Introduction
Small-cell lung cancer (SCLC) is a distinct entity
that accounts for ~15% of all new cases of lung cancer.
Approximately 60–70% of SCLC patients are diagnosed with extensive
disease. Although extensive-disease-SCLC (ED-SCLC) is responsive to
initial chemotherapy, it commonly progresses or relapses within
months and long-term survival is poor (1).
Although various novel anticancer therapies have
been developed for other types of cancer, there have been no
significant advances in the therapeutic approach to ED-SCLC over
the last two decades. To improve the outcome of ED-SCLC, various
chemotherapeutic agents and strategies, including three-drug
combinations (2,3), maintenance or consolidation therapy
beyond 4–6 cycles of standard chemotherapy (4), alternating or sequential combination
therapies (5,6) and dose-intensified regimens (7,8) have been
evaluated. However, none of these approaches demonstrated a
significant advantage over traditional platinum-containing
treatment. In a previous Japanese phase III trial (JCOG9511), the
combination of irinotecan and cisplatin (IP) was found to be
superior to etoposide and cisplatin (EP) for ED-SCLC (9). However, two large North American phase
III trials failed to demonstrate a survival benefit of IP in
comparison with EP (10,11).
Etoposide in combination with a platinum agent
remains one of the standard first-line treatments for ED-SCLC, with
a progression-free survival (PFS) of ~4–6 months and an overall
survival (OS) of 8–11 months (12).
During treatment with EP, patients frequently experience adverse
events (AEs), mainly myelosuppression and febrile neutropenia.
Grade 3–4 neutropenia occurs in 50–80% of the patients,
thrombocytopenia in 10–20% and febrile neutropenia in 5–10%,
requiring dose modifications in a significant proportion of the
patients (10,11). Treatment-related AEs are associated
with treatment delay, reduced quality of life, unnecessary
hospitalization, increase of medical costs and, occasionally,
treatment-related death. Therefore, the design of a more tolerable
regimen to reduce AEs while maintaining acceptable efficacy is
required for ED-SCLC.
Despite the use of several combination chemotherapy
regimens that include etoposide, the optimal dose and schedule of
etoposide administration remains unknown. An intravenous infusion
of etoposide over 1–2 h for 3–5 consecutive days is currently
widely accepted as the routine schedule in the clinical practice
(13). However, several consecutive
days of etoposide infusion require frequent visits to the hospital
or hospitalization, causing inconvenience and additional medical
costs. One-day (24-h) continuous etoposide infusion demonstrated an
inferior clinical outcome compared with a daily 2-h infusion of
etoposide for 5 days in SCLC (14).
However, the effect of multiple 2-h infusions of etoposide within
one day has not been evaluated.
In this study, we amended the dose and schedule of
conventional EP and devised a modified EP, to achieve better
tolerability and convenience. The aim of the present study was to
retrospectively assess the efficacy and safety of this modified EP
as first-line therapy administered to a consecutive series of
ED-SCLC patients at a single institution.
Patients and methods
Patient eligibility
A total of 52 patients were diagnosed with SCLC at
the Inje University Paik Hospital (Busan, Korea) between March,
2010 and January 2014 and ED-SCLC was confirmed in 43 of those
patients. A total of 2 patients requested transfer to other
hospitals for further treatment; 2 patients rejected chemotherapy
treatment and received supportive care alone; and 3 patients were
bedridden, with an Eastern Cooperative Oncology Group performance
status (ECOG PS) of 4 and were not eligible for chemotherapy.
Finally, a total of 36 consecutive patients were treated with the
modified EP as first-line therapy and were included in this
analysis.
This retrospective study was approved by the Local
Ethics Board according to the Good Clinical Practice guidelines and
applicable laws and the principles of the Declaration of
Helsinki.
Treatment
The modified EP employed the lowest practicable EP
dose of conventional EP regimens, including 240 mg/m2
etoposide and 60 mg/m2 cisplatin. One cycle consisted of
21 days. The sequence of chemotherapy administration for the
modified EP was as follows: i) Premedication; ii) two infusions of
120 mg/m2 etoposide diluted in 1,000 ml of 0.9% NaCl and
each administered over 120 min; iii) infusion of 60
mg/m2 cisplatin over 60 min. The patients received the
modified EP as described above for a maximum of 6 cycles, unless
disease progression or unacceptable toxicity occurred. Subsequent
cycles of chemotherapy were permitted only if the absolute
neutrophil count was ≥1.5×109/l, the platelet count was
≥100×109/l, the estimated glomerular filtration rate was
>60 ml/min/1.73 m2 and other treatment-related
non-hematological toxicities (excluding alopecia) had resolved to
≤grade 1. Treatment delay and dose modifications of EP complied
with the general rules according to the grade and duration of
hematological and/or non-hematological toxicities. The available
modified dose level of EP was 180 and 45 mg/m2 for
etoposide and cisplatin, respectively (75% of the initial target
dose of the modified EP regimen). Treatment administration was
recorded and relative dose intensity (RDI) (in
mg/m2/week) was calculated as the total dose/body
surface area divided by the number of weeks between treatment
initiation and the first day of the last treatment plus 3 weeks.
Granulocyte colony-stimulating factor was prescribed for patients
with neutropenia under the discretion of the treating physician,
but prophylactic use was not allowed.
Assessment of the response and
AEs
The following tests were undertaken within 4 weeks
of chemotherapy initiation: A complete history and physical
examination, electrocardiogram, chest X-ray, computed tomography
(CT) of the chest and upper abdomen, magnetic resonance imaging of
the brain, bone scan and positron emission tomography. The response
was assessed with CT scans every 2 cycles of therapy, according to
the Response Evaluation Criteria in Solid Tumors (RECIST), version
1.1 (15). The severity of the AEs
was graded according to the Common Terminology Criteria for Adverse
Events, version 4.0 (16). The
patients received the modified EP for a maximum of 6 cycles, unless
disease progression or unacceptable toxicity occurred. The
toxicities were assessed immediately prior to each treatment cycle
and graded.
Statistical analysis
PFS was defined as the time between treatment
initiation and disease progression, death, or last known follow-up
(whichever occurred first). OS was defined as the interval between
treatment initiation and death or last follow-up. PFS and OS were
considered to have been censored at the last follow-up visit if the
event had not occurred. Response rate (RR) was calculated according
to RECIST 1.1. Kaplan-Meier curves were used to describe OS and
PFS. Data analyses were conducted using Statistical Analysis
Systems software, version 9.1 (SAS Institute Inc. Cary, NC).
Results
Patient characteristics
A total of 36 patients were included in this
analysis. The baseline characteristics at diagnosis are summarized
in Table I. The median age of
patients was 66 years (range, 43–81 years) and 25 (70%) of the
patients were men. The ECOG PS was 0–1 in 21 (58%), 2 in 9 (25%)
and 3 in 6 (17%) patients.
 | Table I.Baseline characteristics of the
patients (n=36). |
Table I.
Baseline characteristics of the
patients (n=36).
| Characteristics | No. of patients
(%) |
|---|
| Age, years |
|
| Median
(range) | 66 (43–81) |
| Gender |
|
| Male | 25 (70.0) |
|
Female | 11 (30.0) |
| ECOG PS |
|
| 0 | 2 (5.0) |
| 1 | 19 (53.0) |
| 2 | 9 (25.0) |
| 3 | 6 (17.0) |
| Metastatic sites |
|
| Adrenal
gland | 5 (14.0) |
| Bone | 15 (42.0) |
|
Brain | 6 (17.0) |
|
Contralateral lung | 9 (25.0) |
|
Liver | 11 (30.0) |
| Malignant
pleural effusion | 14 (39.0) |
Treatment administration
The median number of chemotherapy cycles
administered was 6 (range, 2–6) and 22 (61%) patients completed all
6 cycles. Failure to complete the 6 scheduled cycles was due to
progression during treatment (n=13, 36%) and patient withdrawal
(n=1, 3%). AE-related treatment delays occurred in 7 cycles in 6
patients out of 170 cycles. Dose reduction of etoposide was
required in 3 patients (8%) due to neutropenia and a >25% dose
reduction of etoposide (180 mg/m2) was not necessary. No
dose modification of cisplatin was required. The mean RDI of
etoposide and cisplatin was 94.7 and 98.5% of the planned dose,
respectively.
Treatment efficacy
Of the 36 patients, 35 were evaluable for tumor
response. A total of 24 patients exhibited a confirmed objective
tumor response [overall response rate of 66%, with a complete
response rate of 3% and a partial response (PR) rate of 63%]. Five
(14%) and 7 patients (20%) presented with stable disease (SD) and
progrssive disease (PD), respectively. One patient was excluded
from tumor assessment as he rejected further treatment during cycle
3 and response confirmation by CT was not available (Table II). All 36 patients were assessable
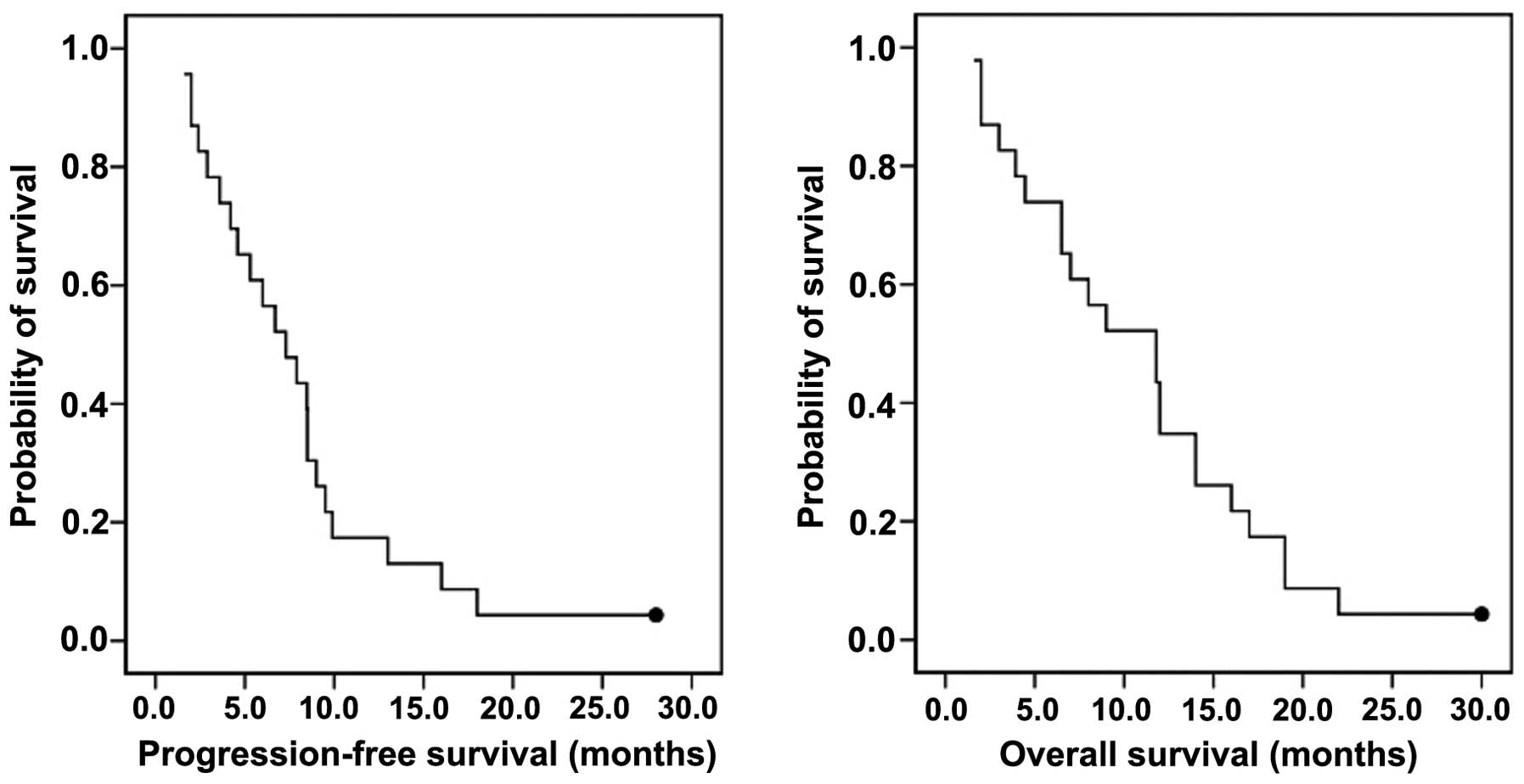
for survival analysis. The median follow-up at the time of the
analysis was 30.1 months. The median OS was 11.8 months [95%
confidence interval (CI): 7.9–15.3] and the PFS was 7.3 months (95%
CI: 5.2–9.7). The survival estimates at 1 year were 35 and 17% for
OS and PFS, respectively (Fig.
1).
 | Table II.Response to treatment
(n=35)a. |
Table II.
Response to treatment
(n=35)a.
| Type of response | No. of patients
(%) |
|---|
| Complete
response | 1 (3.0) |
| Partial response | 22 (63.0) |
| Stable disease | 5 (14.0) |
| Progressive
disease | 7 (20.0) |
Treatment-related toxicity
All 36 patients were evaluable for safety.
Treatment-related toxicities are summarized in Table III. In general, chemotherapy was
well tolerated and the majority of the AEs were grade 1 or 2. Grade
3 neutropenia and anemia were reported in 6 and 8% of the patients,
respectively. Grade 4 hematological toxicities and febrile
neutropenia were not observed during this treatment.
Non-hematological toxicities were also tolerable. There were 2
cases of grade 3 and 1 of grade 4 non-hematological AEs during
treatment. The single case of grade 4 AE was pneumonia without
neutropenia occurring during the first cycle of treatment,
requiring hospitalization and administration of antibiotics. The
patient recovered from the pneumonia and completed 6 cycles of
treatment. There was no reported treatment-related mortality.
 | Table III.Hematological and non-hematological
adverse events. |
Table III.
Hematological and non-hematological
adverse events.
|
| Grade, no. (%) |
|---|
|
|
|
|---|
| Adverse
eventsa | 1 | 2 | 3 | 4 |
|---|
| Leukopenia | 9 (25.0) | 4 (11.0) | 2 (6.0) | 0 (0.0) |
| Neutropenia | 6 (17.0) | 4 (11.0) | 2 (6.0) | 0 (0.0) |
| Anemia | 16 (44.0) | 9 (25.0) | 3 (8.0) | 0 (0.0) |
| TCP | 2 (6.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| FN | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anorexia | 11 (31.0) | 2 (9.0) | 0 (0.0) | 0 (0.0) |
| Nausea | 4 (17.0) | 3 (8.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 3 (8.0) | 1 (3.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 2 (6.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) |
| Mucositis | 3 (8.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 9 (25.0) | 2 (6.0) | 0 (0.0) | 0 (0.0) |
| Dyspnea | 5 (14.0) | 5 (14.0) | 1 (3.0) | 0 (0.0) |
| Weight loss | 3 (8.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AKI | 3 (8.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Infection | 0 (0.0) | 0 (0.0) | 1 (3.0) | 1 (3.0) |
Salvage treatment after
progression
Second-line chemotherapy was administered to 25
patients, 7 (28%) of whom had sensitive SCLC (recurring >3
months after completion of the initial therapy), whereas 18 (72%)
had resistant or refractory SCLC (progressing during chemotherapy
or recurring within 3 months after completion of initial therapy).
A total of 5 patients (20%) achieved a PR, 6 (24%) had SD and 14
(56%) had PD. Third-line chemotherapy was administered to 15
patients and fourth-line chemotherapy to 6 patients (data not
shown).
Discussion
This retrospective analysis assessed the efficacy
and safety of the modified EP combination chemotherapy to estimate
its advantages compared with conventional EP as first-line
treatment in patients with ED-SCLC. The modified EP regimen of the
present study applied the lowest practicable EP dose of
conventional EP regimens to improve tolerance. The administration
of etoposide was performed by multiple infusions within one day for
better convenience, instead of conventional consecutive infusions
over 3–5 days. The length of time for the completion of the
treatment was ~6 h. A total of 36 patients with ED-SCLC were
included in this study and the data suggested that the modified EP
appeared to exhibit favorable tolerability and acceptable efficacy.
The regimen achieved an overall RR of 66%, with a median PFS of 7.3
months and a median OS of 11.8 months. The chemotherapy treatment
was well tolerated, with only 1 case of grade 4 non-hematological
AEs, no grade 4 hematological toxicities and no treatment-related
deaths. The mean RDI of etoposide and cisplatin was measured to be
94.7 and 98.5% of the planned dose, respectively, indicating that
the treatment was conducted without frequent dose modifications or
treatment delays.
The OS of patients with ED-SCLC enrolled in phase
III trials has not improved significantly over the last few years
(17). Recent phase III trials
investigating ED-SCLC (3,10,11,18–20)
revealed a median OS of ~9–11 months, regardless of the treatment
options. Lara et al (11)
reported a pivotal trial including 651 patients comparing the
efficacy of EP and IP as first-line treatment for patients with
ED-SCLC. A total of 327 patients were assigned to the EP arm,
exhibiting a median PFS of 5.2 months and a median OS of 9.1
months. Fink et al (18)
evaluated the efficacy of EP and topotecan/cisplatin as first-line
treatment for patients with ED-SCLC. The EP arm included 345
patients with a median time-to-progression of 5.7 months and a
median OS of 9.5 months. Patients treated with the modified EP
regimen exhibited a median PFS of 7.3 months and a median OS of
11.8 months, which are within the expected range of survival time
for patients with ED-SCLC. Although there are clear limitations to
the present study due to its retrospective nature and small patient
sample, the modified EP appeared to exhibit an acceptable efficacy,
despite employing the lowest practicable dose of cisplatin and
etoposide of conventional EP regimens. To the best of our
knowledge, there have been no clinical data to support that
higher-intensity regimens yield better outcomes in the treatment of
ED-SCLC patients; they are, however, associated with higher
toxicity (2–8). The formulation of a less agressive
regimen may be an alternative practicable approach if the treatment
is equally effective, as a more tolerable regimen may decrease the
incidence of treatment-related toxicities, time of hospitalization
and overall medical costs during the chemotherapy treatment.
Previous clinical trials on EP for ED-SCLC
demonstrated that a significant portion of the patients experienced
high-grade treatment-related toxicities, leading to treatment
delays and dose modifications. In the phase III study of Fink et
al (18), 37.7 and 2.7% of the
334 EP-treated patients developed grade 4 neutropenia and febrile
neutropenia, respectively. Lara et al (11) reported that grade 4 neutropenia and
febrile neutropenia occurred in 48 and 6%, respectively, of 324
patients who were treated with EP. The mean RDI was 78% for
etoposide and 81% for cisplatin, suggesting that frequent dose
reductions and treatment delays were necessary during the
treatment. The retrospective design of the present study did not
allow for accurate and comprehensive assessment of
treatment-related toxicities during the treatment. However, AEs
during EP treatment are usually hematological and associated with
myelosuppression. The hematological toxicities could be reviewed
and evaluated reliably, as complete blood counts were measured
routinely prior to the administration of the chemotherapy and
stored as laboratory medical data. In the present study, there was
no report of grade 4 neutropenia, thrombocytopenia or febrile
neutropenia during treatment with the modified EP, suggesting that
the modified EP may be tolerable as first-line treatment for
patients with ED-SCLC. Considering its low treatment-related
toxicity and acceptable efficacy, modified EP may be a viable
option for elderly or frail patients, in whom AEs during treatment
are a major concern.
We acknowledge a number of limitations and
weaknesses of this study. First, the number of patients in the
analysis was limited, which may not have provided sufficient
evidence to support the efficacy and safety of the modified EP
regimen. Second, survival data were collected retrospectively and
comparisons were made only with historical controls, although the
authors treated patients consecutively during the study period to
minimize selection bias. Third, the toxicity profiles may not be
complete and certain major AEs may have been missed. Therefore, the
present study should be considered as preliminary regarding the
efficacy and safety of modified EP and our results should be
interpreted with caution. Prospective trials including adequate
numbers of ED-SCLC patients must be undertaken to confirm the
results of this study.
To the best of our knowledge, this is the first
study to assess the efficacy and safety of EP combination with
multiple injections of etoposide in one day as first-line treatment
for ED-SCLC. Our data suggest that the modified EP regimen deserves
further clinical evaluation with respect to its efficacy, toxicity
profile and convenience of administration. Based upon the present
study, we are planning to design a prospective randomized clinical
trial to evaluate the therapeutic role of the modified EP compared
with that of conventional EP as first-line treatment in patients
with ED-SCLC.
References
|
1
|
Hann CL and Rudin CM: Management of
small-cell lung cancer: Incremental changes but hope for the
future. Oncology (Williston Park). 22:1486–1492. 2008.PubMed/NCBI
|
|
2
|
Pujol JL, Daurès JP, Rivière A, et al:
Etoposide plus cisplatin with or without the combination of
4′-epidoxorubicin plus cyclophosphamide in treatment of extensive
small-cell lung cancer: A French Federation of Cancer Institutes
multicenter phase III randomized study. J Natl Cancer Inst.
93:300–308. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niell HB, Herndon JE II, Miller AA, et al:
Cancer and Leukemia Group: Randomized phase III intergroup trial of
etoposide and cisplatin with or without paclitaxel and granulocyte
colony-stimulating factor in patients with extensive-stage
small-cell lung cancer: Cancer and Leukemia Group B Trial 9732. J
Clin Oncol. 23:3752–3759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiller JH, Adak S, Cella D, DeVore RF
III and Johnson DH: Topotecan versus observation after cisplatin
plus etoposide in extensive-stage small-cell lung cancer: E7593 - a
phase III trial of the Eastern Cooperative Oncology Group. J Clin
Oncol. 19:2114–2122. 2001.PubMed/NCBI
|
|
5
|
Fukuoka M, Furuse K, Saijo N, Nishiwaki Y,
Ikegami H, Tamura T, Shimoyama M and Suemasu K: Randomized trial of
cyclophosphamide, doxorubicin and vincristine versus cisplatin and
etoposide versus alternation of these regimens in small-cell lung
cancer. J Natl Cancer Inst. 83:855–861. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roth BJ, Johnson DH, Einhorn LH, et al:
Randomized study of cyclophosphamide, doxorubicin and vincristine
versus etoposide and cisplatin versus alternation of these two
regimens in extensive small-cell lung cancer: A phase III trial of
the Southeastern Cancer Study Group. J Clin Oncol. 10:282–291.
1992.PubMed/NCBI
|
|
7
|
Ihde DC, Mulshine JL, Kramer BS, et al:
Prospective randomized comparison of high-dose and standard-dose
etoposide and cisplatin chemotherapy in patients with
extensive-stage small-cell lung cancer. J Clin Oncol. 12:2022–2034.
1994.PubMed/NCBI
|
|
8
|
Thatcher N, Girling DJ, Hopwood P,
Sambrook RJ, Qian W and Stephens RJ: Improving survival without
reducing quality of life in small-cell lung cancer patients by
increasing the dose-intensity of chemotherapy with granulocyte
colony-stimulating factor support: Results of a British Medical
Research Council Multicenter Randomized Trial. Medical Research
Council Lung Cancer Working Party. J Clin Oncol. 18:395–404.
2000.PubMed/NCBI
|
|
9
|
Noda K, Nishiwaki Y, Kawahara M, et al:
Japan Clinical Oncology Group: Irinotecan plus cisplatin compared
with etoposide plus cisplatin for extensive small-cell lung cancer.
N Engl J Med. 346:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanna N, Bunn PA Jr, Langer C, et al:
Randomized phase III trial comparing irinotecan/cisplatin with
etoposide/cisplatin in patients with previously untreated
extensive-stage disease small-cell lung cancer. J Clin Oncol.
24:2038–2043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lara PN Jr, Natale R, Crowley J, et al:
Phase III trial of irinotecan/cisplatin compared with
etoposide/cisplatin in extensive-stage small-cell lung cancer:
Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol.
27:2530–2535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foster NR, Qi Y, Shi Q, Krook JE, Kugler
JW, Jett JR, Molina JR, Schild SE, Adjei AA and Mandrekar SJ: Tumor
response and progression-free survival as potential surrogate
endpoints for overall survival in extensive stage small-cell lung
cancer: Findings on the basis of North Central Cancer Treatment
Group trials. Cancer. 117:1262–1271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hande KR: Etoposide: Four decades of
development of a topoisomerase II inhibitor. Eur J Cancer.
34:1514–1521. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slevin ML, Clark PI, Joel SP, Malik S,
Osborne RJ, Gregory WM, Lowe DG, Reznek RH and Wrigley PF: A
randomized trial to evaluate the effect of schedule on the activity
of etoposide in small-cell lung cancer. J Clin Oncol. 7:1333–1340.
1989.PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: Revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE) v4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmAccessed.
May 28–2009
|
|
17
|
Oze I, Hotta K, Kiura K, Ochi N, Takigawa
N, Fujiwara Y, Tabata M and Tanimoto M: Twenty-seven years of phase
III trials for patients with extensive disease small-cell lung
cancer: Disappointing results. PLoS One. 4:e78352009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fink TH, Huber RM, Heigener DF, et al:
‘Aktion Bronchialkarzinom’ (ABC Study Group): Topotecan/cisplatin
compared with cisplatin/etoposide as first-line treatment for
patients with extensive disease small-cell lung cancer: Final
results of a randomized phase III trial. J Thorac Oncol.
7:1432–1439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmittel A, Sebastian M, Fischer von
Weikersthal L, et al: Arbeitsgemeinschaft Internistische Onkologie
Thoracic Oncology Study Group: A German multicenter, randomized
phase III trial comparing irinotecan-carboplatin with
etoposide-carboplatin as first-line therapy for extensive-disease
small-cell lung cancer. Ann Oncol. 22:1798–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Jong WK, Groen HJM, Koolen MGJ, Biesma
B, Willems LN, Kwa HB, van Bochove A, van Tinteren H and Smit EF:
Phase III study of cyclophosphamide, doxorubicin, and etoposide
compared with carboplatin and paclitaxel in patients with extensive
disease small-cell lung cancer. Eur J Cancer. 43:2345–2350. 2007.
View Article : Google Scholar : PubMed/NCBI
|