Introduction
Small-cell lung cancer (SCLC) accounts for ∼15% of
all lung cancers (1) and is
characterized by a rapid tumor growth rate and early dissemination
to regional lymph nodes and to distant sites (2). The emergence of chemotherapy has
significantly improved quality of life and survival in SCLC
patients. However, long-term survival in SCLC patients has not been
satisfactory (3–5). Etoposide plus platinum (EP) is
currently the standard first-line treatment used in SCLC to obtain
longer overall survival (OS) and progression-free survival,
although numerous chemotherapeutic regimens comprising various
drugs have been investigated and tested in clinical trials
(6, 7).
Ifosfamide is considered to be effective for SCLC,
even when administered alone (8).
Previous studies demonstrated that regimens including ifosfamide
may achieve comparable response rates, survival and safety with the
standard chemotherapy regimens for SCLC (9–12).
Loehrer et al (13) and
Miyamoto et al (14) were
the first to apply ifosfamide in combination with the EP regimen
(IEP) for the treatment of SCLC. Particularly since the Hoosier
Oncology Group initiated a pilot trial to evaluate the efficacy of
IEP in extensive SCLC, reporting encouraging results in terms of
complete response rate (CRR) and median survival time (15), several clinical trials were
performed to further confirm the antitumor activity of ifosfamide
in SCLC when used in combination with standard chemotherapeutic
agents (16–20). Although discrepant conclusions have
been reported by different trials, the IEP regimen is generally
applied in SCLC patients according to the clinician's decision.
A meta-analysis reported that there was no strong
clinical evidence indicating an advantage of other platinum-based
regimens when compared to the EP regimen for patients with
extensive SCLC requiring chemotherapy. Moreover, a clinical study
comparing EP to the IEP regimen in SCLC was included in the
meta-analysis, with the IEP group exhibiting no significant
superiority to the EP group (6).
It has not been clearly determined whether the
addition of ifosfamide to standard regimens is necessary. In order
to evaluate the advantages and disadvantages of ifosfamide for
SCLC, we systematically searched all available published randomized
controlled trials (RCTs) comparing IEP and EP in SCLC. The aim of
this meta-analysis was to synthesize all evidence originating from
direct comparisons and assess the efficacy and tolerability of the
two regimens used as standard first-line treatment for previously
untreated SCLC.
Materials and methods
Search strategy
The Cochrane Library, Embase, MEDLINE and Chinese
Biomedical Literature databases were searched to identify all RCTs
comparing IEP to the EP regimen in patients with histologically
proven SCLC. The following keywords were used: ‘small-cell lung
cancer’ or ‘small-cell lung carcinoma’, ‘etoposide’ and
‘ifosfamide’. The search was limited to ‘randomized controlled
trials’. No limits based on language were imposed.
Selection of studies
Two investigators (H.Z.Y. and Y.M.) independently
assessed the retrieved articles. Any disagreements were resolved by
consensus. The studies included in the meta-analysis were required
to meet all the following criteria: i) the IEP and EP regimens were
compared in previously untreated SCLC patients; and ii) essential
information for the meta-analysis was reported in the RCTs. The
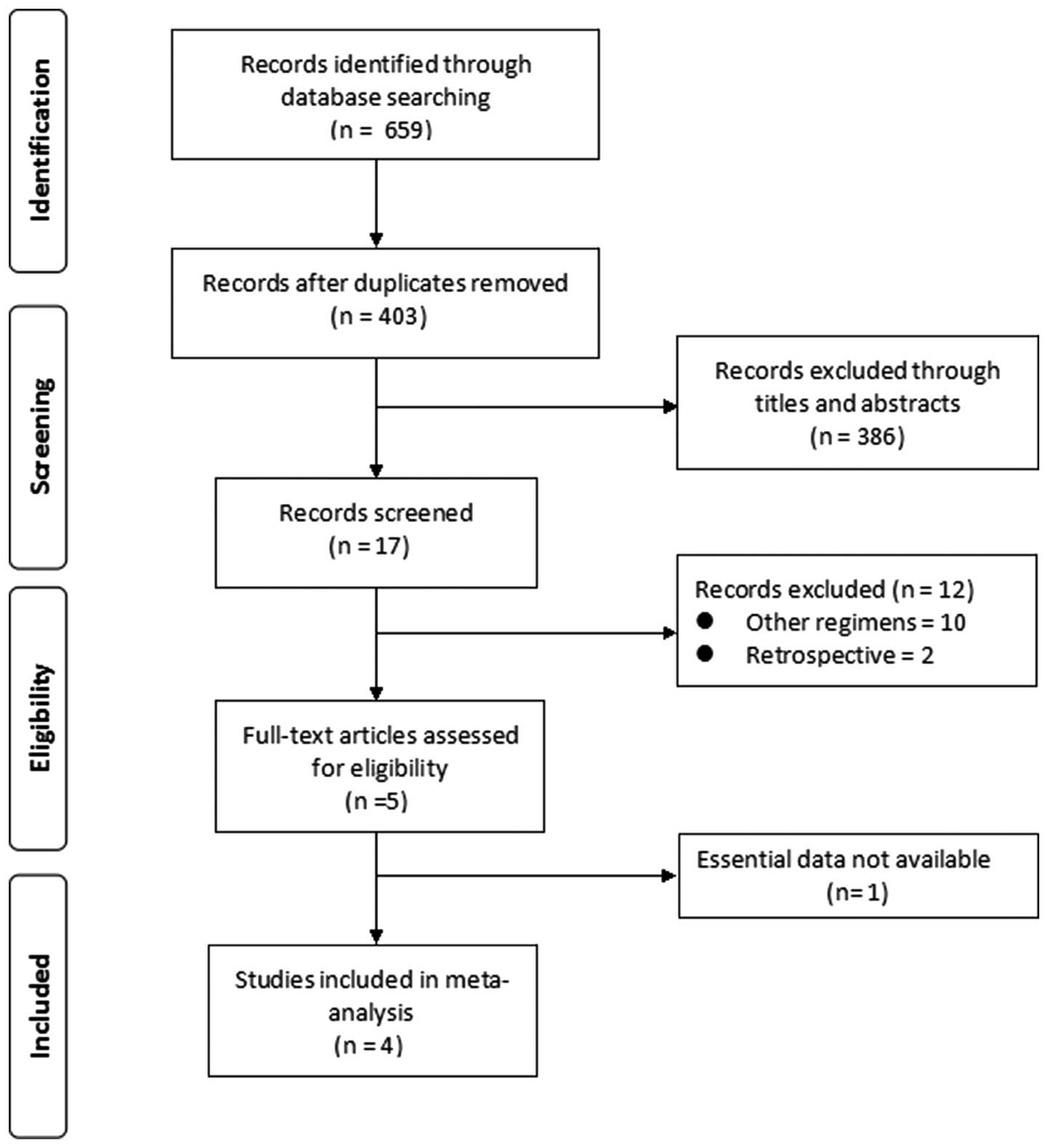
study selection process is summarized in Fig. 1.
Data extraction
The same investigators (H.Z.Y. and Y.M.)
independently extracted relevant data on study characteristics and
examination results by using a standardized form. To resolve any
disagreements between the reviewers, a third reviewer (L.J.M.)
assessed all discrepant items and the majority opinion was used for
analysis.
The quality items assessed were randomization,
allocation concealment, blinding (participants, investigators,
outcome assessors and data analysis) and completeness of
follow-up.
Outcome measures
The efficacy outcomes were CRR, objective response
rate and survival rate. Adverse effects included grade 3/4
hematologic altoxicity and grade 3/4 gastrointestinal toxicity.
Statistical analysis
Review Manager software, version 4.2.8 (The Cochrane
Collaboration) was used for statistical analyses. Relative risks
(RRs) and their 95% confidence intervals (CIs) were calculated
using the Mantel-Haenszel method for dichotomous outcomes. We
estimated heterogeneity between trials by using the Cochran's Q
statistic test and the I2 metric. The fixed-effects
model was generally used for the calculations, unless there was
significant heterogeneity, in which case the random-effects
statistical model was applied. To assess the sources of possible
variation in the study results, we performed a subgroup analysis.
Descriptive techniques were employed to assess adverse effects.
Results
Search results
Our search yielded 659 primary studies, of which 256
were duplicate trials, 10 trials referred to treatments other than
IEP or EP and 2 retrospective trials were detected. Of the 5 trials
considered eligible for assessment, 1 was excluded due to lack of
essential data for the meta-analysis. Finally, 4 RCTs, totaling 447
patients, were included in this meta-analysis (Fig. 1). The quality and characteristics
of the 4 included studies are summarized in Table I.
 | Table I.Characteristics of the 4 tr ia ls
comparing ifosfamide + etoposide + platinum with etoposide +
platinum in patients with previously untreated smal l- cell lung
cancer. |
Table I.
Characteristics of the 4 tr ia ls
comparing ifosfamide + etoposide + platinum with etoposide +
platinum in patients with previously untreated smal l- cell lung
cancer.
| Author | Sample size | Randomization | Allocation
concealment | Blinding | Completeness of
follow-up | Quality | (Refs.) |
|---|
| Miyamoto et
al | 92 | Adequate | Unclear | Unclear | Adequate | C | (14) |
| Loehrer et
al | 171 | Adequate | Adequate | Unclear | Adequate | B | (15) |
| Zhou et
al | 64 | Adequate | Unclear | Unclear | Adequate | C | (19) |
| Wu et al | 120 | Adequate | Unclear | Unclear | Adequate | C | (20) |
Meta-analysis results Response
Response rate was reported in the 4 trials (14, 15,
19, 20) and the meta-analysis indicated that
IEP was superior to the EP regimen in terms of overall response;
however, the difference was not significant (RR=1.07, 95% CI:
0.97-1.19). There was no heterogeneity (I2 =0%, P=0.50)
and the fixed-effects model was used to pool RR for overall
response. The results are shown in Fig. 2.
Survival rate
RRs for 1-year survival rate data were available
from 3 trials including 324 patients (14, 15,
19). The meta-analysis revealed
no significant difference (RR=1.22, 95% CI: 0.96-1.55) between the
IEP and EP regimens. There was no heterogeneity (I2 =0%,
P=0.81) and the fixed-effects model was used to obtain the pooled
RR for 1-year survival rate. The results are shown in Fig. 3.
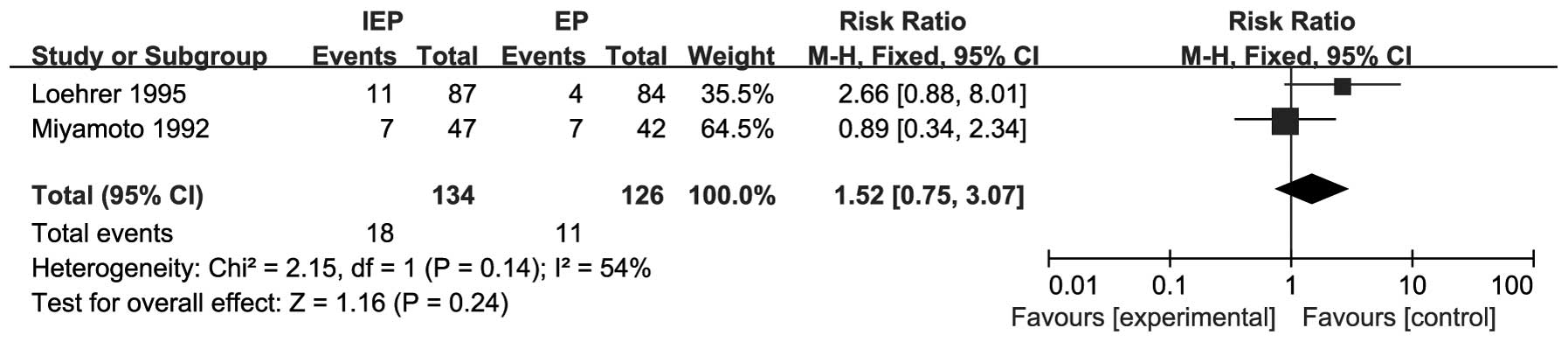
RRs for 2-year survival rate data were available
from 2 trials including 260 patients (14, 15). The pooled RR for the 2-year
survival rate revealed that IEP may prolong OS in SCLC patients;
however, the difference was not significant (RR=1.52, 95% CI:
0.75-3.07). There was significant heterogeneity (I2=54%,
P=0.14) and the pooled RR for 2-year survival rate was calculated
using the random-effects model. The results are shown in Fig. 4.
Adverse effects
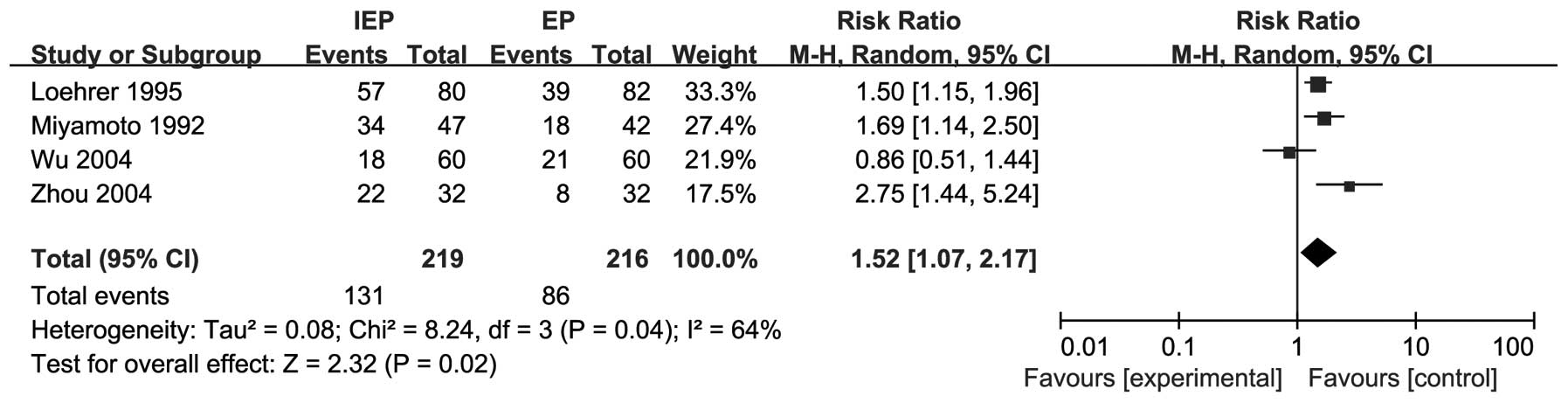
All 4 trials reported grade 3/4 hematological
toxicities and the IEP regimen was more frequently associated with
grade 3/4 neutropenia compared to the EP regimen (RR=1.52, 95% CI:
1.07-2.17). There was significant heterogeneity (I2
=64%, P=0.04) and the pooled RR for grade 3/4 neutropenia was
calculated using the random-effects model. The results are shown in
Fig. 5.
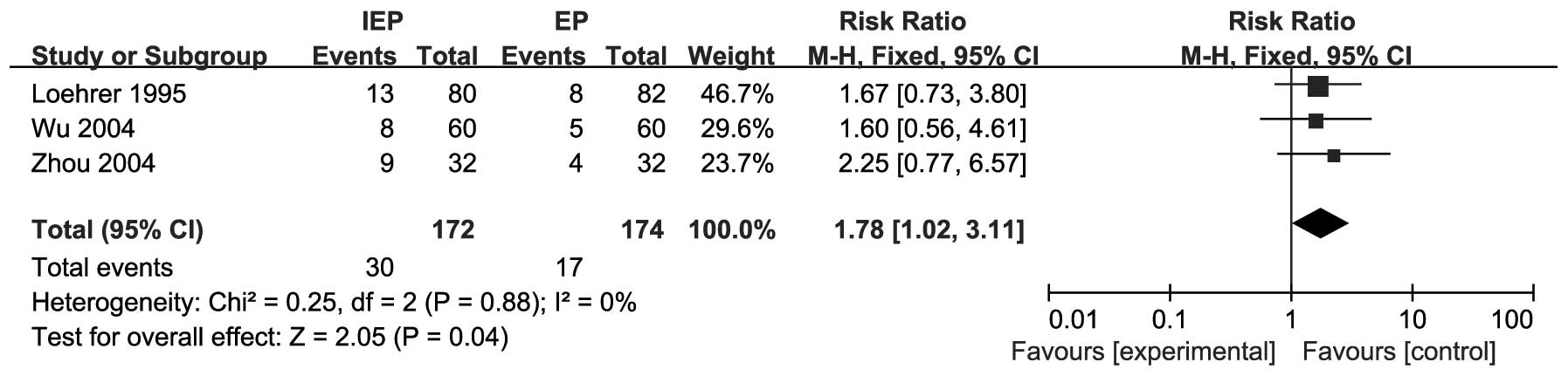
Non-hematological toxicities were reported in 3
trials (15, 19, 20). The pooled data demonstrated that
grade 3/4 vomiting was more common with IEP compared to the EP
regimen (RR=1.78, 95% CI: 1.02-3.11). There was no heterogeneity
(I2=0%, P=0.88) and the fixed-effects model was used to
obtain the pooled RR for grade 3/4 vomiting. The results are shown
in Fig. 6.
Discussion
Chemotherapy is an essential component of the
treatment of all SCLC patients. although the prognosis of SCLC has
improved significantly, long-term survival remains <10%.
According to the American National Comprehensive Cancer Network
guidelines, the most common standard first-line therapy for SCLC is
the EP regimen (1–5). To obtain an optimal response, more
regimens were investigated by adding new drugs to the standard
chemotherapy regimen and several clinical trials were conducted to
confirm the efficacy and safety of those new regimens in SCLC
(9–20).
The IEP regimen is considered as an alternative
first-line treatment option for SCLC by an increasing number of
physicians; however, it is our opinion that the superiority of IEP
to EP requires further clinical confirmation, as different RCTs
reported inconsistent results (14, 15,
19, 20). Loehrer et al (15) first reported a group study
comparing the IEP to the EP regimen in 163 patients with extensive
SCLC and observed significant differences in terms of
time-to-progression and OS in favor of the IEP arm. However,
Miyamoto et al (14)
reported that IEP was not superior to EP chemotherapy in SCLC.
Subsequently, Zhou et al (19) and Wu et al (20) further confirmed the results of
Loehrer et al in their clinical studies; in addition, Wu
et al (20) proved that IEP
may also used as salvage chemotherapy for patients with SCLC who
failed to respond to EP chemotherapy.
The objective of this meta-analysis was to compare
the efficacy and safety of IEP to that of EP in patients with
previously untreated SCLC. A total of 4 RCTs involving 447 patients
were included. Response rate and grade 3/4 neutropenia were
reported in all 4 trials. One-year survival rate was reported by 3
studies and 2-year survival rate by 2 studies. Grade 3
vomiting/nausea were reported by 3 studies. The meta-analysis
results demonstrated that IEP failed to achieve a higher response
rate and longer survival time, but induced more severe grade 3/4
neutropenia and vomiting compared to the EP regimen.
In conclusion, IEP was not found to be superior to
the EP regimen in the first-line treatment of SCLC; in addition, it
induced more severe hematological and gastrointestinal toxicities.
Therefore, SCLC patients did not obtain any benefit from the
addition of ifosfamide to the EP regimen and the role of ifosfamide
in multimodality treatment regimens requires further
investigation.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masters G: The clinical presentation of
small cell lung cancerLung Cancer: Principles and Practice. Pass
HI, Carbone DP, Johnson DH, Minna JD and Turrisi AT III: 3rd.
Lippincott Williams & Wilkins; Philadelphia, PA: pp. 304–314.
2005
|
|
3
|
Jackman DM and Johnson BE: Small-cell lung
cancer. Lancet. 366:1385–1396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turrisi AT III, Kim K, Blum R, et al:
Twice-daily compared with once-daily thoracic radiotherapy in
limited small-cell lung cancer treated concurrently with cisplatin
and etoposide. N Engl J Med. 340:265–271. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sorensen M, Pijls-Johannesma M, Felip E,
et al: Small-cell-lung cancer: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 21 (Suppl
5):v120–v125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sambrook RJ and Girling DJ: A national
survey of the chemotherapy regimens used to treat small cell lung
cancer (SCLC) in the United Kingdom. Br J Cancer. 84:1447–1452.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang L, Yang KH, Guan QL, et al:
Cisplatin plus etoposide versus other platin-based regimens for
patients with extensive small-cell lung cancer: a systematic review
and meta-analysis of randomised, controlled trials. Intern Med J.
42:1297–1309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brade WP, Herdrich K and Varini M:
Ifosfamide - pharmacology, safety and therapeutic potential. Cancer
Treat Rev. 12:1–47. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Havemann K, Wolf M, Drings P, et al:
Experience of a German multicenter study group with ifosfamide in
small cell lung cancer. Semin Oncol. 16 (Suppl 3):9–18.
1989.PubMed/NCBI
|
|
10
|
Thatcher N, Cerny T, Stout R, et al:
Ifosfamide, etoposide, and thoracic irradiation therapy in 163
patients with unresectable small cell lung cancer. Cancer.
60:2382–2387. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolf M, Havemann K, Holle R, et al:
Cisplatin/etoposide versus ifosfamide/etoposide combination
chemotherapy in small-cell lung cancer: a multicenter German
randomized trial. J Clin Oncol. 5:1880–1889. 1987.PubMed/NCBI
|
|
12
|
Evans WK, Stewart DJ, Shepherd FA, et al:
VP-16, ifosfamide and cisplatin (VIP) for extensive small cell lung
cancer. Eur J Cancer. 30A:299–303. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loehrer PJ, Rynard S, Ansari R, et al:
Etoposide, ifosfamide, and cisplatin in extensive small cell lung
cancer. Cancer. 69:669–673. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyamoto H, Nakabayashi T, Isobe H, et al:
A phase III comparison of etoposide/cisplatin with or without added
ifosfamide in small-cell lung cancer. Oncology. 49:431–435. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Loehrer PJ Sr, Ansari R, Gonin R, et al:
Cisplatin plus etoposide with and without ifosfamide in extensive
small-cell lung cancer: a Hoosier Oncology Group study. J Clin
Oncol. 13:2594–2599. 1995.PubMed/NCBI
|
|
16
|
Wolff AC, Ettinger DS, Neuberg D, et al:
Phase II study of ifosfamide, carboplatin, and oral etoposide
chemotherapy for extensive-disease small-cell lung cancer: an
Eastern Cooperative Oncology Group pilot study. J Clin Oncol.
13:1615–1622. 1995.PubMed/NCBI
|
|
17
|
Glisson B, Lee JS, Palmer J, et al:
Cisplatin, ifosfamide, and prolonged oral etoposide in the
treatment of patients with extensive small cell lung carcinoma.
Cancer. 82:301–308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hand S, Baker J, Smith AP, et al:
Outpatient intensive chemotherapy for small cell lung cancer: five
years experience of modified ‘ICE’ ifosfamide carboplatin and
etoposide. Clin Oncol (R Coll Radiol). 14:367–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou H, Wang A, Huang Z, et al: Randomized
clinical trial of IEP and EP regimens in the treatment of patients
with small cell lung cancer. Chin J Lung Cancer. 7:240–242.
2004.(In Chinese).
|
|
20
|
Wu C, Qi H, Dai Y, et al: Therapeutic
efficacy of chemotherapy with VIP for small cell lung cancer. Chin
J Lung Cancer. 7:151–153. 2004.(In Chinese).
|