We would like
to thank Christine F. Wogan in the Division of Radiation Oncology
at MD Anderson Cancer Center for editorial assistance.
References
|
1
|
Jia G, Luo SQ, Li CD and Ma ZY: Long-term
effect of chemotherapy combined with radiotherapy in treatment of
intracranial germinoma: report of 39 cases. Chin Med J. 83:198–200.
2003.(In Chinese).
|
|
2
|
Echevarria ME, Fangusaro J and Goldman S:
Pediatric central nervous system germ cell tumors: a review.
Oncologist. 13:690–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang HW, Wu YH, Hsieh JY, et al: Pediatric
primary central nervous system germ cell tumors of different
prognosis groups show characteristic miRNome traits and chromosome
copy number variations. BMC Genomics. 11:1322010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaing TH, Wang HS, Hung IJ, et al:
Intracranial germ cell tumors: a retrospective study of 44
children. Pediatr Neurol. 26:369–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huh SJ, Shin KH, Kim IH, Ahn YC, Ha SW and
Park CI: Radiotherapy of intracranial germinomas. Radiother Oncol.
38:19–23. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aoyama H, Shirato H, Kakuto Y, et al:
Pathologically-proven intracranial germinoma treated with radiation
therapy. Radiother Oncol. 47:201–205. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogawa K, Shikama N, Toita T, et al:
Long-term results of radiotherapy for intracranial germinoma: a
multi-institutional retrospective review of 126 patients. Int J
Radiat Oncol Biol Phys. 58:705–713. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shim KW, Kim TG, Suh CO, et al: Treatment
failure in intracranial primary germinomas. Childs Nerv Syst.
23:1155–1161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eom KY, Kim IH, Park CI, et al: Upfront
chemotherapy and involved-field radiotherapy results in more
relapses than extended radiotherapy for intracranial germinomas:
modification in radiotherapy volume might be needed. Int J Radiat
Oncol Biol Phys. 71:667–671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho J, Choi JU, Kim DS and Suh CO:
Low-dose craniospinal irradiation as a definitive treatment for
intracranial germinoma. Radiother Oncol. 91:75–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jennings MT, Gelman R and Hochberg F:
Intracranial germ-cell tumors: natural history and pathogenesis. J
Neurosurg. 63:155–167. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoffman HJ, Otsubo H, Hendrick EB, et al:
Intracranial germ-cell tumors in children. J Neurosurg. 74:545–551.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rychly B, Sidlova H and Danis D: The 2007
World Health Organisation classification of tumours of the central
nervous system, comparison with 2000 classification. Cesk Patol.
44:35–36. 2008.(In Slovak). PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung DI, Harisiadis L and Chang CH:
Midline pineal tumors and suprasellar germinomas: highly curable by
irradiation. Radiology. 128:745–751. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanamori M, Kumabe T, Saito R, et al:
Optimal treatment strategy for intracranial germ cell tumors: a
single institution analysis. J Neurosurg Pediatr. 4:506–514. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JW, Kim WC, Cho JH, et al: A
multimodal approach including craniospinal irradiation improves the
treatment outcome of high-risk intracranial nongerminomatous germ
cell tumors. Int J Radiat Oncol Biol Phys. 84:625–631. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chao CK, Lee ST, Lin FJ, Tang SG and Leung
WM: A multivariate analysis of prognostic factors in management of
pineal tumor. Int J Radiat Oncol Biol Phys. 27:1185–1191. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wolden SL, Wara WM, Larson DA, Prados MD,
Edwards MS and Sneed PK: Radiation therapy for primary intracranial
germ-cell tumors. Int J Radiat Oncol Biol Phys. 32:943–949. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu YW, Huang PI, Wong TT, et al: Salvage
treatment for recurrent intracranial germinoma after reduced-volume
radiotherapy: a single-institution experience and review of the
literature. Int J Radiat Oncol Biol Phys. 84:639–647. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogawa K, Yoshii Y, Shikama N, et al:
Spinal recurrence from intracranial germinoma: risk factors and
treatment outcome for spinal recurrence. Int J Radiat Oncol Biol
Phys. 72:1347–1354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shibamoto Y, Takahashi M and Abe M:
Reduction of the radiation dose for intracranial germinoma: a
prospective study. Br J Cancer. 70:984–989. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen MJ, Santos Ada S, Sakuraba RK, et al:
Intensity-modulated and 3D-conformal radiotherapy for
whole-ventricular irradiation as compared with conventional
whole-brain irradiation in the management of localized central
nervous system germ cell tumors. Int J Radiat Oncol Biol Phys.
76:608–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YW, Huang PI, Ho DM, et al: Change in
treatment strategy for intracranial germinoma: long-term follow-up
experience at a single institute. Cancer. 118:2752–2762. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khatua S, Dhall G, O'Neil S, et al:
Treatment of primary CNS germinomatous germ cell tumors with
chemotherapy prior to reduced dose whole ventricular and local
boost irradiation. Pediatr Blood Cancer. 55:42–46. 2010.PubMed/NCBI
|
|
26
|
Paximadis P, Hallock A, Bhambhani K, et
al: Patterns of failure in patients with primary intracranial
germinoma treated with neoadjuvant chemotherapy and radiotherapy.
Pediatr Neurol. 47:162–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kersh CR, Constable WC, Eisert DR, et al:
Primary central nervous system germ cell tumors. Effect of
histologic confirmation on radiotherapy. Cancer. 61:2148–2152.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sawamura Y: Current diagnosis and
treatment of central nervous system germ cell tumours. Curr Opin
Neurol. 9:419–423. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kochi M and Ushio Y: Chemo-radiotherapy
for malignant brain tumors. Cancer & chemotherapy. 29:669–676.
2002.(In Japanese).
|
|
30
|
Matsutani M: Pineal germ cell tumors. Prog
Neurol Surg. 23:76–85. 2009.PubMed/NCBI
|