Introduction
Radiotherapy is crucial for the management of head
and neck cancer, either as definitive or post-surgical adjuvant
treatment (1). Radiation-induced
mucositis is the most common toxicity, which often interrupts the
planned course of treatment and significantly reduces a patient's
quality of life (QOL) during the treatment (2). Amifostine has been reported to
prevent severe radiation-induced toxicities in head and neck cancer
patients and is the only radioprotectant that has been approved by
the US Food and Drug Administration (3). However, the daily use of amifostine
is limited due to its hematological and gastrointestinal toxicity.
Therefore, there is no standardized treatment for
chemoradiotherapy-induced mucositis.
Polaprezinc (PZ; Zeria Pharmaceutical Co., Ltd.,
Tokyo, Japan) is an antiulcer drug and a chelate compound
consisting of a zinc ion, L-carnosine, a β-alanine dipeptide and
L-histidine. It has been reported that PZ has antioxidant
properties and scavenges free radicals (4–7). We
previously reported the efficacy of PZ for acute radiation
proctitis in an animal model and demonstrated that PZ exerts
anti-inflammatory effects following radiation exposure (8). Watanabe et al (9) recently reported that PZ is effective
for mucositis induced by chemoradiotherapy in head and neck cancer
patients. Swallowing is often difficult for patients with
mucositis, including an oral rinse. In addition, the direct effects
of PZ on the mucosa remain unclear, although PZ is likely to exert
direct anti-inflammatory effects on the damaged mucosa (8). Therefore, we prepared a PZ oral rinse
using a new protocol, which yields a rinse with a good stability
and a stronger adhesion activity, to enhance the direct effects of
PZ on the oral and oropharyngeal mucosa (9).
In the present study, we investigated the
feasibility of the newly designed PZ oral rinse in head and neck
cancer patients in a prospective clinical trial and also assessed
the efficacy of PZ for radiation-induced mucositis in a
case-matched study with a larger number of patients.
Materials and methods
Patients
A total of 32 patients with newly diagnosed head and
neck cancer who underwent radiotherapy at the Hospital of Hyogo
College of Medicine between March, 2010 and February, 2013 were
enrolled in this prospective study [PZ (+) group]. Adult patients
with pathologically confirmed head and neck cancer, who were
scheduled to receive radical radiotherapy and who provided written
informed consent, were considered eligible for participation in
this study. Patients who received postoperative radiotherapy for
head and neck cancer were excluded. This study was approved by the
Ethics Committee of Hyogo College of Medicine (no. 791).
Preparation of the PZ oral rinse and
treatment schedule
The preparation of and treatment with the PZ oral
rinse was previously described in detail (10). In brief, PZ was specifically
prepared for the present study as an oral rinse using carboxyvinyl
polymer (HIVISWAKO105; Wako Pure Chemical Industries, Ltd., Osaka,
Japan) as a base. The pH was adjusted to ∼7.0 with sodium hydrogen
carbonate (Yoshida Pharmaceutical Co., Ltd., Tokyo, Japan). PZ was
suspended to provide a final concentration of 37.5 mg/10 ml,
according to the standard dose of PZ for the treatment of gastric
ulcer (150 mg/day). We confirmed the stability of the PZ oral rinse
prior to the treatment of patients as previously described
(10). A 10-ml aliquot of the PZ
oral rinse was used four times a day. The oral rinse was retained
for 1 min and was then spat out without swallowing. No ingestion of
food or drink was permitted for 30 min following treatment. The
treatment with the PZ oral rinse was repeated during the entire
course of radiotherapy. A mouthwash with sodium gualenate hydrate
was also concomitantly used, but no other mucosal protective agents
were permitted during this prospective study. Toxicity was assessed
according to the Common Terminology Criteria for Adverse Events,
version 3.0 (11).
Radiotherapy
All the patients were placed in the supine position
and scanned using an Aquilion LB computed tomography (CT) unit
(Toshiba, Ohtawara, Japan). The CT dataset was transferred to the
XiO treatment planning system (Elekta, Stockholm, Sweden), to
outline the volumes of interest. Radiotherapy was performed using a
three-dimensional conformal radiotherapy technique, which was
typically performed using 4-MV photons. The planned radiotherapy
was delivered with a dose of 200 cGy once daily using a linac
machine (Primus MD2; Siemens, Munich, Germany). Patients with oral
cancer received a total prescription dose of 60 Gy combined with
concurrent arterial infusion chemotherapy. Patients with laryngeal
and pharyngeal cancer received a total prescription dose of 66 Gy
combined with concurrent chemotherapy.
Case-matched analysis
To evaluate the efficacy of the PZ oral rinse, a
case-matched cohort analysis was performed in the present study.
Thirty patients with head and neck cancer who underwent
radiotherapy at the Hospital of Hyogo College of Medicine between
January, 2009 and January, 2010 were retrospectively reviewed based
on their medical records and analyzed [PZ (-) group] and compared
to the present cohort. The patients in the PZ (-) group underwent
radiotherapy using the same protocol as that used in the
prospective study of the PZ oral rinse. A mouthwash with sodium
gualenate hydrate was used as supportive treatment, but the
patients in the PZ (-) group did not receive PZ. One patient from
the prospective cohort who discontinued the PZ oral rinse on the
third day and dropped out of the chemoradiotherapy protocol due to
a severe gastric ulcer that developed during the fifth week was
excluded from the comparative analysis.
Statistical analysis
The data are expressed as medians and range, unless
otherwise indicated. A statistical analysis was performed to
compare the two groups. Parametric data were analyzed using a
t-test and non-parametric variables were analyzed by a two-tailed
Fisher's test. The Chi-square test was first used to assess the
trend in more than two groups. The Fisher's exact test was then
used to assess the significance of the differences between each
group and the other groups. The outcomes were estimated with
Kaplan-Meier statistics and compared using the log-rank test. The
Prism 6.0b software (Graph Pad Software, Inc., La Jolla, CA, USA)
was used for the statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Feasibility of the PZ oral rinse
A total of 32 patients, including 29 men and 3
women, were enrolled in this prospective study. The median patient
age was 67 years (range, 41–86 years). The patient characteristics
are listed in Table I. One patient
reported discomfort in the pharynx on the third day after treatment
initiation. One patient developed nausea on the 22nd day and
complained of the same symptom when sodium gualenate hydrate was
used as a mouthwash without PZ. Both patients discontinued the PZ
oral rinse. The remaining 30 patients (93.8%) reported no
complaints due to the PZ oral rinse and continued using the PZ oral
rinse during the entire course of radiotherapy. No elevated
transaminase levels were observed upon completion of the
radiotherapy course.
 | Table I.Characteristics of the patients in the
prospective study. |
Table I.
Characteristics of the patients in the
prospective study.
| Characteristics | No. (n=32) |
|---|
| Age, years |
|
|
Median | 67 |
|
Range | 41-86 |
| Gender |
|
| Male | 29 |
|
Female | 3 |
| Tumor location |
|
| Oral
cavity | 6 |
|
Nasopharynx | 9 |
|
Oropharynx | 5 |
|
Hypopharynx | 5 |
|
Larynx | 7 |
|
| Clinical stage |
|
| I | 6 |
| II | 7 |
| III | 7 |
| IVA | 11 |
| IVC | 1 |
| Pathological
type |
|
| Squamous
cell carcinoma | 31 |
|
Lymphoepithelial
carcinoma | 1 |
| Total radiotherapy
dose, Gy |
|
| 66 | 24 |
| 62 | 1 |
| 60 | 7 |
| Chemotherapy |
|
|
CBDCA | 8 |
| TXT | 18 |
|
CDDPa | 6 |
Efficacy of PZ for radiation mucositis
in head and neck cancer patients
The patient characteristics of the PZ (+) and PZ (-)
groups are listed in Table II.
There were no significant differences in gender, age, tumor
location or total dose of radiotherapy between the two groups. The
sequential changes due to acute radiation mucositis during the
course of radiotherapy are shown in Figs. 1 and 2. The PZ oral rinse significantly reduced
the incidence of symptoms and the macroscopic findings and promoted
recovery from radiation-induced mucosal damage, although no
significant differences were observed between the groups.
 | Table II.Characteristics of the patients in the
case-matched analysis. |
Table II.
Characteristics of the patients in the
case-matched analysis.
| Characteristics | PZ (+) (n=31) | PZ (-) (n=30) | P-value |
|---|
| Age, years |
|
|
0.87 |
|
Median | 67 | 67 |
|
|
Range | 41-86 | 21-80 |
|
| Gender |
|
|
0.47 |
| Male | 28 | 25 |
|
|
Female | 3 | 5 |
|
| Follow-up term,
months |
|
|
0.41 |
| Mean | 25 | 24 |
|
|
Range | 0-46 | 0-60 |
|
| Tumor location |
|
|
0.14 |
| Oral
cavity | 6 | 6 |
|
|
Nasopharynx | 9 | 2 |
|
|
Oropharynx | 5 | 4 |
|
|
Hypopharynx | 5 | 10 |
|
|
Larynx | 6 | 7 |
|
| Primary
unknown | 0 | 1 |
|
| Clinical stage |
|
|
0.20 |
| I | 5 | 2 |
|
| II | 7 | 5 |
|
| III | 7 | 8 |
|
| IVA | 11 | 14 |
|
| IVC | 1 | 1 |
|
| Pathological
type |
|
|
0.35 |
| Squamous
cell carcinoma | 30 | 27 |
|
|
Othersa | 1 | 3 |
|
| Total radiotherapy
dose, Gy |
|
|
0.17 |
| 66 | 24 | 18 |
|
| 60 | 7 | 12 |
|
| Chemotherapy |
|
|
0.0057 |
|
CBDCA | 8 | 2 |
0.081 |
| TXT | 17 | 16 |
1.00 |
|
CDDPb | 6 | 7 |
0.76 |
|
CDDP+5FU | 0 | 2 |
0.24 |
|
None | 0 | 3 |
0.11 |
PZ does not affect survival
outcome
PZ has been reported to reduce the reactive oxygen
species in the damaged mucosa (6).
The negative effects of PZ on cancer therapy have been less
extensively investigated, although the use of a radioscavenger may
reduce the efficacy of radiotherapy in patient with malignant
diseases. To address this issue, we analyzed and compared the
survival outcomes of patients treated with and without the PZ oral
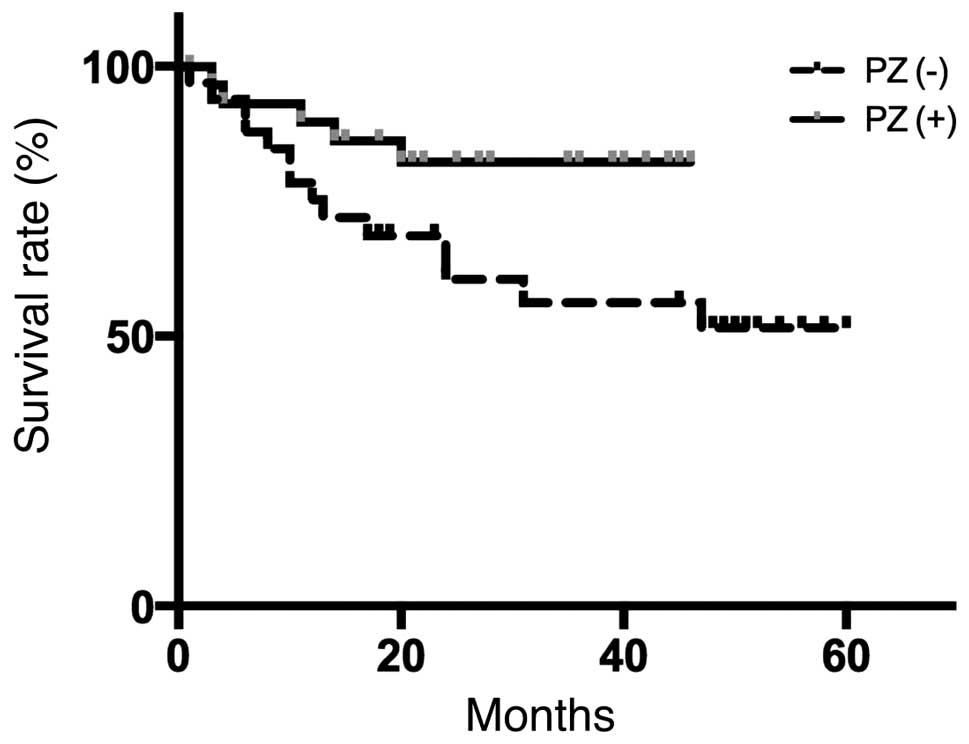
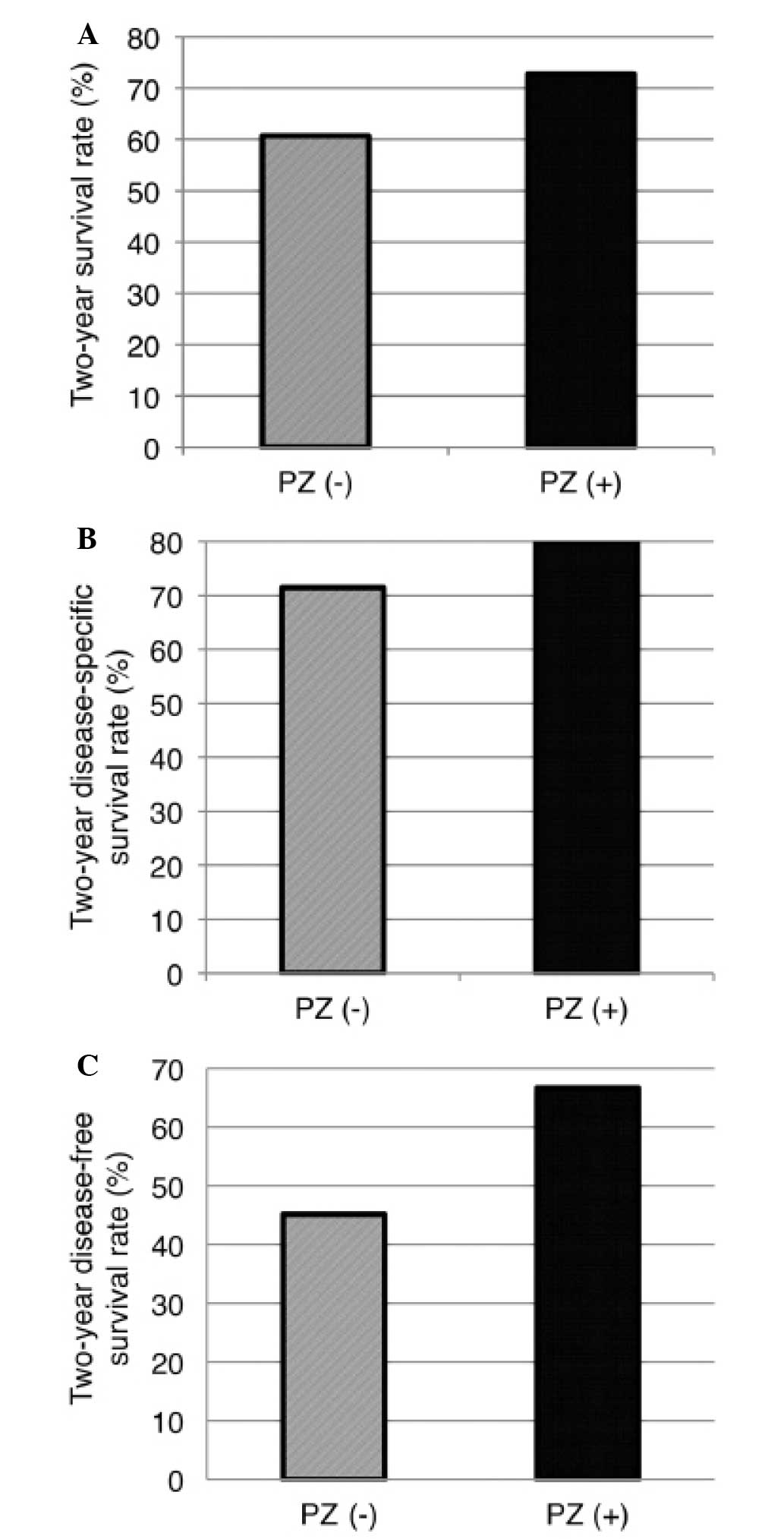
rinse. The overall patient survival is presented in Fig. 3 and the survival, disease-specific
survival and disease-free survival rates at 2 years after treatment
are shown in Fig. 4. There were no
significant differences in overall survival, 2-year survival,
2-year disease-specific survival or disease-free survival rates
during follow-up between the two groups. However, the PZ (+) group
exhibited a tendency for better survival outcomes. In addition, the
patients with a follow-up period of at least 1 year were analyzed
in terms of late toxicity. The incidence of osteonecrosis of the
jaw was 7.7 and 8.0% in the 26 patients in the PZ (+) group and in
the 25 patients in the PZ (-) group, respectively (P=0.97) (data
not shown).
Discussion
Although radiotherapy is highly effective for head
and neck cancer, painful oral mucositis frequently develops, which
results in compromised oral food intake, thus leading to a
requirement for enteral nutrition or intravenous hyperalimentation.
Benzydamin has been reported to reduce the frequency of severe
mucositis in head and neck cancer patients treated with lower doses
of radiotherapy in a randomized controlled clinical trial (11). However, there are no established
agents for the prevention of radiation-induced mucositis in
patients with head and neck cancer receiving radical radiation
therapy. We herein demonstrated that PZ oral rinse was highly
tolerable and exhibited a good efficacy against
chemoradiotherapy-induced oral mucositis in a higher number of head
and neck cancer patients compared to previous reports (9, 10).
The survival outcomes of patients who receive PZ as
supportive treatment are poorly understood (9). We were therefore concerned whether
the survival outcome would be affected by PZ, as it is known that
radioscavangers may decrease the efficacy of chemoradiotherapy. Of
note, the patients in the PZ (+) group exhibited better clinical
outcomes compared to those in the PZ (-) group. Watanabe et
al (9) reported that the oral
food intake during radiotherapy was significantly improved with PZ
treatment. Our data appear to be consistent with this previous
report. In our study, the findings suggested that the use of PZ
reduced the severity of mucositis induced by radiotherapy and
improved the QOL, without negatively affecting the clinical
outcomes. The improved QOL associated with PZ treatment was
associated with improved patient health as a result of better
nutrition. Therefore, the present study suggested that the use of
PZ in patients with malignancies is acceptable.
It has been reported that PZ exerts its effects via
a variety of cytoprotective mechanisms, including suppression of
lipid peroxidation, reduction of the levels of various cytokines,
inhibition of superoxide generation and promotion of the
restoration of the gastric mucosa (4–7). In
addition, PZ was recently reported to reduce radiation-induced
damage and exert anti-inflammatory effects on the normal rectum of
rats (8). Zinc supplementation has
been reported to decrease the severity of radiation-induced
mucositis in patients with head and neck cancer (12, 13). In addition, PZ, which is a chelate
compound consisting of a zinc ion, L-carnosine, a β-alanine
dipeptide and L-histidine, has been reported to be more effective
in terms of superoxide scavenging activity compared to zinc sulfate
(5). Therefore, the efficacy of PZ
for radiation-induced mucositis appears to be promising.
It was recently reported that PZ reduced the
severity of radiotherapy-induced mucositis in a randomized
controlled clinical trial (9).
However, the intake of PZ may have affected the results, since PZ
may also act as a zinc supplement. PZ was not swallowed in the
present study, in order to avoid the potential effects of PZ intake
and to directly evaluate the effects of PZ on the oral mucosa. The
PZ oral rinse was prepared with carboxyvinyl polymer as a base, in
order to increase its stability and adherence to the mucosa
(10). As a result, using the PZ
oral rinse without swallowing exhibited good efficacy against
radiation-induced mucositis and promoted mucosal healing in a
retrospective analysis with a larger number of patients. Therefore,
our data indicate that PZ exerts direct effects on the mucosa.
There were several limitations to the present study.
We reported the outcomes following radiotherapy for head and neck
cancer patients evaluated in a retrospective analysis. However, to
the best of our knowledge, our study included the largest number of
patients evaluated thus far and the treatment was performed at a
single institution with high consistency. Although the follow-up
period was insufficient to definitely evaluate survival outcome and
late toxicity, we were able to confirm that the outcome was not
inferior in patients who received the PZ oral rinse. Based on the
present study, the PZ oral rinse may reduce the severity of acute
mucositis caused by radiotherapy and significantly improve the
patients' QOL. To fully elucidate the efficacy of the PZ oral
rinse, future clinical trials with a longer follow-up period and a
prospective randomized controlled study are required.
In conclusion, we performed a clinical trial
comparing the effects of PZ on the incidence of severe oral
mucositis and the symptoms induced by radiotherapy in patients with
head and neck cancer. PZ was found to be highly effective in
reducing the severity of mucositis and contributed to a better QOL
for the patients, with no severe adverse effects and without
compromising the tumor response to radiotherapy.
Acknowledgements
The authors would like to thank Drs Kota Kida,
Takeshi Mohri, Kosuke Sagawa, Nobuhiro Uwa, Nobuo Saeki and
Tomonori Terada from the Department of Otolaryngology, Hyogo
College of Medicine (Nishinomiya, Hyogo, Japan) and Drs Kuniyasu
Moridera and Kazuma Noguchi from the Department of Oral and
Maxillofacial Surgery, Hyogo College of Medicine (Nishinomiya,
Hyogo, Japan). We would also like to thank Ms. Sayaka Takahashi and
Ms. Saori Kondo for their assistance with the data collection. This
study was supported in part by unrestricted funding from Zeria
Pharmaceutical Co., Ltd., (Tokyo, Japan).
References
|
1
|
National Comprehensive Cancer Network.
NCCN Guidelines Head and Neck Cancers version 2. 2014.
|
|
2
|
Trotti A, Bellm LA, Epstein JB, et al:
Mucositis incidence, severity and associated outcomes in patients
with head and neck cancer receiving radiotherapy with or without
chemotherapy: a systematic literature review. Radiother Oncol.
66:253–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gu J, Zhu S, Li X, Wu H, Li Y and Hua F:
Effect of amifostine in head and neck cancer patients treated with
radiotherapy: a systematic review and meta-analysis based on
randomized controlled trials. PLoS One. 9:e959682014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshikawa T, Naito Y, Tanigawa T, Yoneta T
and Kondo M: The antioxidant properties of a novel zinc-carnosine
chelate compound, N-(3-aminopropionyl)-L-histidinato zinc. Biochim
Biophys Acta. 1115:15–22. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshikawa T, Yamaguchi T, Yoshida N, et
al: Effect of Z-103 on TNB-induced colitis in rats. Digestion.
58:464–468. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Omatsu T, Naito Y, Handa O, et al:
Reactive oxygen species-quenching and anti-apoptotic effect of
polaprezinc on indomethacin-induced small intestinal epithelial
cell injury. J Gastroenterol. 45:692–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Opoka W, Adamek D, Plonka M, et al:
Importance of luminal and mucosal zinc in the mechanism of
experimental gastric ulcer healing. J Physiol Pharmacol.
61:581–591. 2010.PubMed/NCBI
|
|
8
|
Doi H, Kamikonya N, Takada Y, et al:
Efficacy of polaprezinc for acute radiation proctitis in a rat
model. Int J Radiat Oncol Biol Phys. 80:877–884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe T, Ishihara M, Matsuura K, Mizuta
K and Itoh Y: Polaprezinc prevents oral mucositis associated with
radiochemotherapy in patients with head and neck cancer. Int J
Cancer. 127:1984–1990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakayama M, Fujiwara M, Nakamura T, et al:
Stability of polaprezinc-containing oral rinse and its clinical
effectiveness against radiotherapy-induced oral mucositis. Iyakuhin
Johogaku. 15:133–138. 2013.
|
|
11
|
Palazzi M, Tomatis S, Orlandi E, et al:
Effects of treatment intensification on acute local toxicity during
radiotherapy for head and neck cancer: prospective observational
study validating CTCAE, version 3.0, scoring system. Int J Radiat
Oncol Biol Phys. 70:330–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Epstein JB, Silverman S, Paggiarino DA, et
al: Benzydamine HCl for prophylaxis of radiation-induced oral
mucositis: results from a multicenter, randomized, double-blind,
placebo-controlled clinical trial. Cancer. 92:875–885. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ertekin MV, Koc M, Karslioglu I and Sezen
O: Zinc sulfate in the prevention of radiation-induced
oropharyngeal mucositis: a prospective, placebo-controlled,
randomized study. Int J Radiat Oncol Biol Phys. 58:167–174. 2004.
View Article : Google Scholar : PubMed/NCBI
|