Introduction
The lung is a main site of metastatic disease for
the majority of solid tumors and local treatments have been playing
an emerging role in combination with systemic therapies. In
oligometastatic (1–3 pulmonary nodules) and clinically selected
patients (good performance status and absent or stable
extra-thoracic disease), surgery can be considered as the standard
option, with good results in terms of local control and survival
rate (1).
Patients with pulmonary metastases often receive
multiple surgeries due to the fact that not all the metastatic
disease is Wdetectable at first presentation and the high
likelihood of recurrence. Therefore, invasive therapies have been
explored to treat patients with oligometastatic disease in an
effort to improve the long-term survival rate and quality of life
in patients who are not able to undergo surgery. Radiofrequency
ablation (RFA) is one such technique that generates tissue heating
to necrose tumors in situ and has been proved to be safe and
effective in treating selected patients with certain solid tumors
unsuitable for surgical resection (2).
In addition, several studies have shown that RFA was
as effective as surgical resection in selected patients with
primary pulmonary tumors (3–5).
Patients and methods
Patient characteristics
Between January 2011 and September 2014, 67 patients
with 115 lung metastases were enrolled in the prospective trial.
Patient characteristics are listed in Table I. The decision to perform RFA was made
by a multidisciplinary team and approved by the Ethics Committee of
the Fudan University Shanghai Cancer Center and the patients.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Patient
characteristics | n |
|---|
| Patients | 67 |
| Lesions | 115 |
| Gender |
|
| Male | 38 |
|
Female | 29 |
| Age, years |
|
|
>65 | 15 |
| ≤65 | 52 |
| Pulmonary
metastases |
|
| 1 | 36 |
| 2 | 14 |
| 3 | 17 |
| Prior chemotherapy
for metastatases |
|
| Yes | 47 |
| No | 20 |
| Presence of
extrathoracic disease |
|
| Yes | 33 |
| No | 34 |
| Primary tumor |
|
| CRC | 26 |
| HCC | 5 |
|
NSCLC | 13 |
| GCC | 3 |
| STC | 7 |
| RCC | 2 |
| EC | 7 |
| GC | 1 |
| BC | 3 |
The selection criteria for RFA were: i) 1–3 lung
metastases at the treatment time, with a maximum tumor diameter
<50 mm; ii) minimum distance was 10 mm apart from the big
trachea, primary bronchi, esophagus, great vessels and heart; iii)
medically inoperable or patients refused surgery; iv) absent or
controlled extra-thoracic disease [at computed tomography (CT) or
positron emission tomography (PET)-CT or magnetic resonance imaging
confirmed prior to RFA].
All the patients had biopsies of their metastatic
lung lesions proving metastatic disease prior to RFA and their
grade of Eastern Cooperative Oncology Group were grade 0–1.
Patients who had coagulation disorders, severe heart or pulmonary
failure, or uncontrolled infection were excluded from the
trial.
RFA
The equipment used for RFA of lung lesions consisted
of the radiofrequency generator (CelonLab POWER), cold circulation
pump (Celon Aquaflow Ⅲ), radiofrequency needle electrode (Celon
proSurge: T20, T30 and T40 is an electrode length of 20, 30 and 40
mm respectively, and maximum output power of 20, 30 and 40 W;
Olympus Surgical Technologies Europe, Hamburg, Germany).
The puncture point, direction, depth and the needle
electrode type and number were confirmed by the CT scan (120 kV,
100 mA, 3 mm thickness) and 3-dimentional reconstruction prior to
treatment. Puncturing was subsequently performed, and the CT scan
confirmed whether the needle was at the right place, prior to
connecting the radiofrequency generator and commencing RFA.
Ablation finished when ground-glass opacity was 5–10 mm away from
the tumor boundary. Subsequently, the needles were withdrawn and
the CT scan was performed again to observe the occurrence of
pneumothorax, hemorrhage and other complications.
Follow-up and assessment
All the patients on the trial had a CT carried out 1
month after RFA and this served as a new basis for the comparison
of future scans. Subsequently, CT scans were obtained within ~3, 6,
9 and 12 months after RFA and every 6 months after 1 year. PET-CT
was recommended at the physician's discretion. The criteria of the
assessment of local lesion were abiding by the modified Response
Evaluation Criteria in Solid Tumors (mRECIST) (6). Local control defined that the target
lesion had not progressed during the follow-up period and was
analyzed by every lesion.
Statistical methods
Primary endpoint of the clinical study was local
control, and the secondary endpoints were overall survival (OS),
progression-free survival (PFS) and treatment-related toxicity.
Local control was calculated by χ2 test,
and PFS and OS were calculated using the Kaplan-Meier method. Cox
proportional hazards was used to calculate the hazard ratios (HRs)
and 95% confidence interval (CI) in multivariate analysis. All
P-values are two-sided and P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
carried out with the Statistical Package for the Social Sciences
v19 (SPSS; IBM Corp, Armonk, NY, USA).
Results
Local tumor control
In the study, all the punctures were successful.
According to mRECIST, 101 in 115 (87.8%) lesions were confirmed as
complete response (CR) in the follow-up, 3 (2.6%) were partial
response (PR), 5 (4.3%) were stable disease, 6 (5.2%) were
progression disease and 4 of these 6 patients received
radiotherapy, with the other 2 receiving RFA again and obtaining
CR.
PFS and OS
Median follow-up after RFA was 24 months (range,
3–39). A total of 41 patients succumbed to disease progression in
the lung (other sites of lung) and extrapulmonary sites and 2 were
lost during follow-up. In total, 24 patients were remained.
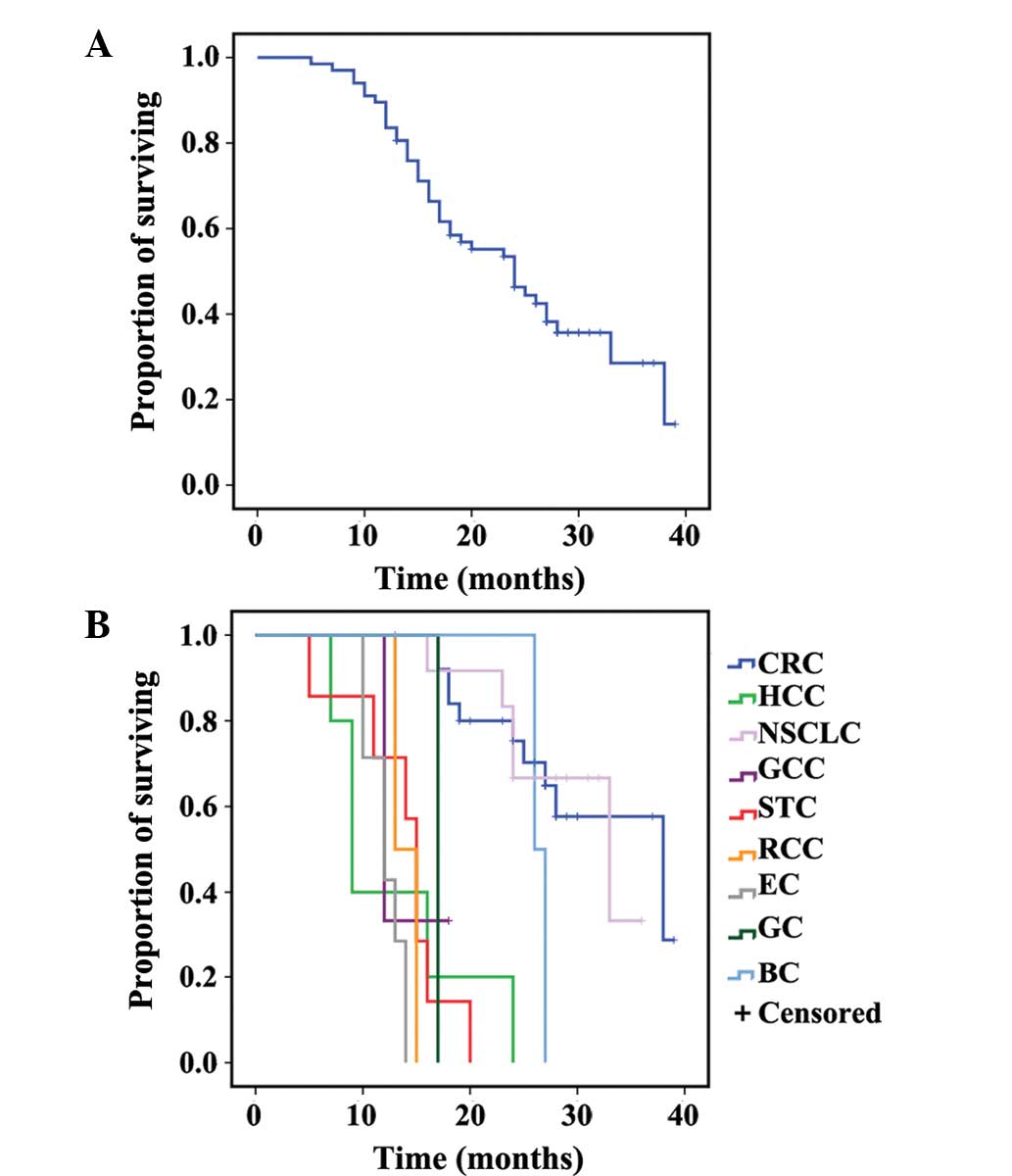
The median PFS from RFA was 14 months (95% CI,
1.6–16.4). The 6-, 12- and 18-month PFS rates were 82.1, 55.7 and
27.5%, respectively. The median OS from RFA was 24 months (95% CI,
18.2–29.8). The 1-, 2- and 3-year OS rates were 83.6, 46.3 and
14.3%, respectively. Primary tumor was significantly correlated to
PFS and OS on multivariate analysis, and the other variates showed
no significance difference (Figs. 1
and 2, and Tables II and III).
 | Table II.Multivariate analysis. |
Table II.
Multivariate analysis.
|
| PFS | OS |
|---|
|
|
|
|
|---|
| Factors | HR | P-value | HR | P-value |
|---|
| Gender | 0.596 | 0.092 | 0.632 | 0.186 |
| Age, years | 1.133 | 0.743 | 1.591 | 0.269 |
| Mets, n | 1.361 | 0.091 | 1.279 | 0.213 |
| Prior chemo | 0.835 | 0.586 | 1.341 | 0.401 |
| Extra D | 0.967 | 0.916 | 0.758 | 0.463 |
| Primary | 1.213 | 0.002 | 1.290 | 0.001 |
 | Table III.PFS and OS of different primary
tumors. |
Table III.
PFS and OS of different primary
tumors.
|
| PFS, months | OS, months |
|---|
|
|
|
|
|---|
| Primary | Median | 6 | 12 | 18 | Median | 1 | 2 | 3 |
|---|
| CRC | 18 | 100.0 | 72.9 | 48.6 | 38 | 92.0 | 75.3 | 28.8 |
| HCC | 6 | 20.0 | – | – | 9 | 20.0 | – | – |
| LC | 16 | 83.9 | 71.9 | 24.0 | 33 | 91.7 | 66.7 | – |
| GCC | 7 | 33.3 | – | – | 12 | 33.3 | – | – |
| STC | 8 | 42.9 | – | – | 15 | 57.1 | – | – |
| RCC | 5 | – | – | – | 13 | 50.0 | – | – |
| EC | 8 | 57.1 | – | – | 12 | 42.9 | – | – |
| GC | 17 | – | – | – | 17 | – | – | – |
| BC | 15 | 100.0 | 66.7 | – | 26 | 50.0 | – | – |
| All patients | 14 | 82.1 | 55.7 | 27.5 | 24 | 83.6 | 46.3 | 14.3 |
Complications
There was no periprocedural mortality in the trial.
The main complications and relative treatments are listed in
Table IV.
 | Table IV.Main complications and treatment. |
Table IV.
Main complications and treatment.
| Complications | n (%) | Treatment |
|---|
| Pneumothorax | 8 (11.9) | 2 patients
percutaneous chest tube |
| Pneumorrhagia | 6 (9.0) | Intravenous
injection thrombin |
| Pleural
effussion | 7 (10.4) | Combined by
infection, antibiotics and percutaneous chest tube |
| Fever | 7 (10.4) | Indometacin
suppositories anal plug |
| Thoracalgia | 6 (9.0) | Self-healing |
|
Aerodermectasia | 2 (3.0) | Self-healing |
| Emesia | 1 (1.5) | Self-healing |
Discussion
The lung is a main site of metastatic disease for
the majority of solid tumors, and local ablation techniques, such
as radiofrequency, cryotherapy (7)
and microwave (8), were widely used
for patients who were not candidates for surgery.
Hiraki et al (3) applied RFA to non-small cell lung cancer
(NSCLC) and evaluated the role of RFA in the treatment of
early-stage NSCLC and concluded that RFA may currently be reserved
for early-stage NSCLC patients who are unfit for sublobar resection
or stereotactic body radiotherapy (SBRT). Schlijper et al
(1) reviewed 27 studies and
identified that the treatment-related mortality rates for RFA and
surgery were 0 and 1.4–2.4%, respectively, whereas morbidity rates
were reported inconsistently but appeared to be the lower for
surgery. The study by Ochiai et al (9) was conducted to compare the clinical
outcomes of RFA with those of SBRT. The RFA and SBRT groups showed
a similar 3-year local tumor progression (9.6 vs. 7.0%, p=0.746)
and OS rates (86.4 vs. 79.6%, p=0.738).
In recent studies, RFA was certificated with
efficacy and safety to lung metastases. The Matsui et al
(10) retrospective study showed that
RFA was a promising treatment option for patients with pulmonary
metastases from esophageal cancer. Baba et al (11,12)
applied RFA to pulmonary metastases from gastrointestinal cancers
and esophageal squamous cell carcinoma. Nakamura et al
(13) and Koelblinger et al
(14) applied RFA in elderly patients
with lung metastases from musculoskeletal sarcomas and concluded
that elderly sarcoma patients with lung metastases should always be
considered for either metastasectomy or RFA. In particular, Petre
et al (15) and Hiraki et
al (16) studied the OS of
patients with lung metastases treated by RFA and identified that
short- to mid-term survival after RFA appears to be promising and
is 85–95% in 1 year and 45–55% in 3 years.
RFA as a radical means can destroy all tumor or
normal cells in its ablation extent and no tumors reoccur
theoretically. Due to the special site and shape of tumors, the
ablation was not complete in certain patients. In the present
study, the local control rate was that 87.8% (101/115) of lesions
were confirmed CR, 3 (2.6%) were PR, and only 6 (5.2%) progressed.
During the observational period, no periprocedural mortality
occurred. The median follow-up of PFS was 14 months and the
progression reasons were outside the RFA site, including distant
intrapulmonary, and intra- and extrapulmonary progression.
Therefore, we can conclude that the local lesions were well
controlled by RFA.
In the present study, primary tumor was
significantly correlated to PFS and OS on multivariate analysis. It
is well known that each tumor has its own biological
characteristics and its prognosis is different from other tumors.
PFS or OS in the study was in accordance with the biological
characteristics; the patients of colorectal cancer survived
longer.
However, the outcome of the 1-, 2- and 3-year OS was
83.6, 46.3 and 14.3%, respectively. The outcome was not as good as
other similar studies. The selection criteria of the patients was
not the same as others, the ratio of ≥2 pulmonary metastases and
having extrathoracic diseases was higher compared to other studies,
and the two factors were risk factors in uinvariate analysis
although they had no statistical significance in multivariate
analysis. Petre et al (15)
found that lesions >1.5 cm in size had a higher risk of local
progression compared to those ≤1.5 cm. Baschnagel et al
(17) identified that the 3-year
survival rate of patients with the lung as the only known site of
metastatic disease treated with SBRT was 71 vs. 58% in patients who
had extrathoracic disease treated prior or subsequent to SBRT.
Therefore, more patients should be enrolled to confirm that ≥2
pulmonary metastases and having extrathoracic disease were the risk
factors. Another possible reason was the different treatment
following progression, as there was no treatment guideline in this
stage and the majority inclined to adopt systematic chemotherapy.
In the study, 70.1% (47/67) patients have received chemotherapy to
lung metastases and the lesions were residual disease following
chemotherapy, the sensitivity of next line chemotherapy to
progression following RFA was lower than first line chemotherapy.
These were possibly the reasons for the low survival rate.
In conclusion, RFA is safe for patients and can be
considered as a promising treatment option for patients with
pulmonary metastases.
References
|
1
|
Schlijper RC, Grutters JP, Houben R,
Dingemans AM, Wildberger JE, Van Raemdonck D, Van Cutsem E,
Haustermans K, Lammering G, Lambin P, et al: What to choose as
radical local treatment for lung metastases from colo-rectal
cancer: Surgery or radiofrequency ablation? Cancer Treat Rev.
40:60–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valls C, Ramos E, Leiva D, Ruiz S,
Martinez L and Rafecas A: Safety and efficacy of ultrasound-guided
radiofrequency ablation of recurrent colorectal cancer liver
metastases after hepatectomy. Scand J Surg. 2014.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hiraki T, Gobara H, Iguchi T, Fujiwara H,
Matsui Y and Kanazawa S: Radiofrequency ablation for early-stage
nonsmall cell lung cancer. Biomed Res Int. 2014:1520872014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iguchi T, Hiraki T, Gobara H, Fujiwara H,
Matsui Y, Soh J, Toyooka S, Kiura K and Kanazawa S: Percutaneous
radiofrequency ablation of lung cancer presenting as ground-glass
opacity. Cardiovasc Intervent Radiol. 2014.(Epub ahead of
print).
|
|
5
|
Crabtree T, Puri V, Timmerman R, Fernando
H, Bradley J, Decker PA, Paulus R, Putnum JB Jr, Dupuy DE and
Meyers B: Treatment of stage I lung cancer in high-risk and
inoperable patients: Comparison of prospective clinical trials
using stereotactic body radiotherapy (RTOG 0236), sublobar
resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG
Z4033). J Thorac Cardiovasc Surg. 145:692–699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Littrup PJ, Duan Y, Zhang Y, Feng
H and Nie Z: Thoracic masses treated with percutaneous cryotherapy:
Initial experience with more than 200 procedures. Radiology.
235:289–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolf FJ, Grand DJ, Machan JT, Dipetrillo
TA, Mayo-Smith Smith and Dupuy DE: Microwave ablation of lung
malignancies: Effectiveness, CT findings, and safety in 50
patients. Radiology. 247:871–879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ochiai S, Yamakado K, Kodama H, Nomoto Y,
Ii N, Takaki H and Sakuma H: Comparison of therapeutic results from
radiofrequency ablation and stereotactic body radiotherapy in
solitary lung tumors measuring 5 cm or smaller. Int J Clin Oncol.
2014.(Epub ahead of print). View Article : Google Scholar
|
|
10
|
Matsui Y, Hiraki T, Gobara H, Fujiwara H,
Iguchi T, Shirakawa Y, Fujiwara T, Toyooka S and Kanazawa S:
Percutaneous radiofrequency ablation for pulmonary metastases from
esophageal cancer: Retrospective evaluation of 21 patients. J Vasc
Interv Radiol. 25:1566–1572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baba Y, Watanabe M, Yoshida N, Kawanaka K,
Yamashita Y and Baba H: Radiofrequency ablation for pulmonary
metastases from gastrointestinal cancers. Ann Thorac Cardiovasc
Surg. 20:99–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baba Y, Watanabe M, Kawanaka K, Iwagami S,
Ishimoto T, Iwatsuki M, Yoshida N, Yamashita Y and Baba H:
Radiofrequency ablation for pulmonary metastases from esophageal
squamous cell carcinoma. Dis Esophagus. 27:36–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura T, Matsumine A, Yamakado K, Takao
M, Uchida A and Sudo A: Clinical significance of radiofrequency
ablation and metastasectomy in elderly patients with lung
metastases from musculoskeletal sarcomas. J Cancer Res Ther.
9:219–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koelblinger C, Strauss S and Gillams A:
Outcome after radiofrequency ablation of sarcoma lung metastases.
Cardiovasc Intervent Radiol. 37:147–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petre EN, Jia X, Thornton RH, Sofocleous
CT, Alago W, Kemeny NE and Solomon SB: Treatment of pulmonary
colorectal metastases by radiofrequency ablation. Clin Colorectal
Cancer. 12:37–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hiraki T, Gobara H, Iguchi T, Fujiwara H,
Matsui Y and Kanazawa S: Radiofrequency ablation as treatment for
pulmonary metastasis of colorectal cancer. World J Gastroenterol.
20:988–996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baschnagel AM, Mangona VS, Robertson JM,
Welsh RJ, Kestin LL and Grills IS: Lung metastases treated with
image-guided stereotactic body radiation therapy. Clin Oncol (R
Coll Radiol). 25:236–241. 2013. View Article : Google Scholar : PubMed/NCBI
|