Introduction
Renal cell carcinoma (RCC) is characterized by a
highly resistant phenotype to conventional non-surgical therapeutic
modalities; therefore, immunotherapy with cytokines was the
previous mainstay of treatment for metastatic RCC (mRCC), although
only limited disease control was achieved with this treatment, with
a median overall survival (OS) of ~1 year (1). However, novel molecular-targeted agents
have been developed based on intensive research of the molecular
mechanisms underlying the progression of RCC and the recent
introduction of these agents has revolutionized the therapeutic
strategy against mRCC (2).
Of the several types of molecular-targeted agents,
tyrosine kinase inhibitors (TKIs), which mainly ACT BY inactivating
vascular endothelial growth factor (VEGF)-related pathways, are
considered to exhibit powerful antitumor activities against mRCC,
based on the outcomes of pivotal randomized clinical trials
(3–6).
Therefore, TKIs currently play a central role in the treatment of
patients with mRCC, particularly as first-line standard of care
(7). Furthermore, the excellent
antitumor activities of TKIs against mRCC were confirmed by various
studies evaluating these agents in routine clinical settings
(8–12). For example, Gore et al
(8) reported the efficacy and safety
profile of sunitinib in a global expanded-access trial for patients
with mRCC, with results similar to those of phase III clinical
trials. Considering these findings, it may be crucial to assess the
detailed prognostic effect of TKIs introduced as first-line agents
against mRCC in order to provide optimal patient counseling,
risk-directed therapy and clinical trial design in the era of
molecular-targeted therapy.
Three types of TKIs are applicable for patients with
TKI-naive RCC in Japan (13);
sorafenib and sunitinib were approved in 2008 and have been widely
recognized as efficacious systemic agents for the treatment of
mRCC, while pazopanib became available in 2014. However, there have
been no studies including a comparatively large number of Japanese
patients treated with TKIs as first-line therapy for mRCC.
Considering these findings, we retrospectively reviewed our
experience with the use of TKIs as first-line molecular-targeted
agents in a total of 271 consecutive Japanese mRCC patients in a
routine clinical setting and analyzed the oncological outcomes in
order to identify prognostic factors in this cohort of
patients.
Patients and methods
Patients
This study included a total of 271 consecutive
Japanese patients with TKI-naive mRCC who were treated with either
sorafenib or sunitinib as first-line molecular-targeted therapy for
≥2 months between April, 2008 and September, 2013 in a routine
clinical setting at our institutions. Of these 271 patients, 26
were not treated by radical nephrectomy, but underwent needle
biopsies of either the primary or metastatic tumor to determine the
histological subtype; therefore, all the included patients were
pathologically diagnosed with primary RCC. Prior to participation
in this study, informed consent was obtained from each patient and
the study design was approved by the Research Ethics Committee of
our institutions.
TKI administration
In this series, all the patients initially received
either 400 mg of sorafenib twice daily with a continuous dosing
schedule, or 50 mg of sunitinib once daily in repeated 6-week
cycles (4 weeks on, followed by 2 weeks off). Treatment with TKIs
was continued until disease progression or the development of
intolerable adverse events (AEs). As a rule, TKI dose modification
was conducted in cases with treatment-related GRADE ≥3 AEs as
follows: the dose of sorafenib was reduced from 800 to 400 mg once
daily, followed by additional dose reduction to a single 400 mg
dose every other day, while the dose reduction of sunitinib was
from 50 to 37.5 mg once daily and then to 25 mg once daily.
Informed consent was obtained prior to treatment modification for
all the patients.
Evaluation
As baseline evaluations, the clinicopathological
examination, performance status (PS) and risk classification were
assessed based on the union for international cancer control TNM
classification system, Karnofsky PS scale and Memorial
Sloan-Kettering Cancer Center (MSKCC) risk classification (14), respectively. Prior to the initiation
of TKI treatment, radiological evaluation of all the patients was
conducted by computed tomography (CT) SCANs of the brain, chest and
abdomen and radionuclide bone scan. As a rule, tumor measurements
were performed by CT prior TO and every 12 weeks following TKI
introduction. The response to treatment and the severity of the AEs
were analyzed by the treating physician based on the Response
Evaluation Criteria in Solid Tumors, version 1.0 and the National
Cancer Institute Common Terminology Criteria for Adverse Events,
version 3.0, respectively.
Statistical analysis
All the statistical analyses were performed using
Statview 5.0 software (Abacus Concepts, Inc., Berkeley, CA, USA)
and P<0.05 was considered to indicate a statistically
significant difference. The OS rates were calculated with the
Kaplan-Meier method and the differences were determined by the
log-rank test. The prognostic significance of certain factors was
assessed using the Cox proportional hazards regression model.
Results
Patient characteristics
The detailed baseline characteristics of the 271
patients included in this study are summarized in Table I. Of these 271 patients, 172 (63.5%)
and 99 (36.5%) were treated with sorafenib and sunitinib,
respectively. The tumor response to TKIs in the 271 patients was as
follows: 3 (1.1%), 47 (17.3%) and 182 (67.2%) patients exhibited a
complete response (CR), partial response (PR) and stable disease,
respectively, for ≥6 weeks; however, the remaining 39 patients
(14.4%) exhibited progressive disease. Therefore, the objective
response and clinical benefit rates on TKI treatment were 18.4 and
85.6%, respectively (data not shown).
 | Table I.Patient characteristics according to
overall survival. |
Table I.
Patient characteristics according to
overall survival.
|
| Overall survival |
|---|
|
|
|
|---|
| Characteristics | Total (n=271) | Deceased (n=126) | Alive (n=145) | P-value |
|---|
| Age, years (%) | | | | 0.40 |
| ≤65 | 126(46.5) | 62(49.2) | 64(44.1) | |
|
>65 | 145(53.5) | 64(50.8) | 81(55.9) | |
| Gender(%) | | | | 0.22 |
| Male | 215(79.3) | 104(82.5) | 111(76.6) | |
|
Female | 56(20.7) | 22(17.5) | 34(23.4) | |
| Prior
immunotherapy(%) | | | | 0.79 |
| Yes | 172(63.5) | 81(64.3) | 91(62.8) | |
| No | 99(36.5) | 45(35.7) | 54(37.2) | |
| MSKCC
classification(%) | | | | <0.001 |
| Favorable
or intermediate | 227(83.8) | 92(83.0) | 135(93.1) | |
| Poor | 44(16.2) | 34(27.0) | 10(6.9) | |
| C-reactive protein,
mg/dl(%) | | | | <0.001 |
| ≥1.0 | 187(69.0) | 61(48.4) | 126(86.9) | |
|
<1.0 | 84(31.0) | 65(51.6) | 19(13.1) | |
| Metastatic
organ(%) | | | | |
| Lung | 177(65.3) | 83(65.9) | 94(64.8) | 0.85 |
| Lymph
node | 69(25.5) | 43(34.1) | 26(17.9) | 0.0023 |
| Bone | 55(20.3) | 32(25.4) | 23(15.9) | 0.052 |
|
Liver | 31(11.4) | 25(19.8) | 6(4.1) | <0.001 |
|
Brain | 21(7.7) | 13(10.3) | 8(5.5) | 0.14 |
| Histological
subtype(%) | | | | 0.17 |
| Clear
cell carcinoma | 231(85.2) | 103(81.7) | 128(88.3) | |
|
Other | 40(14.8) | 23(18.3) | 17(11.7) | |
| Sarcomatoid
characteristics(%) | | | | 0.58 |
| Yes | 32(11.8) | 24(19.0) | 8(5.5) | |
| No | 239(88.2) | 102(81.0) | 137(94.5) | |
Overall survival
During the observation period of this study (median,
19 months; range, 2–64 months), 126 patients (46.5%) succumbed to
the disease. As shown in Fig. 1, the
median OS in this series was 33.1 months and the 1- and 3-year OS
rates were 77.3 and 48.8%, respectively. Table I shows the distribution of several
parameters according to the OS. The clinicopathological
characteristics that were significantly correlated with poor OS
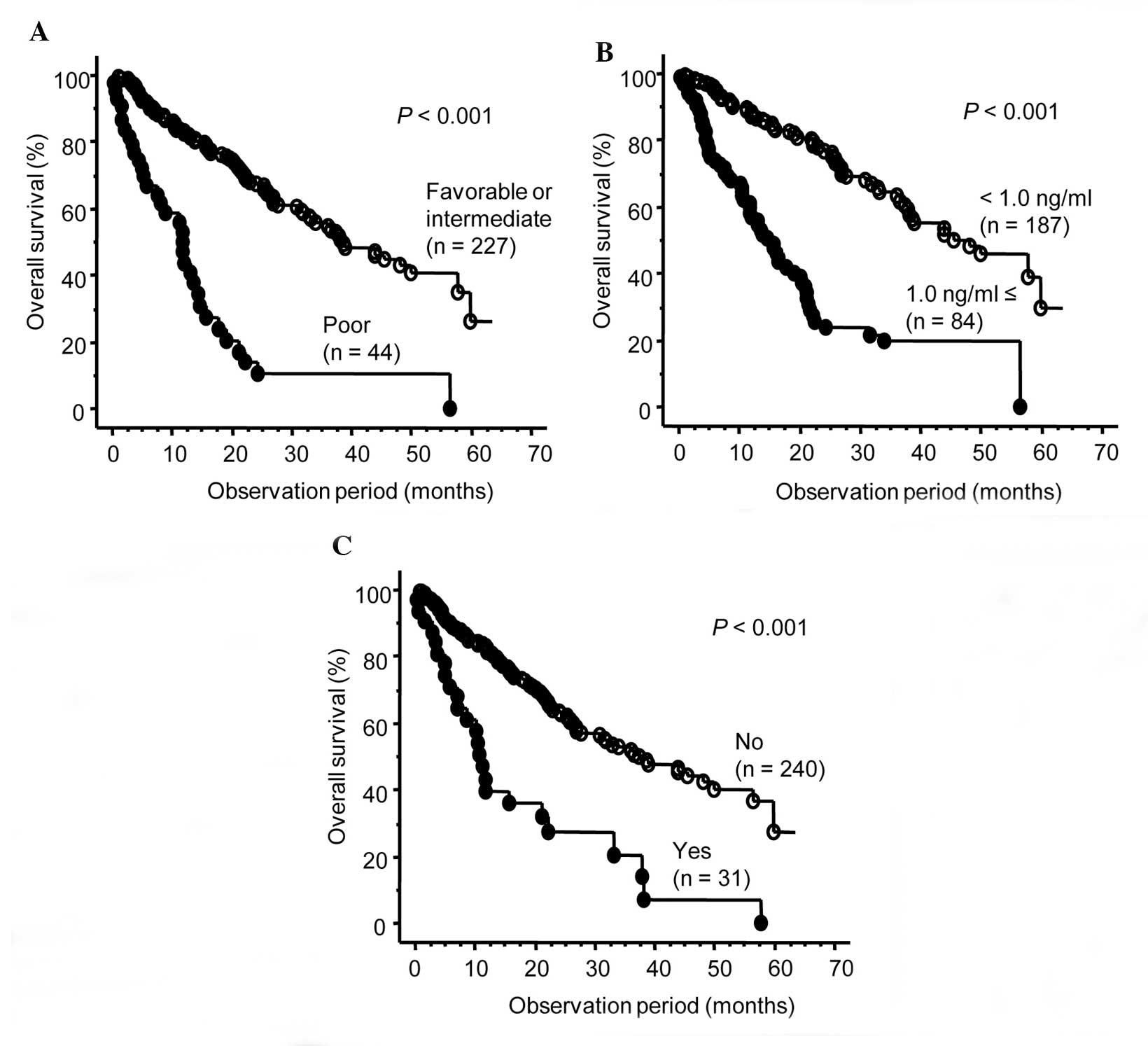
included a poor-prognosis group based on MSKCC classification,
elevated C-reactive protein (CRP) levels, presence of lymph node
metastasis and presence of liver metastasis.
Uni- and multivariate analyses of the
association between various factors and overall survival
The effect of several clinicopathological factors on
the OS in these 271 patients was analyzed by uni- and multivariate
analyses using the Cox proportional hazards regression model
(Table II). the univariate analysis
identified prior nephrectomy, MSKCC classification, CRP level,
lymph node, bone and liver metastases, histological subtype and
sarcomatoid characteristics as significant predictors of OS.
Furthermore, of these 7 factors, only MSKCC classification, CRP
level and liver metastasis were found to be independently
associated with OS in the multivariate analysis. The OS curves
according to these independent OS predictors are presented in
Fig. 2. There were significant
differences in OS with respect to all 3 factors.
 | Table II.Uni- and multivariate analyses of
association between various factors and overall survival in 271
patients with metastatic renal cell carcinoma who were treated with
tyrosine kinase inhibitor. |
Table II.
Uni- and multivariate analyses of
association between various factors and overall survival in 271
patients with metastatic renal cell carcinoma who were treated with
tyrosine kinase inhibitor.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | Hazard ratio | P-value | Hazard ratio | P-value |
|---|
| Age, years (<60
vs. ≥60) | 1.02 | 0.92 | – | – |
| Gender (male vs.
female) | 1.47 | 0.15 | – | – |
| Prior immunotherapy
(yes vs. no) | 1.37 | 0.10 | – | – |
| MSKCC classification
(poor vs. favorable or intermediate) | 4.31 | <0.001 | 1.73 | 0.038 |
| C-reactive protein,
mg/dl (≥1.0 vs. <1.0) | 3.69 | <0.001 | 2.80 | <0.001 |
| Lung metastasis (yes
vs. no) | 1.16 | 0.45 | – | – |
| Lymph node
metastasis (yes vs. no) | 1.59 | 0.014 | 1.12 | 0.57 |
| Bone metastasis
(yes vs. no) | 1.67 | 0.026 | 1.14 | 0.51 |
| Liver metastasis
(yes vs. no) | 3.08 | <0.001 | 2.37 | 0.0030 |
| Brain metastasis
(yes vs. no) | 1.09 | 0.78 | – | – |
| Histological
subtype (clear cell carcinoma vs. others) | 1.66 | 0.030 | 1.04 | 0.88 |
| Sarcomatoid
characteristics (yes vs. no) | 2.45 | <0.001 | 1.55 | 0.082 |
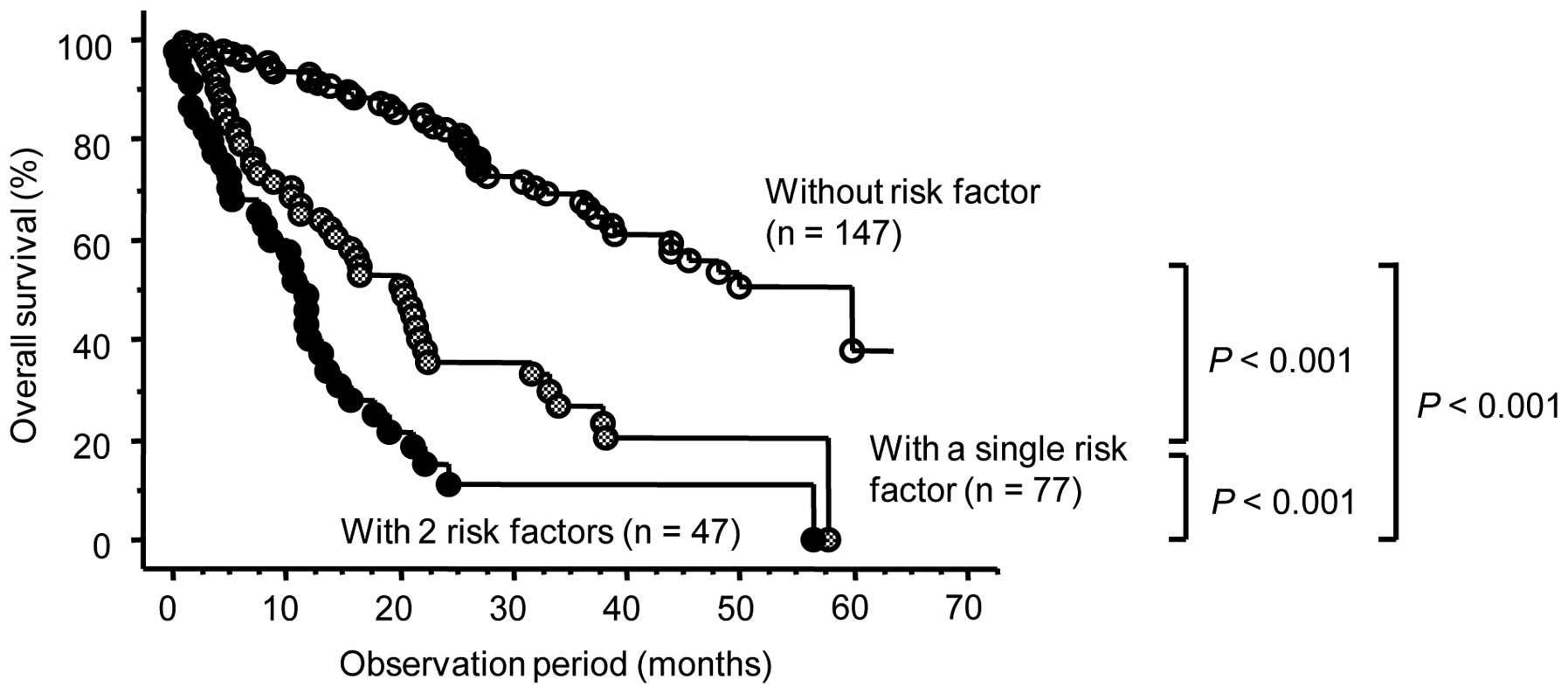
To more accurately predict OS in this patient
cohort, we classified the 271 patients into 3 groups based on
positivity for the 3 independent risk factors for OS identified by
the multivariate analysis. Overall, 46 of the 147 patients (31.3%)
who were negative for all 3 risk factors, 45 of the 77 patients
(58.3%) who were positive for a single risk factor and 35 of the 47
patients (74.5%) who were positive for 2 or 3 risk factors
succumbed to the disease. As shown in Fig. 3, there were significant differences in
OS among these 3 groups.
Discussion
As a result of the pivotal randomized phase III
clinical trials, TKIs are currently considered as a new reference
standard of care for the first-line treatment of mRCC, excluding
patients classified into the poor-prognosis group. For example,
sorafenib achieved significantly longer progression-free survival
(PFS) compared TO placebo in patients with TKI-naive mRCC following
failure of one systemic therapy, the majority of whom had received
cytokine-based treatment (3).
Similarly, sunitinib exhibited efficacy superior to that of
interferon-α (IFN-α) as first-line therapy for mRCC, with a median
PFS of 11 vs. 5 months, respectively (4). Furthermore, the therapeutic efficacy of
TKIs observed in clinical trials was subsequently confirmed in
several studies targeting mRCC patients treated with TKIs in
routine clinical practice (8–12). To date, however, there have not been
any data from a large number of Japanese patients with mRCC treated
with TKIs as first-line molecular-targeted therapy; therefore, we
retrospectively investigated the oncological outcomes in a total of
271 patients who received either sorafenib or sunitinib for
TKI-naive mRCC.
In this series, a total of 50 patients were
classified as exhibiting CR or PR, resulting in an objective
response rate of 18.4%. This outcome may be explained as follows:
This study included a larger proportion of patients receiving
sorafenib, which is characterized by a significantly lower response
rate compared to that of sunitinib; in addition, it is generally
difficult to achieve a response rate to TKIs in a routine clinical
setting superior to that in a clinical trial, due to the presence
of a certain proportion of patients with unfavorable
characteristics in the general MRCC patient population, who do not
meet the inclusion criteria for clinical trials (15). In fact, the response rates to
sorafenib and sunitinib were reported to be 9.8 and 30.7%,
respectively, in phase III clinical trials (3,4), while
expanded-access trials reported response rates to sorafenib and
sunitinib of 4.0 and 17.4%, respectively (8,10). In
addition, the median OS of this series was 33.1 months, which is
also very favorable, even when compared to the OS reported in a
clinical trial (28.4 months) as well as an expanded-access study
(18.4 months) in patients receiving sunitinib (4,10).
Collectively, these findings strongly suggest the usefulness of
first-line TKI therapy in Japanese patients without prior treatment
with a molecular-targeted agent.
It is of interest to identify factors associated
with the prognosis of Japanese mRCC patients treated with TKIs as
the first-line molecular-targeted therapy in an actual clinical
setting. In this series, of the 7 significant predictors of OS
identified by univariate analysis, the MSKCC classification, CRP
level and liver metastasis were found to be independently
correlated with OS. The prognostic significance of these parameters
has already been reported in several previous studies (11,13–17). The
common findings regarding prognostic indicators across various
studies suggest the significant effect of the underlying biology of
mRCC on disease control, even in the era of molecular-targeted
therapy.
Another point of interest is to develop a system
that allows a more precise assessment of the prognostic risk in
individual patients with mRCC receiving TKIs as first-line
molecular-targeted therapy, since such a tool would be useful for
counseling patients and planning therapeutic options and follow-up
schedule. To date, the most widely used system is the MSKCC
classification model that may facilitate prognostic
individualization in mRCC patients who have received systemic
therapy (14); however, despite being
validated in the era of molecular-targeted therapy (18), this model was developed based on data
from patients treated with IFN-α in a clinical trial. To overcome
such possible limitations of the MSKCC classification, Heng et
al (11) presented a novel model
to assess the prognosis of TKI-naive mRCC patients treated with
VEGF-targeted agents in routine clinical practice. In this series,
we evaluated the ability to predict the prognosis of mRCC patients
following TKI introduction by combining 3 independent risk factors
identified in this study (i.e., MSKCC classification, CRP level and
liver metastasis) and demonstrated that it is possible to stratify
OS according to the positivity for these factors. However, to draw
a definitive conclusion regarding the significance of our model, it
requires prospective validation based on data from a larger patient
sample.
Although it was not a major objective of this study
to compare the treatment efficacy between the sorafenib and
sunitinib groups, this assessment may help guide decisions on the
therapeutic strategy for patients with TKI-naive mRCC. It is
currently hypothesized that the majority of Japanese mRCC patients,
who are classified into a favorable or an intermediate risk group,
are initially treated with sunitinib. Until recently, however,
sorafenib and sunitinib tended to be separately administered to
mRCC patients with comparatively favorable and unfavorable
characteristics, respectively (19),
due to several background factors in Japan as follows: Even after
the introduction of molecular-targeted agents, immunotherapy was
still likely to be administered to mRCC patients, particularly to
those classified into the favorable risk group and due to the
delayed approval of temsirolimus, a suitable agent for patients
classified into the poor-prognosis group (20), this category of patients was
preferably treated with sunitinib. Although this trend appeared to
be marked in this study as well, there was no significant
difference in the OS between the sorafenib and sunitinib groups and
the OS in the sunitinib group with unfavorable characteristics,
such as elevated CRP level, appeared to be superior to that in the
sorafenib group (data not shown). Considering these findings, it is
highly recommended to administer sunitinib as a first-line systemic
agent to the majority of mRCC patients, apart from those in the
poor-prognosis group.
There were several limitations to this study. First,
although this series may include the largest number of Japanese
mRCC patients who received TKIs as first-line molecular-targeted
agents, this was a retrospective study conducted in a routine
clinical setting; therefore, the findings of this study require
confirmation in an external cohort. Second, as described above, the
indication for the administration of either sorafenib or sunitinib
was not determined according to strictly established criteria,
which may have affected the findings of this study. Third, it may
be necessary to re-analyze the outcomes of first-line TKI therapy
after the accumulation of data from patients who received
pazopanib, a recently approved TKI with a potential activity
against systemic therapy-naive RCC. Finally, this study focused on
prognostic issues; however, the usefulness of each agent should be
evaluated more comprehensively considering other characteristics,
such as those associated with AEs, quality of life and health
economy.
In conclusion, to the best of our knowledge, this is
the first study to systematically assess the oncological outcome
with TKIs introduced as first-line molecular-targeted agents for
Japanese patients with mRCC and the outcomes presented in this
study appear to be encouraging with respect to cancer control by
TKIs for Japanese mRCC patients based on real-world clinical
practice. Furthermore, the OS of Japanese mRCC patients receiving
TKIs as first-line molecular targeted therapy may be precisely
stratified according to the positivity for independent prognostic
risk factors identified by multivariate analysis, including the
MSKCC classification, CRP level and liver metastasis. Accordingly,
these findings strongly suggest the utility of TKIs in the majority
of systemic therapy-naive mRCC patients; however, it is necessary
to perform an external validation study to draw definitive
conclusions regarding the issues presented in this study.
References
|
1
|
Parton M, Gore M and Eisen T: Role of
cytokine therapy in 2006 and beyond for metastatic renal cell
cancer. J Clin Oncol. 24:5584–5592. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Figlin R, Sternberg C and Wood CG: Novel
agents and approaches for advanced renal cell carcinoma. J Urol.
188:707–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Escudier B, Eisen T, Stadler WM, et al:
Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J
Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Sunitinib versus interferon alfa in metastatic renal-cell
carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rini BI, Escudier B, Tomczak P, et al:
Comparative effectiveness of axitinib versus sorafenib in advanced
renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet.
378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Motzer RJ, Hutson TE, Cella D, et al:
Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N
Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patard JJ, Pignot G, Escudier B, et al:
ICUD-EAU International Consultation on Kidney Cancer 2010:
treatment of metastatic disease. Eur Urol. 60:684–690. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gore ME, Szczylik C, Porta C, et al:
Safety and efficacy of sunitinib for metastatic renal-cell
carcinoma: an expanded-access trial. Lancet Oncol. 10:757–763.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harshman LC, Xie W, Bjarnason GA, et al:
Conditional survival of patients with metastatic renal-cell
carcinoma treated with VEGF-targeted therapy: a population-based
study. Lancet Oncol. 13:927–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beck J, Procopio G, Bajetta E, et al:
Final results of the European Advanced Renal Cell Carcinoma
Sorafenib (EU-ARCCS) expanded-access study: a large open-label
study in diverse community settings. Ann Oncol. 22:1812–1823. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heng DY, Xie W, Regan MM, et al:
Prognostic factors for overall survival in patients with metastatic
renal cell carcinoma treated with vascular endothelial growth
factor-targeted agents: results from a large, multicenter study. J
Clin Oncol. 27:5794–5799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choueiri TK, Duh MS, Clement J, et al:
Angiogenesis inhibitor therapies for metastatic renal cell
carcinoma: effectiveness, safety and treatment patterns in clinical
practice-based on medical chart review. BJU Int. 105:1247–1254.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyake H, Miyazaki A, Harada K, et al:
Assessment of efficacy, safety and quality of life of 110 patients
treated with sunitinib as first-line therapy for metastatic renal
cell carcinoma: experience in real-world clinical practice in
Japan. Med Oncol. 31:9782014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motzer RJ, Bacik J, Murphy BA, et al:
Interferon-alfa as a comparative treatment for clinical trials of
new therapies against advanced renal cell carcinoma. J Clin Oncol.
20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ainsworth NL, Lee JS and Eisen T: Impact
of anti-angiogenic treatments on metastatic renal cell carcinoma.
Expert Rev Anticancer Ther. 9:1793–1805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You D, Jeong IG, Ahn JH, et al: The value
of cytoreductive nephrectomy for metastatic renal cell carcinoma in
the era of targeted therapy. J Urol. 185:54–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beuselinck B, Vano YA, Oudard S, et al:
Prognostic impact of baseline serum C-reactive protein in patients
with metastatic renal cell carcinoma (RCC) treated with sunitinib.
BJU Int. 114:81–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon WA, Cho IC, Yu A, et al: Validation
of the MSKCC and Heng risk criteria models for predicting survival
in patients with metastatic renal cell carcinoma treated with
sunitinib. Ann Surg Oncol. 20:4397–4404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyake H, Kusuda Y, Harada K, et al:
Third-line sunitinib following sequential use of cytokine therapy
and sorafenib in Japanese patients with metastatic renal cell
carcinoma. Int J Clin Oncol. 18:81–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hudes G, Carducci M, Tomczak P, et al:
Temsirolimus, interferon alfa, or both for advanced renal-cell
carcinoma. N Engl J Med. 356:2271–2281. 2007. View Article : Google Scholar : PubMed/NCBI
|