Introduction
Anaplastic thyroid cancer (ATC) is a rare entity,
accounting for 1–3% of all thyroid cancer cases. ATC is one of the
most aggressive malignancies in humans. Multimodal therapies,
including surgery, radiation and chemotherapy, are generally used
to manage this highly malignant disease. However, the prognosis is
poor and the majority of the patients succumb to the disease within
a year, with a median survival time of <6 months from the
initial diagnosis (1–3). Recently, new approaches to treating ATC
by targeted molecular markers have been developed to overcome
therapeutic resistance (4–6). We previously demonstrated the efficacy
of gefitinib, a tyrosine kinase inhibitor (TKI) of the epidermal
growth factor receptor (EGFR), on an ATC cell line, as a potential
novel therapeutic strategy. However, we observed that gefitinib was
not effective in regulating cell growth in a different cell line
that exhibited an altered EGFR-initiated signal transduction
pathway (7). A mutation of the
phosphoinositide-3-kinase, catalytic, α polypeptide gene (PI3KCA)
was later identified in the gefitinib-resistant cell line (8). In the present study, we attempted to
regulate the downstream effector of EGFR-Akt-mammalian target of
rapamycin (mTOR) pathway by an mTOR inhibitor, everolimus, in a
gefitinib-resistant cell line and to demonstrate the mechanism to
overcome resistance to EGFR-targeted therapy.
Materials and methods
Chemicals
Everolimus (RAD001) was provided by novartis (Basel,
Switzerland). Paclitaxel was purchased from Wako Pure Chemical
Industries, Ltd. (Osaka, Japan).
Cell lines and cell cultures
We used a panel of 8 authentic human ATC cell lines,
including 2 cell lines (OCUT-2 and ACT-1) that were used in our
previous experiment (7). OCUT-1-6
were established and characterized in our laboratory. A mutation of
B-Raf V600E was found in OCUT-1-5 and a N-Ras mutation was found in
the OCUT-6 and ACT-1 cell lines (9).
OCUT-2 was known to carry a mutation of the PI3CA gene (8). ACT-1 was kindly provided by Dr S. Ohata
of Tokushima University. Each cell line was cultured in Dulbecco's
Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), 100 IU/ml penicillin and 100 mg/ml streptomycin at
37°C with 5% CO2 in a humidified incubator.
MTT assay
The inhibitory effects of everolimus on the
viability of these cell lines were measured by the MTT assay
(7). Cells (1×103) were
seeded in each well of a 96-well plastic culture plate and left
overnight under the same conditions. The cells were then treated
with the intended doses of everolimus for 3 days. After the
incubation period, MTT was added to a final concentration of 0.5
mg/ml and the cells were incubated again for 2 h under the same
conditions. The culture plate was centrifuged at 200 × g for 5 min
and the supernatant was removed. Dimethyl sulfoxide was added for
reaction and the absorbency was measured with a model 550
microplate reader (Bio-Rad Laboratories, Hercules, CA, USA) and
calculated using the supplied software. The experiments were
performed three times independently, in triplicate each time and
the average values of the three independent experiments were
calculated.
The effect of everolimus on cell viability following
paclitaxel treatment was also measured by the MTT assay. OCUT-2
cells were exposed to 1 nM of everolimus 1 h before, concomitantly,
or 1 h after treatment with 1–100 nM of paclitaxel for 72 h.
Effect of everolimus on tumor cell
proliferation under EGF stimulation in vitro
Cells (5×104) were spread onto a 10-mm
plastic dish and left overnight. The cells were then cultured in
DMEM without FBS. One nmol of EGF (no. 26190U, Upstate, Lake
Placid, NY, USA) was added to a plate to stimulate the EGFR of the
cells. The efficacy of everolimus (1 and 10 nM) was investigated by
immediate addition following EGF exposure. The cells were counted
after 48 h of incubation. The experiments were performed
independently in triplicate.
Cell cycle analysis by flow
cytometry
Flow cytometry was used to measure the DNA content
of individual cells, which allowed us to assess the cell-cycle
profiles of the cells treated with everolimus. In preparation for
flow cytometry, cells treated with 2 nM of everolimus for 16–72 h
were collected following brief trypsinization, washed with
phosphate-buffered saline and fixed with 70% cold ethanol. The
samples were then treated with ribonuclease (R6513; Sigma-Aldrich
Corp., Saint Louis, MO, USA), stained with 10 mg/ml propidium
iodide and analyzed by a facscan cell sorter (Becton Dickinson,
Mountain View, CA, USA). Cell cycle distributions were quantified
using Cellquest software (Becton Dickinson).
Results
Growth inhibitory effect of
everolimus
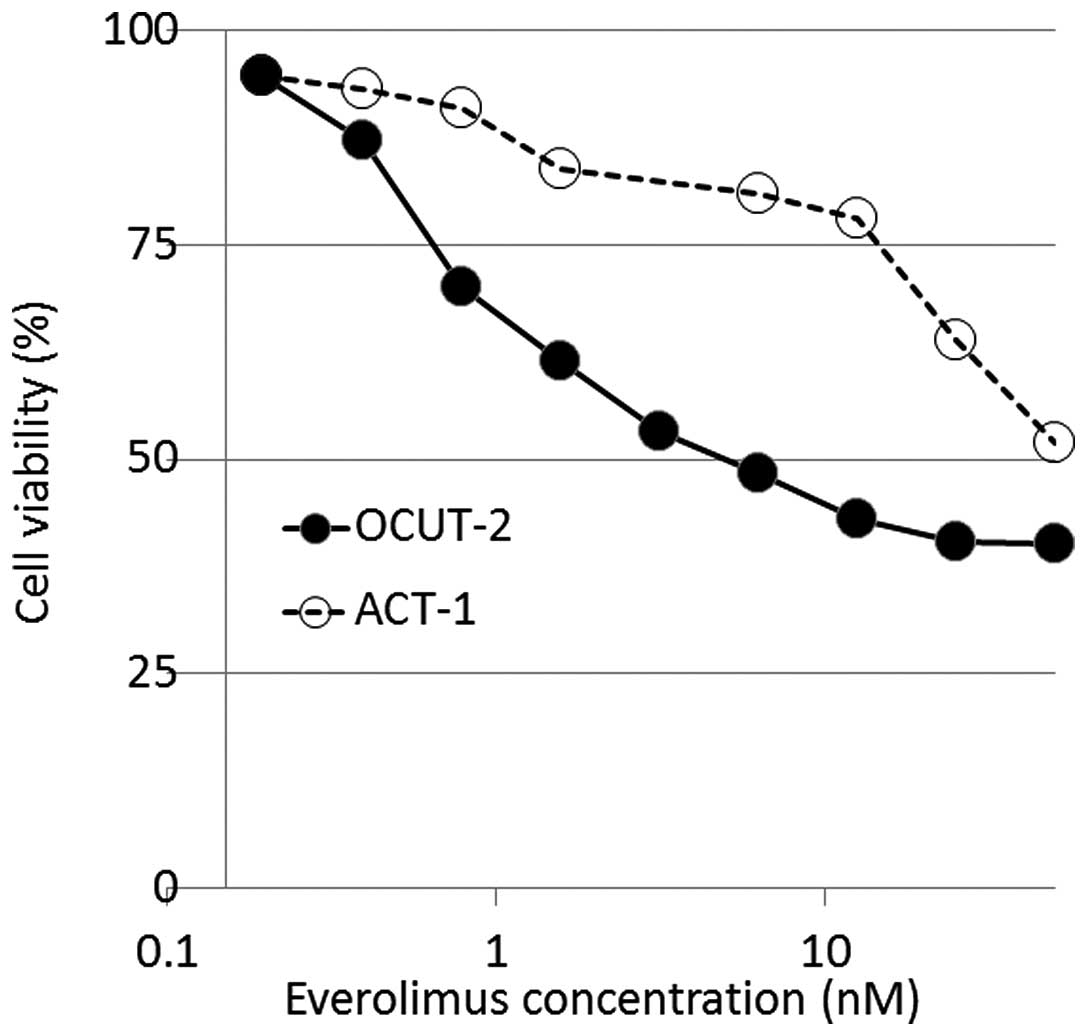
A similar growth inhibitory effect of everolimus was
observed in all the cell lines, except for OCUT-2. The 30 and 50%
inhibitory concentrations of everolimus ranged from 8.4 to 23.5 and
from 19 to >100 nM, respectively (Table I). However, in OCUT-2, everolimus
achieved a significant growth inhibition compared to that in other
cell lines, with 30 and 50% inhibitory concentrations of 0.8 and 5
nM, respectively. The maximal growth inhibitory effect of
everolimus on OCUT-2 cells was demonstrated at the concentration of
~20 nM, where ~60% of the cells were growth-inhibited and no
further effect was observed by increasing the concentration
(Fig. 1). There was a significant
difference in the sensitivity to everolimus between the ACT-1 and
OCUT-1, −3, −4, −5 and −6 cell lines (data not shown).
 | Table I.Inhibitory concentrations (30 and 50%)
of everolimus in different cell lines. |
Table I.
Inhibitory concentrations (30 and 50%)
of everolimus in different cell lines.
| Cell line | Known gene
mutations | IC30 (nM) | IC50 (nM) |
|---|
| OCUT-1C | B-Raf | 14 | –a |
| OCUT-1F | B-Raf | 8.5 | 80 |
| OCUT-2 | B-Raf, PI3KCA and
EGFR | 0.8 | 5 |
| OCUT-3 | B-Raf | 2.5 | 19 |
| OCUT-4 | B-Raf | 23.5 | –a |
| OCUT-5 | B-Raf | 8.4 | 24 |
| OCUT-6 | N-Ras | 14 | –a |
| ACT-1 | N-Ras | 17 | 80 |
The proliferation of cancer cells in reaction to EGF
clearly differed between the ACT-1 and OCUT-2 cell lines. ACT-1
cells exhibited a significant upregulation of proliferation by
stimulation with 1 nM of EGF, as previously reported (7). There was no change in OCUT-2 cell
proliferation by EGF stimulation. Everolimus achieved a significant
inhibition of cell proliferation in the OCUT-2 cell line, even
under EGF stimulation. A concentration of 1 nM was sufficient to
achieve maximal growth inhibition in OCUT-2 cells. By contrast,
ATC-1 cell proliferation was significantly stimulated by EGF, but
not affected by exposure to 1 nM of everolimus. A high
concentration (10 nM) of everolimus was required to achieve a
partial inhibition of the upregulated cellular growth of ACT-1
stimulated by EGF (Fig. 2).
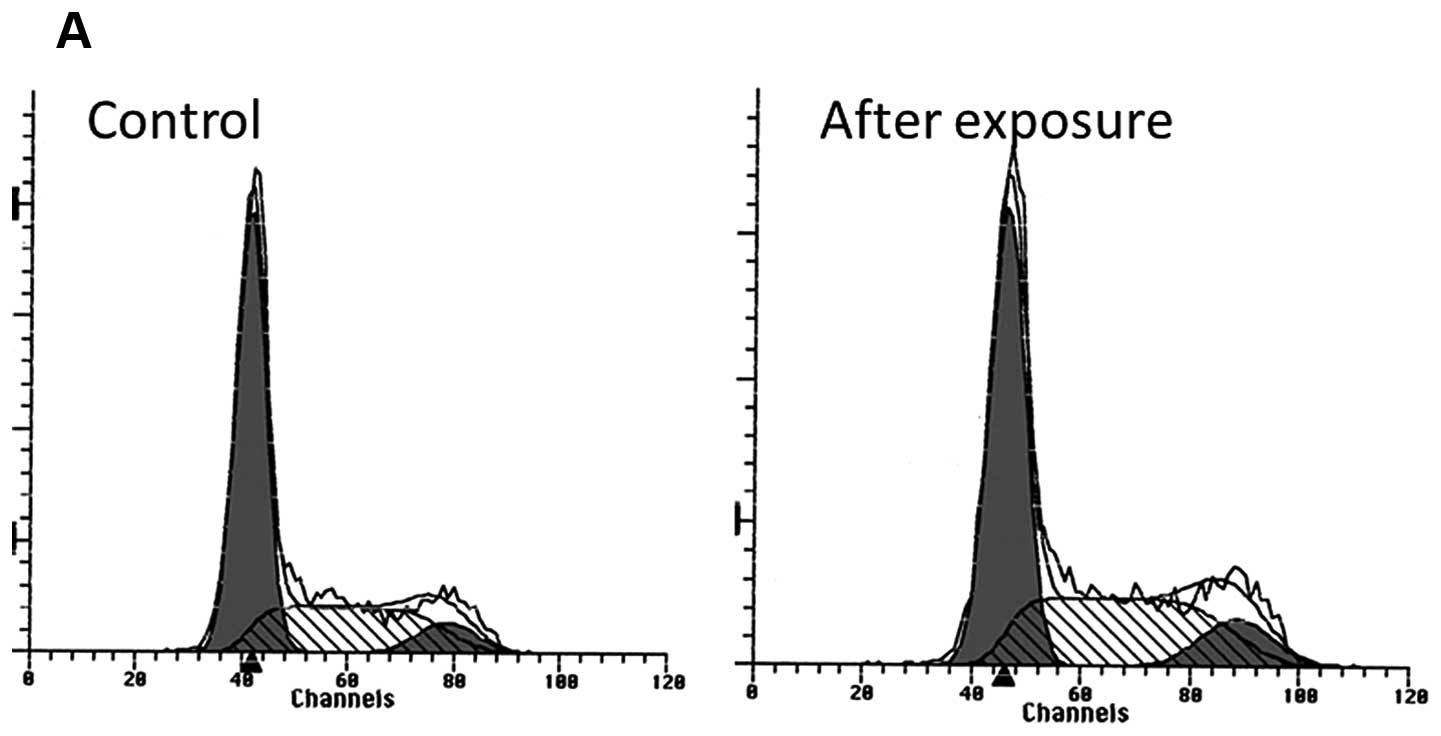
G2M cell cycle arrest was observed after a 16-h
exposure to everolimus (1 nM), with a 19% increased ratio of cells
in the G2M phase (32–39%). The G2M arrest continued until 72 h
after exposure (Fig. 3).
An additive effect of everolimus onto the
cytotoxicity of paclitaxel was demonstrated at a dose of 1 nM.
There was a 32% decrease in cell viability following exposure of
OCUT-2 cells to 1 nM of paclitaxel in the presence of 1 nM of
everolimus compared to paclitaxel alone. The additive effect of
everolimus was more pronounced when it was administered prior to
paclitaxel exposure (Fig. 4).
Discussion
Conventionally, ATC has been managed with a
multimodal therapeutic approach by combining surgery, chemotherapy
and external beam radiation. However, the effect of these
approaches is limited due to the highly aggressive nature of the
tumor and rapid acquisition of resistance to treatment. To overcome
this resistance, molecular-targeted approaches have been applied as
a possible novel therapeutic strategy. To date, there has been some
success in managing this disease (4–6). EGFR is a
well known cell membranous receptor and has been reported to be
highly expressed in ATC cells (10).
Thus, we attempted to manage the disease by inhibiting the kinase
activity of EGFR by gefitinib in a previous study and demonstrated
the significant efficacy of this TKI in inhibiting cancer cell
growth. In the ACT-1 cell line, the cell-proliferating signal
through EGFR to mitogen-activated protein kinase kinase (MEK) was
clearly inhibited by gefinitib (7).
Furthermore, this significant effect was not observed in OCUT-2, a
cell line exhibiting a lower level of EGFR expression.
There are two major pathways inducing the
proliferation of cancer cells downstream of cell surface EGFR,
namely the Raf/Ras/MEK and PI3K/Akt/mTOR pathways. Alterations in
both pathways have often been observed in thyroid cancer (11–15).
B-Raf, Ras, PI3KCA and phosphatase and tensin homolog mutations
have also been reported in ATC (16–19).
Several cell lines harbor one of these gene mutations. By contrast,
OCUT-2 is a unique cell line exhibiting alterations in both B-Raf
and PI3KCA genes, resulting in completely aberrant proliferation
signal generation independent of EGFR-mediated signaling (9). The results of our previous study may,
thus, be interpreted as that the efficacy of EGFR-targeted therapy
may be determined by the signaling status downstream of EGFR.
Therefore, in this study, we attempted to inhibit mutated
PI3KCA-generated aberrant growth signaling by an mTOR inhibitor to
overcome gefitinib resistance.
A significantly more prominent inhibitory effect on
cell proliferation was demonstrated by everolimus in the OCUT-2
cell line, compared to that in other cell lines not carrying
alterations in the PI3K/Akt/mTOR pathway, as expected. This
observation may indicate the significance of detecting alterations
in the PI3K/Akt/mTOR signaling pathway as an indicator of the
possible efficacy of everolimus. According to the results of the
effects on ACT-1, everolimus was only able to impair part of the
proliferation signal generated from EGFR when the signaling pathway
was intact. By contrast, a major signal for proliferation was
blocked in OCUT-2 cells harboring an aberrantly activated
PI3K/Akt/mTOR pathway by a PI3KCA mutation. In addition, OCUT-2
also carrying an active B-Raf mutation, which should cause
alternative generation of the proliferating signal in the absence
of the PI3K/Akt/mTOR axis, resulted in the incomplete impairment of
cell growth.
We observed a marginal increase in the number of
cells in the S and G2M phase following exposure to everolimus in
the OCUT-2 cell line, suggesting G2M cell cycle arrest. Everolimus
is known to cause G0G1 arrest by inhibiting the expression of
cyclin D1. However, a very high concentration (5,000–20,000 nM) of
everolimus was reportedly required to achieve G0G1 arrest (20,21)
compared to the concentration we used in the present experiment.
The concentration of everolimus we used in this study was 2 nM,
since we were unable to achieve additional inhibition in cell
proliferation when increasing the dose to >20 nM in the initial
experiments investigating the effect of everolimus on cell
viability. The DNA histogram analysis did not identify any cell
population with lower or fragmented DNA contents, suggesting
apoptosis. These observations may indicate that the effect of
everolimus is expressed as a moderate cell cycle arrest in G2M, but
not as cell killing within physiological doses.
In the present study, we demonstrated that
everolimus inhibited cell growth in cancer cells harboring an
altered PI3K/Akt/mTOR signaling pathway. This effect was limited to
cell growth arrest and no complete cytotoxicity was observed. As
stated earlier, alterations in the PI3K/Akt/mTOR pathway in ATC was
not a major genetic abnormality, compared to B-Raf- or Ras-mediated
pathway. Farstino et al (22)
suggested the possible mechanism of LKB1-mediated mTOR pathway
upregulation in thyroid carcinoma harboring the B-Raf mutation.
Dual inhibition of the Raf/Ras/MEK and PI3K/Akt/mTOR pathways was
attempted, with promising preclinical results (23). A combination of a cytotoxic drug with
everolimus may be another practical choice to increase the efficacy
of treatment. We observed an additive effect of paclitaxel to that
of everolimus in the OCUT-2 cell line. Paclitaxel exerts its
cytotoxic effect by inhibiting the polymerization of tubulin in the
G2M phase. Further studies are required to maximize the inhibitory
effect of everolimus on the mTOR pathway.
In conclusion, a significant growth inhibitory
effect of everolimus on a gefitinib-resistant ATC cell line was
demonstrated. A possible correlation between the efficacy of
everolimus and PI3KCA gene mutation requires further investigation
using additional ATC samples with accurate information on genetic
alterations. This study indicated the significance of identifying
target molecules, or applying target-oriented therapeutic
strategies in managing patients with highly malignant ATC.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Scientific Research (Kakenhi no. 25461992).
References
|
1
|
Smallridge RC: Approach to the patient
with anaplastic thyroid carcinoma. J Clin Endocrinol Metab.
97:2566–2572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akaishi J, Sugino K, Kitagawa W, et al:
Prognostic factors and treatment outcomes of 100 cases of
anaplastic thyroid carcinoma. Thyroid. 21:1183–1189. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugitani I, Miyauchi A, Sugino K, Okamoto
T, Yoshida A and Suzuki S: Prognostic factors and treatment
outcomes for anaplastic thyroid carcinoma: ATC research consortium
of Japan cohort study of 677 patients. World J Surg. 36:1247–1254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cabanillas ME, Waguespack SG, Bronstein Y,
Williams MD, Feng L, Hernandez M, Lopez A, Sherman SI and Busaidy
NL: Treatment with tyrosine kinase inhibitors for patients with
differentiated thyroid cancer: the M. D. Anderson experience. J
Clin Endocrinol Metab. 95:2588–2595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benvenga S: Emerging therapies in sight
for the fight against dedifferentiated thyroid cancer. J Clin
Endocrinol Metab. 96:347–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosove MH, Peddi PF and Glaspy JA: BRAF
V600E inhibition in anaplastic thyroid cancer. N Engl J Med.
368:684–685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nobuhara Y, Onoda N, Yamashita Y, Yamasaki
M, Ogisawa K, Takashima T, Ishikawa T and Hirakawa K: Efficacy of
epidermal growth factor receptor-targeted molecular therapy in
anaplastic thyroid cancer cell lines. Br J Cancer. 92:1110–1116.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu D, Hou P, Liu Z, Wu G and Xing M:
Genetic alterations in the phosphoinositide 3-kinase/Akt signaling
pathway confer sensitivity of thyroid cancer cells to therapeutic
targeting of Akt and mammalian target of rapamycin. Cancer Res.
69:7311–7319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Onoda N, Nakamura M, Aomatsu N, Noda S,
Kashiwagi S and Hirakawa K: Establishment, characterization and
comparison of seven authentic anaplastic thyroid cancer cell lines
retaining clinical features of the original tumor. World J Surg.
38:688–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wiseman SM, Masoudi H, Niblock P, Turbin
D, Rajput A, Hay J, Bugis S, Filipenko D, Huntsman D and Gilks B:
Anaplastic thyroid carcinoma: expression profile of targets for
therapy offers new insights for disease treatment. Ann Surg Oncol.
14:719–729. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rusinek D, Szpak-Ulczok S and Jarzab B:
Gene expression profile of human thyroid cancer in relation to its
mutational status. J Mol Endocrinol. 47:R91–R103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing M, Alzahrani AS, Carson KA, et al:
Association between BRAF V600E mutation and mortality in patients
with papillary thyroid cancer. JAMA. 309:1493–1501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim TH, Park YJ, Lim JA, et al: The
association of the BRAF(V600E) mutation with prognostic factors and
poor clinical outcome in papillary thyroid cancer: a meta-analysis.
Cancer. 118:1764–1773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garcia-Rostan G, Zhao H, Camp RL, Pollan
M, Herrero A, Pardo J, Wu R, Carcangiu ML, Costa J and Tallini G:
Ras mutations are associated with aggressive tumor phenotypes and
poor prognosis in thyroid cancer. J Clin Oncol. 21:3226–3235. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vasko V, Ferrand M, Di Cristofaro J,
Carayon P, Henry JF and de Micco C: Specific pattern of RAS
oncogene mutations in follicular thyroid tumors. J Clin Endocrinol
Metab. 88:2745–2752. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santarpia L, El-Naggar AK, Cote GJ, Myers
JN and Sherman SI: Phosphatidylinositol 3-kinase/akt and
ras/raf-mitogen-activated protein kinase pathway mutations in
anaplastic thyroid cancer. J Clin Endocrinol Metab. 93:278–284.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Hou P, Ji M, Guan H, Studeman K,
Jensen K, Vasko V, El-Naggar AK and Xing M: Highly prevalent
genetic alterations in receptor tyrosine kinases and
phosphatidylinositol 3-kinase/akt and mitogen-activated protein
kinase pathways in anaplastic and follicular thyroid cancers. J
Clin Endocrinol Metab. 93:3106–3116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xing M: Genetic alterations in the
phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer.
Thyroid. 20:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
García-Rostán G, Costa AM, Pereira-Castro
I, Salvatore G, Hernandez R, Hermsem MJ, Herrero A, Fusco A,
Cameselle-Teijeiro J and Santoro M: Mutation of the PIK3CA gene in
anaplastic thyroid cancer. Cancer Res. 65:10199–10207. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang ZG, Fukazawa T, Nishikawa T, et al:
RAD001 offers a therapeutic intervention through inhibition of mTOR
as a potential strategy for esophageal cancer. Oncol Rep.
23:1167–1172. 2010.PubMed/NCBI
|
|
21
|
Xu DZ, Geng QR, Tian Y, et al: Activated
mammalian target of rapamycin is a potential therapeutic target in
gastric cancer. BMC Cancer. 10:5362010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Faustino A, Couto JP, Pópulo H, Rocha AS,
Pardal F, Cameselle-Teijeiro JM, Lopes JM, Sobrinho-Simões M and
Soares P: mTOR pathway overactivation in BRAF mutated papillary
thyroid carcinoma. J Clin Endocrinol Metab. 97:E1139–E1149. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin N, Jiang T, Rosen DM, Nelkin BD and
Ball DW: Dual inhibition of mitogen-activated protein kinase kinase
and mammalian target of rapamycin in differentiated and anaplastic
thyroid cancer. J Clin Endocrinol Metab. 94:4107–4112. 2009.
View Article : Google Scholar : PubMed/NCBI
|