Introduction
Liver resection is the only treatment that offers
hope of long-term survival and a cure for patients with colorectal
liver metastasis (CRLM). Systemic neoadjuvant chemotherapy (NAC)
may be used prior to conversion surgery and achieve R0 resection,
even in patients with initially unresectable CRLM.
Oxaliplatin-based chemotherapy plays a central role in the
treatment of CRLM in Japan. However, oxaliplatin-based chemotherapy
has been associated with sinusoidal obstruction syndrome (SOS), a
condition characterized by hepatic sinusoidal dilatation,
hepatocyte atrophy, perisinusoidal fibrosis and nodular
regenerative hyperplasia (1). These
histological changes have been observed in ≤40% of patients treated
with oxaliplatin-based regimens undergoing liver resection
(2–6).
SOS is a cause for concern prior to major hepatectomy, as it is
associated with increased perioperative morbidity and prolonged
hospital stay (7). Clinically, the
diagnostic factors associated with SOS include portal hypertension,
splenomegaly, thrombocytopenia, abnormal indocyanine green (ICG)
retention rate and elevations in the levels of liver enzymes,
bilirubin and hyaluronic acid (8–12).
Pathologically, SOS is characterized by disruption of the
sinusoidal endothelium, collagen deposition in the perisinusoidal
space, fibrosis, particularly around the central vein (in zone
III), with dilatation of the sinusoidal space and congestion
(13). The significant association
between the sinusoidal endothelium and platelets (14) suggests platelet involvement in SOS and
that oxaliplatin-based chemotherapy affects platelets in the liver.
The aim of the present study was to assess the effects of
oxaliplatin-based NAC on platelets in the liver.
Material and methods
Patients
Between January, 2005 and December, 2010, 32
patients with CRLM underwent surgery at the Department of
Gastroenterologic Surgery, Kanazawa University Hospital (Kanazawa,
Japan), including 17 patients who received oxaliplatin-based NAC
(NAC group) and 15 who did not (control group). The patient records
were retrospectively assessed and the factors evaluated included
platelet count and indocyanine green (ICG) retention rate at 15
min. Spleen volume was determined from computed tomography scans
using a spleen index (15), which was
calculated as 0.8 times the product of the long and short diameters
of the maximum cut surface of the spleen.
Written informed consent was obtained from all the
patients prior to their enrollment in the study. Treatment
protocols were approved by the local Medical Ethics Committee.
Pathological specimens
Formalin-fixed, paraffin-embedded specimens were
retrieved from the surgical pathology files of the Pathology
Department of Kanazawa University Hospital.
Immunohistochemical examination
Immunohistochemical staining was performed using the
Dako EnVision system, which uses dextran polymers conjugated with
horseradish peroxidase (Dako, Carpinteria, CA, USA), thus avoiding
any contamination by endogenous biotin. The tissues were fixed in
10% formaldehyde in phosphate-buffered saline, embedded in paraffin
and cut into 5-mm tissue sections. The sections were deparaffinized
in xylene and rehydrated in a graded ethanol series. Endogenous
peroxidases were blocked by immersing the sections in 3%
H2O2 in 100% methanol for 20 min at room
temperature. Antigen retrieval was achieved by microwaving the
sections at 95°C for 10 min in 0.001 M citrate buffer (pH 6.7).
After blocking endogenous peroxidases, the sections were incubated
with Protein Block Serum-Free (Dako) at room temperature for 10 min
to block non-specific staining. The sections were subsequently
incubated for 2 h at room temperature with a 1:50 dilution of mouse
monoclonal antibody against CD42b (cat. no. EPR6995; Abcam, Tokyo,
Japan). Peroxidase activity was detected with the enzyme substrate
3-amino-9-ethylcarbazole. As negative controls, the sections were
incubated with Tris-buffered saline without the primary antibody.
Samples in which ≥10% of the tumor cells were slightly
counterstained with Meyer's hematoxylin were defined as positive.
Positive expression was defined as staining of >5% of the
cells.
Statistical analysis
Categorical variables were compared using the
Chi-square test. All the tests were two-tailed and P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Between January, 2005 and December, 2010, 32
patients with CRLM (25 men and 7 women) underwent surgery. Of
those, 17 patients (13 men and 4 women) of mean age 64.3 years
(range, 48–78 years), underwent oxaliplatin-based NAC (NAC group),
whereas the remaining 15 patients (12 men and 3 women) of mean age
63.0 years (range, 37–83 years) underwent surgical resection alone
(control group). The platelet levels were significantly lower in
the NAC compared to those in the control group. The ICG retention
rate differed between the two groups, although the difference was
not statistically significant. The splenic index, a measure of
spleen size, was higher in the NAC compared to that in the control
group, but the difference was not significant (Table I). However, the spleens of the
patients in the NAC group were significantly enlarged following
oxaliplatin-based chemotherapy (Table
II).
 | Table I.Common biomarkers of sinusoidal
obstruction syndrome. |
Table I.
Common biomarkers of sinusoidal
obstruction syndrome.
| Markers | NAC (n=17) | Control (n=15) | P-value |
|---|
| ICG 15 (%) |
10.9±6.7 |
8.6±7.8 | NS |
| Platelet count
(x104) |
16.9±5.5 |
22.7±8.8 | <0.05 |
| Spleen index
(cm2) |
29.6±11.2 |
27.2±10.4 | NS |
 | Table II.Spleen index of NAC cases (n=17). |
Table II.
Spleen index of NAC cases (n=17).
| Measurement | Prior to NAC | After NAC | P-value |
|---|
| Spleen index
(cm2) | 25.6±10.4 | 29.6±11.2 | <0.01 |
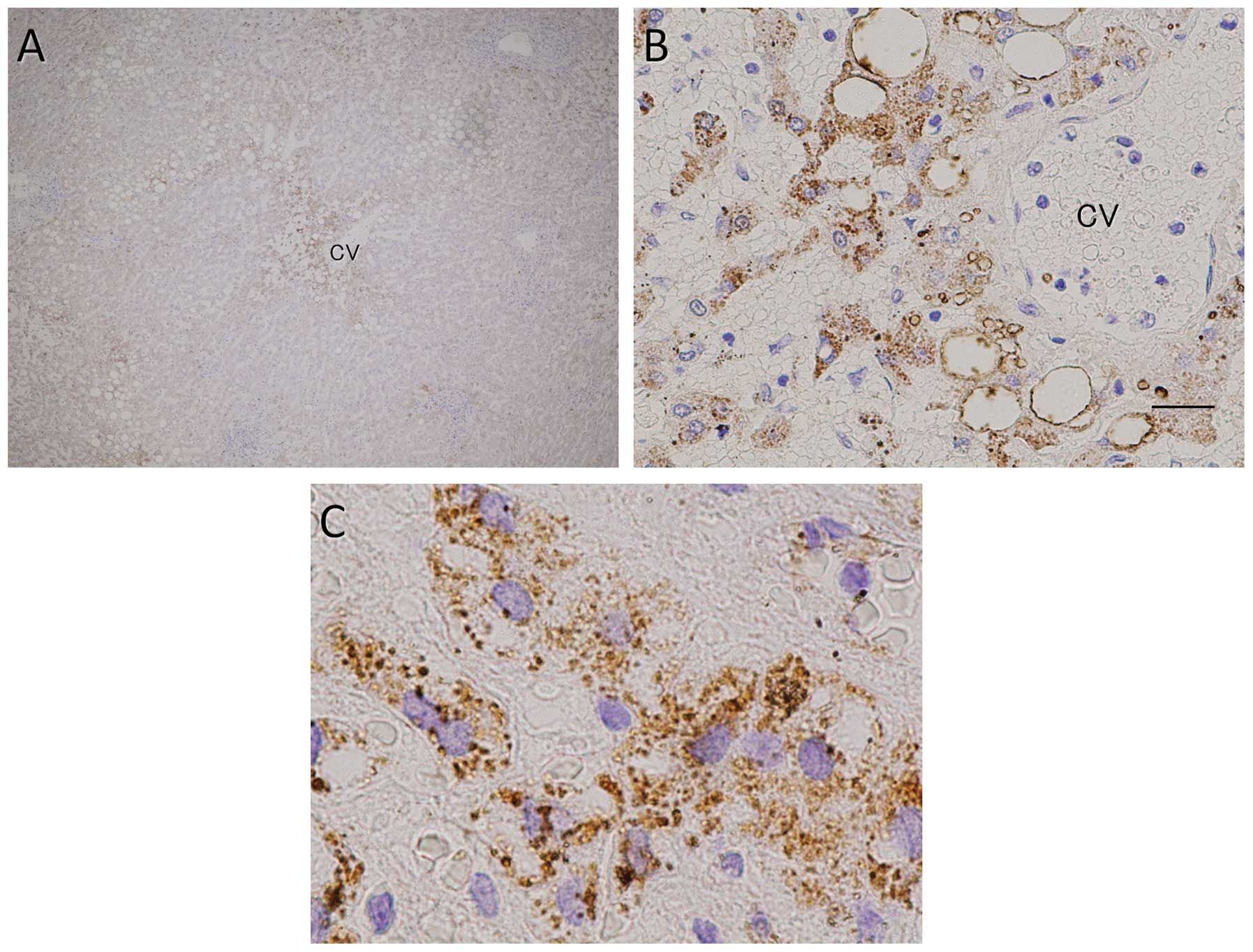
Immunohistochemical examination for
CD42b
Tissue specimens surgically resected from 30
patients with CRLM (17 from the NAC and 13 from the control group),
were immunohistochemically assayed for CD42b expression (Table III). The platelet counts were higher
in the NAC compared to those in the control group, particularly in
zone III (P=0.009). Moreover, platelet numbers were found to be
correlated with the severity of SOS; specimens from patients with
mild cases of SOS showed a few platelets in the sinusoidal space,
whereas specimens from patients with severe SOS showed high
platelet numbers in contact with hepatocytes, particularly in zone
III (Fig. 1).
 | Table III.Immunohistochemical detection of CD42b
in the resected liver. |
Table III.
Immunohistochemical detection of CD42b
in the resected liver.
| Zone | NAC, no. (%)
(n=15) | Control, no. (%)
(n=12) | P-value |
|---|
| I | 4 (26.7) | 1 (8.3) | NS |
| II | 7 (46.7) | 3 (25.0) | NS |
| III | 9 (60.0) | 1 (8.3) | 0.009 |
Discussion
SOS, also referred to as hepatic veno-occlusive
disease, is a fatal hepatic injury that occurs predominantly
following drug or toxin exposure. SOS may present in an acute,
subacute or chronic form, usually with abdominal pain and
hepatosplenomegaly, with evidence of portal hypertension, serum
liver enzyme elevations and jaundice. Liver histology reveals
sinusoidal obstruction in zone III, with hepatocyte necrosis and
hemorrhage. Agents that cause SOS include cancer chemotherapeutic
agents, particularly alkylating agents such as busulfan,
cyclophosphamide and platinum coordination complexes - carboplatin,
cisplatin and oxaliplatin. The diagnosis of SOS is usually based on
its typical clinical presentation, which is characterized by
hepatomegaly, ascites, portal hypertension, weight gain and
jaundice (16), or exclusion of other
causes of liver injury. In bone marrow transplant recipients, the
usual differential diagnosis includes graft vs. host disease,
sepsis, other forms of drug-induced liver injury and viral
hepatitis. The diagnosis is usually supported by imaging, revealing
changes typical of sinusoidal obstruction. Liver biopsy is
diagnostic, but not usually necessary, and may be difficult due to
concurrent coagulopathy or thrombocytopenia (13).
Oxaliplatin has been found to induce hepatic
sinusoidal injury (HSI), defined as disruption of the sinusoidal
endothelium and collagen deposition in the perisinusoidal space.
HSI may result in portal hypertension, splenomegaly and, finally,
thrombocytopenia. However, our finding of an early change in
platelet levels suggests that thrombocytopenia occurs at an earlier
stage.
Platelets are invisible on tissue samples stained
with hematoxylin and eosin, as they have no nuclei. Immunostaining
of the platelet surface marker CD42b (glycoprotein Ibα), may be
used to visualize the presence of platelets (17). Normal liver tissues exhibit
maintenance of hepatocyte structure, the space of Disse and
sinusoidal endothelium, with blood cells, including platelets,
present only in the sinusoidal spaces. We observed that, in
patients with mild SOS, a few platelets were present in the
sinusoidal spaces and the hepatocytes remained intact. In patients
with severe SOS, however, platelets were observed in contact with
hepatocytes, particularly in zone III, with hepatocyte
destruction.
These results suggested that the disruption of
sinusoidal endothelial cells by oxaliplatin allows activated
platelets to migrate into the space of Disse and aggregate. These
activated platelets secrete various growth factors, including
platelet-activating factor (PAF), thromboxane (TX)A2,
thrombospondin, vascular endothelial growth factor (VEGF)-A and
plasminogen activator inhibitor (PAI)-1 (18), which may cause the liver injury
observed in SOS. PAF and TXA2 may cause central vein occlusion and
portal hypertension (19).
Transforming growth factor (TGF)-β, activated by thrombospondin
(20), causes collagen deposition in
the perisinusoidal space and inhibits substance exchange in the
space of Disse, resulting in bilirubin elevation and an abnormal
ICG retention rate. PAI-1 and TGF-β interfere with liver
regeneration by suppressing hepatocyte growth factor (21). VEGF-A, which usually acts as a
vasodilator, may paradoxically act as a vasoconstrictor under
conditions of endothelial failure (22). Moreover, bevacizumab, an antibody
against VEGF-A, was found to protect against SOS in patients
administered oxaliplatin-based chemotherapy for CRLM (23–25).
In summary, treatment of CRLM patients with
oxaliplatin-based NAC induces thrombocytopenia, along with platelet
aggregation in zone III. This extravasated platelet aggregation in
the space of Disse, particularly around the central vein, may play
an important role in the development of SOS. Moreover, antiplatelet
drugs may prevent the onset of SOS in patients administered
oxaliplatin-based chemotherapy for CRLM.
References
|
1
|
Rubbia-Brandt L, Audard V, Sartoretti P,
et al: Severe hepatic sinusoidal obstruction associated with
oxaliplatin-based chemotherapy in patients with metastatic
colorectal cancer. Ann Oncol. 15:460–466. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hubert C, Fervaille C, Sempoux C, et al:
Prevalence and clinical relevance of pathological hepatic changes
occurring after neoadjuvant chemotherapy for colorectal liver
metastases. Surgery. 147:185–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vauthey JN, Pawlik TM, Ribero D, et al:
Chemotherapy regimen predicts steatohepatitis and an increase in
90-day mortality after surgery for hepatic colorectal metastases. J
Clin Oncol. 24:2065–2072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamandl D, Klinger M, Eipeldauer S, et al:
Sinusoidal obstruction syndrome impairs long-term outcome of
colorectal liver metastases treated with resection after
neoadjuvant chemotherapy. Ann Surg Oncol. 18:421–430. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robinson SM, Wilson CH, Burt AD, Manas DM
and White SA: Chemotherapy-associated liver injury in patients with
colorectal liver metastases: a systematic review and meta-analysis.
Ann Surg Oncol. 19:4287–4299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aloysius MM, Zaitoun AM, Beckingham IJ, et
al: The pathological response to neoadjuvant chemotherapy with
FOLFOX-4 for colorectal liver metastases: a comparative study.
Virchows Arch. 451:943–948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakano H, Oussoultzoglou E, Rosso E, et
al: Sinusoidal injury increases morbidity after major hepatectomy
in patients with colorectal liver metastases receiving preoperative
chemotherapy. Ann Surg. 247:118–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jardim DL, Rodrigues CA, Novis YAS, Rocha
VG and Hoff PM: Oxaliplatin-related thrombocytopenia. Ann Oncol.
23:1937–1942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miura K, Nakano H, Sakurai J, et al:
Splenomegaly in FOLFOX-naive stage IV or recurrent colorectal
cancer patients due to chemotherapy-associated hepatotoxicity can
be predicted by the aspartate aminotransferase to platelet ratio
before chemotherapy. Int J Oncol. 16:257–263. 2011. View Article : Google Scholar
|
|
10
|
Krieger PM, Tamandl D, Herberger B, et al:
Evaluation of chemotherapy-associated liver injury in patients with
colorectal cancer liver metastases using indocyanine green
clearance testing. Ann Surg Oncol. 18:1644–1650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato S, Nakano H, Ishida Y and Otsubo T:
The aspartate aminotransferase to platelet ratio before
chemotherapy predicts adverse events for FOLFOX and XELOX regimens
including bevacizumab as the first-line therapy for stage IV,
recurrent and metastatic colorectal cancer. J Gastrointest Oncol.
4:203–209. 2013.PubMed/NCBI
|
|
12
|
Narita M, Oussoultzoglou E, Chenard MP, et
al: Liver injury due to chemotherapy-induced sinusoidal obstruction
syndrome is associated with sinusoidal capillarization. Ann Surg
Oncol. 19:2230–2237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morine Y, Shimada M and Utsunomiya T:
Evaluation and management of hepatic injury induced by
oxaliplatin-based chemotherapy in patients with hepatic resection
for colorectal liver metastasis. Hepatol Res. 44:59–69. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lalor PF, Herbert J, Bicknell R and Adams
DH: Hepatic sinusoidal endothelium avidly binds platelets in an
integrin-dependent manner, leading to platelet and endothelial
activation and leukocyte recruitment. Am J Physiol Gastrointest
Liver Physiol. 304:G469–G478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koga T: Correlation between sectional area
of the spleen by ultrasonic tomography and actual volume of the
removed spleen. J Clin Ultrasound. 7:119–120. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campos-Varela I, Castells L, Dopazo C, et
al: Transjugular intrahepatic portosystemic shunt for the treatment
of sinusoidal obstruction syndrome in a liver transplant recipient
and review of the literature. Liver Transpl. 18:201–205. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taaning E: Platelet immunology. ELISA for
detection of platelet antibodies, platelet-specific antigens and
platelet glycoproteins. Dan Med Bull. 39:343–354. 1992.PubMed/NCBI
|
|
18
|
Battinelli EM, Markens BA and Italiano JE:
Release of angiogenesis regulatory proteins from platelet alpha
granules: modulation of physiologic and pathologic angiogenesis.
Blood. 118:1359–1369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui S, Shibamoto T, Liu W, Takano H and
Kurata Y: Effects of platelet-activating factor, thromboxane A2 and
leukotriene D4 on isolated perfused rat liver. Prostaglandins Other
Lipid Mediat. 80:35–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Venkatraman L, Chia SM, Narmada BC, et al:
Plasmin triggers a switch-like decrease in thrombospondin-dependent
activation of TGF-β1. Biophys J. 103:1060–1068. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Narmada BC, Chia SM, Tucker-Kellogg L and
Yu H: HGF regulates the activation of TGF-β1 in rat hepatocytes and
hepatic stellate cells. J Cell Physiol. 228:393–401. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parenti A, Brogelli L, Filippi S, Donnini
S and Ledda F: Effect of hypoxia and endothelial loss on vascular
smooth muscle cell responsiveness to VEGF-A: role of
flt-1/VEGF-receptor-1. Cardiovasc Res. 55:201–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ribero D, Wang H, Donadon M, et al:
Bevacizumab improves pathologic response and protects against
hepatic injury in patients treated with oxaliplatin-based
chemotherapy for colorectal liver metastases. Cancer.
110:2761–2767. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rubbia-Brandt L, Lauwers GY, Wang H, et
al: Sinusoidal obstruction syndrome and nodular regenerative
hyperplasia are frequent oxaliplatin-associated liver lesions and
partially prevented by bevacizumab in patients with hepatic
colorectal metastasis. Hepatology. 56:430–439. 2010.
|
|
25
|
van der Pool AEM, Marsman HA, Verheij J,
et al: Effect of bevacizumab added preoperatively to oxaliplatin on
liver injury and complications after resection of colorectal liver
metastases. J Surg Oncol. 106:892–897. 2012. View Article : Google Scholar : PubMed/NCBI
|