Introduction
Thyroid nodules are commonly encountered lesions and
have been observed in 50% of autopsied patients (1). The estimated annual incidence rate of
0.1% in the US suggests 300,000 newly diagnosed nodules as of 2005
(2). Although only 1 of 20 clinically
identified nodules is malignant (3),
it is important to exclude the presence of a malignant thyroid
lesion (2,4).
Previous studies have demonstrated the feasibility
of contrast-enhanced ultrasound (CEUS) for the differentiation of
benign and malignant thyroid nodules (5). Nemec et al (6) reported that the complete CEUS data of 42
patients (73.8% benign and 26.2% malignant nodules) revealed a
significant difference in enhancement between benign and malignant
nodules. Furthermore, CEUS demonstrated a sensitivity of 76.9%,
specificity of 84.8% and accuracy of 82.6%. Quantitative analysis
of CEUS using a microbubble contrast agent allows the
differentiation of benign and malignant thyroid nodules and may
potentially serve, in addition to grey-scale and Doppler
ultrasound, as an adjunctive tool in the assessment of patients
with thyroid nodules. Hornung et al (7) reported that CEUS represents a highly
sensitive method for the detection of the microvascularization of
thyroid carcinomas. Future studies should compare these findings to
benign pathologies in order to establish CEUS as a standard
diagnostic procedure in the preoperative evaluation of suspicious
thyroid nodules. Zhang et al (8) reported that CEUS enhancement patterns
were different in benign and malignant lesions. Ring enhancement
was predictive of benign lesions, whereas heterogeneous enhancement
was helpful for detecting malignant lesions.
The ultrasonic imaging characteristics of 68
patients with thyroid carcinoma proved by pathology were
retrospectively analyzed in the present study. The correlation of
real-time CEUS characteristic was mainly discussed in association
with the lesion size.
Materials and methods
Study population
The study was approved by the Ethics Committee of
Shanghai Pudong New Area People Hospital (Pudong, Shanghai, China),
and patient consent was obtained. A total of 158 thyroid tumor
cases were examined with CEUS in the hospital between January 2012
and August 2014. In total, 68 patients with thyroid carcinoma
confirmed by pathology were recruited in the study. There were 31
male and 37 female patients with a mean age of 39.2±12.8 years
(range, 24–75 years) except thyroid diffuse diseases. All the 68
cases were solitary lesion with a mean diameter of 18±9 mm (max,
5–32 mm). Written informed consent was obtained from all the
patients prior to the exam. All the cases were diagnosed by
histological evaluation: Papillary carcinoma was diagnosed in 53
patients, follicular carcinoma in 11, papillary follicular
carcinoma in 3 and medullary carcinoma in 1.
Ultrasound techniques
Siemens Sequoia 512 color Doppler ultrasound
(Siemens, Bavaria, Germany) with the contrast pulse sequencing
(CPS) image condition, probe model 15L8Ws and a frequency of 7 MHz
were used in the examination. All the patients were examined in the
same conditions of mechanical index, depth, gain and time gain
compensation. The contrast agent was SonoVue® (SonoVue, Bracco,
Italy); 25 mg lyophilized powder with 5 ml saline configured as a
suspension, artificially agitated well.
Image analysis
Subjects lay supine on the examination Table with
their neck hyper-extended. All the examinations were performed by
the same experienced operator. A two-dimensional high-frequency
probe was used to observe location, size and Doppler flow signals
of thyroid nodules. The largest section of the lesions was selected
as the ultrasound imaging section, and when possible, the whole
image and the surrounding area of the thyroid nodule were observed.
The focus point was the place at the trailing edge of the lesion,
and the gain was adjusted to reveal only the lesion boundaries.
Subsequently, CPS was initiated by bolus injection through the
elbow vein with 2.5 ml contrast agent, washed with 5 ml saline.
When CPS started, the operator fixed the probe and asked the
patient to avoid swallowing. The whole dynamic imaging process was
stored on the machine's hard disk and ultrasound workstation for
subsequent memorial processing and analysis. Real-time ultrasound
contrast images were analyzed by the physicians with 5 years of
ultrasound scanning experience. Each physician was blinded to the
results of another physician. A senior physician analyzed the
images when there were different opinions. The physicians discussed
the images to provide the final agreement. In total there were 68
cases of thyroid cancer divided into 3 groups by the maximum
diameter of the nodules; <10, 10–20 and >20 mm. On observing
the real-time ultrasound imaging process, the following data and
analysis interpretation angiography characteristics were recorded:
i) Absolute enhancement beginning time: From contrast agent bolus
injected to contrast agent appearance; ii) relative enhancement
beginning time: Contrast normal thyroid lesions began to increase
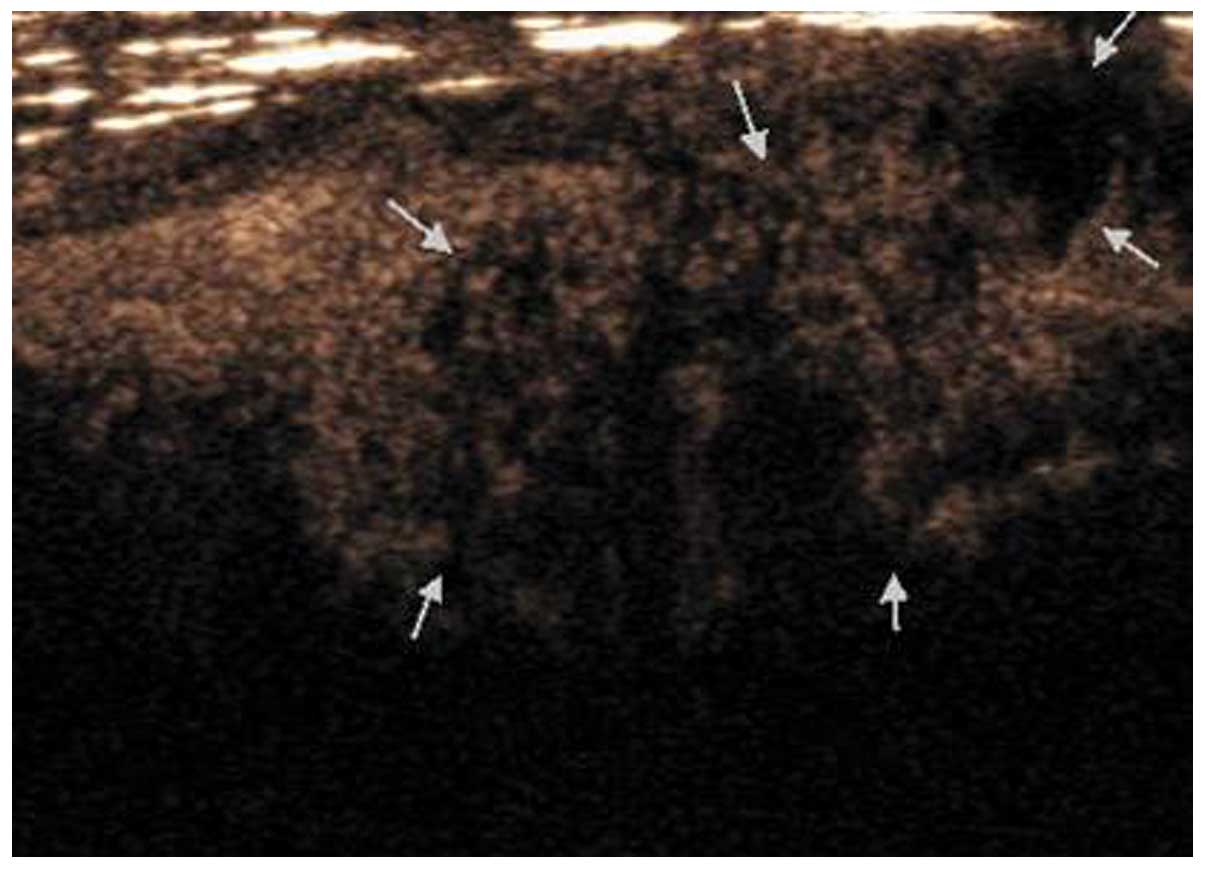
early or late; iii) uniformity of enhancement: Homogeneous or
heterogeneous enhancement (Fig. 1);
iv) the existence of perfusion defects (Fig. 2); v) enhanced sequence: Concentric,
eccentric or diffuse; vi) intensity of enhancement (compared to the
surrounding normal thyroid): High, equal or low enhancement; vii)
enhancement of lesion border: Clear, less clear or ill boundary
(Fig. 3); For iii)-vii) characters
were analyzed in contrast to the peak.
Statistical analysis
The SAS 8.0 statistical software (SAS Institute
Inc., Cary, NC, USA) was used for data analysis. Measurement data
are expressed as mean ± standard deviation. Analysis of variance
was used in the absolute enhancement beginning time of thyroid
cancer. Enhancement characteristics were analyzed by the
χ2 test, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Absolute enhancement beginning time of
normal thyroid and thyroid cancer
The contrast agent began to increase 8–17 sec after
injection in the normal thyroid tissue, with an average of
12.28±1.16 sec. In total, 68 cases of thyroid cancer were enhanced.
The absolute enhancement beginning time for the diameter of
carcinoma being <10, 10–20 and >20 mm was 15.21±3.62,
14.88±3.45 and 14.59±3.17 sec, respectively. There was no
statistical significance among the 3 groups (P>0.05).
Association between relative
enhancement beginning time and size of thyroid cancer
The 3 groups of thyroid cancer enhanced later than
the surrounding normal thyroid (Table
I). There was no statistical significance among the 3 groups
(P>0.05).
 | Table I.Association between relative
enhancement beginning time and size of thyroid nodules. |
Table I.
Association between relative
enhancement beginning time and size of thyroid nodules.
|
| Tumor diameter,
mm |
|
|
|---|
|
|
|
|
|
|---|
| Relative enhancement
beginning time | <10, n | 10–20, n | >20, n | χ2 | P-valuea |
|---|
| Cases | 21 | 35 | 12 |
|
|
| Earlier | 2 | 3 | 1 | 0.0192 | 0.9905 |
| Equal | 6 | 9 | 3 | 0.0712 | 0.9650 |
| Later | 13 | 23 | 8 | 0.1079 | 0.9475 |
Association between enhancement
characteristics and size of thyroid cancer
All the 3 groups of thyroid cancer showed
heterogeneous and concentric enhancement (Table II). There was no statistical
significance among the 3 groups (P>0.05). In lesions with
diameters <10 mm or between 10–20 mm, insignificant enhancement
was usually observed, while in lesions with diameter >20 mm,
hyper-enhancement was usually observed. The difference was
statistically significant (P<0.05). With the increase of
diameter, the perfusion defects of nodules increased by 28.57,
54.29 and 75.00%, respectively. The difference was statistically
significant (P<0.05).
 | Table II.Association between enhancement
characteristics and size of thyroid cancer. |
Table II.
Association between enhancement
characteristics and size of thyroid cancer.
|
| Tumor diameter,
mm |
|
|
|---|
|
|
|
|
|
|---|
| Enhancement
characteristics | <10, n | 10–20, n | >20, n | χ2 | P-valuea |
|---|
| Cases | 21 | 35 | 12 |
|
|
| Uniformity of
enchancement |
|
|
|
|
|
|
Homogeneous | 3 | 4 | 2 | 0.0099 | 0.9209 |
|
Heterogeneous | 18 | 31 | 10 |
|
|
| Perfusion
defects | 6 | 19 | 9 | 6.9695 | 0.0083 |
| Enhanced
sequence |
|
|
|
|
|
|
Concentric | 13 | 23 | 8 | 0.0576 | 0.9716 |
|
Eccentric | 5 | 4 | 1 |
|
|
|
Diffuse | 3 | 8 | 3 |
|
|
| Intensity of
enhancement |
|
|
|
|
|
| High | 1 | 3 | 7 | 14.4743 | 0.0001 |
|
Equal | 3 | 5 | 3 |
|
|
| Low | 17 | 27 | 2 |
|
|
| Enhancement of lesion
border |
|
|
|
|
|
|
Clear | 1 | 4 | 2 | 0.7126 | 0.3986 |
| Less
clear | 5 | 11 | 2 |
|
|
| Ill | 15 | 20 | 8 |
|
|
Discussion
A tumor is a type of typical vascular-dependent
lesion. Small blood vessels with diameters <40 µm can be
detected by CEUS. Previously, clear results have been achieved in
studying tumor vascular perfusion features by CEUS for focal liver
lesions, but the study of its application in thyroid nodules was
only preliminary (9–12). In the present study, real-time CEUS
images for 68 cases of patients with thyroid cancer were
retrospectively analyzed with the main purpose of exploring the
association between characteristics of thyroid carcinomas in
real-time CEUS and tumor sizes.
In the present study, the absolute enhancement
beginning time for different lesions were not statistically
significant, as the enhancement beginning time following injection
of the contrast agent could be affected by various factors, such as
differences in time of contrast agent bolus injection among
different operators and difference in intimate circulation of the
contrast agent among different individuals. Therefore, relative
enhancement beginning time was used to evaluate the enhancement
time of different lesions. The majority of lesions enhanced later
than the surrounding thyroid gland and the differences in features
of enhancement for lesions among different groups were not
statistically significant. The predominant enhancement pattern in
different groups was heterogeneous concentric enhancement, and the
majority of lesions showed less clear or poorly defined margins
following enhancement. The study by Zheng et al (13) reported that 35 thyroid carcinomas
presented three enhancement patterns with CEUS. These were type I:
23 lesions enhanced in a pattern of ring with centripetal fill-in,
however, the central part had no contrast agent filling; type II: 5
lesions enhanced regularly and homogeneously; and type III: 7
lesions enhanced irregularly and homogeneously.
We believe that the above enhancement features are
in accordance with the pathological features. Vascular pathological
anatomy for thyroid cancer is complicated. Generally, neovascular
could be divided into surrounding and central area, which showed
different vascular distribution characteristics. Blood vessels in
the surrounding area are relatively concentrated and the tumor
usually grows infiltratively outward, which leads to the formation
of less clear or poorly defined margins following enhancement;
while blood vessels in the central area are relatively less
concentrated. Therefore, the difference in the abundance of blood
vessels between the surrounding and central area are the main
reason for concentric enhancement. For the growth heterogeneity and
neovascular damage caused by malignant infiltration, thyroid cancer
is always combined with fibrosis and hyalinization degeneration.
The original vascular networks would be damaged, which results in
coexistence of abundant blood supply in certain areas and
inabundant blood supply in other areas. Existence of various
arteriovenous fistulas enhanced the imbalance of vascular networks
(14). All the abovementioned reasons
contribute to the heterogeneous enhancement in CEUS for thyroid
cancer.
As indicated in the present study, the incidence of
perfusion defect within lesions increased with the increase of
lesion diameter, which is consistent with the growth feature of
malignant tumor. A previous study reported that during the growth
of the malignant tumor, the doubling time for vascular endothelial
cells and tumor cells is different, which indicates that the tumor
growth speed is much higher than the formation speed of
microvessels (15). The increase of
the microvessel number is relatively slower and the incidence is
more evident with the growth of the tumor. With the growth of the
tumor, the blood supply in the lesion is poorer, so the incidence
of part or complete defect is higher, and this results in a higher
rate of local perfusion defects.
Bartolotta et al (5) identified that the enhancement pattern of
thyroid nodules in CEUS was closely associated with nodule size,
which was indicated as an inabundant blood supply in CEUS for
malignant lesions <10 mm, few nodular enhancements for 10–20 mm
lesion and diffuse enhancement for lesions >20 mm. With a larger
sample, the enhancement features in CEUS for groups with different
lesion sizes were analyzed in this study. The results indicate
that, in groups with a lesion diameter <10 mm and between 10–20
mm, CEUS mainly showed a not significant enhancement, but mainly
hyper-enhancement in the group with a lesion size >20 mm. The
following may be the reasons for this: Growth of the tumor consists
of two stages, from the slow-growing stage without blood vessels
(pre-vascular phase) to fast-growing stage with blood vessels
(vascular phase). Without or with fewer blood vessels, the
enhancement observed in CEUS for relatively smaller tumors was
mainly insignificant enhancement. When the tumor grows quickly,
various new blood vessels form under the introduction of multiple
angiogenic factors to meet the requirements of fast growth. With
complicated vascular network, hyper-enhancement was usually
observed in relatively large thyroid cancer lesions.
In conclusion, there is a certain correlation
between enhancement features in CEUS for thyroid cancer and lesion
size. In lesions with diameters <10 mm or between 10–20 mm,
insignificant enhancement was usually observed, while in lesions
with diameters >20 mm, hyper-enhancement was usually observed.
The incidence of perfusion defects within the lesion increases with
larger lesion diameters. Real-time CEUS can provide valuable
information for clinical diagnosis.
Acknowledgements
The study was supported by grants from Pudong New
Area Health Plan Board of Health Science and Technology Project in
Shanghai (no. PW2014A-23), Pudong New Area leading talents training
plan (no. PWR12012-02), Shanghai Health Bureau research projects
(no. 20134059) and Shanghai Pudong Science and Technology
Innovation Fund (no. PKJ2012-Y56).
References
|
1
|
Mortensen JD, Woolner LB and Bennett WA:
Gross and microscopic findings in clinically normal thyroid glands.
J Clin Endocrinol Metab. 15:1270–1280. 1995. View Article : Google Scholar
|
|
2
|
Gharib H, Papini E, Paschke R, Duick DS,
Valcavi R, Hegedüs L and Vitti P: American Association of Clinical
Endocrinologists, Associazione Medici Endocrinologi and European
Thyroid Association medical guidelines for clinical practice for
the diagnosis and management of thyroid nodules: Executive summary
of recommendations. J Endocrinol Invest. 33:51–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hegedus L: Clinical practice. The thyroid
nodule. N Engl J Med. 351:1764–1771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frates MC, Benson CB, Charboneau JW, Cibas
ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB,
Goellner JR, Hay ID, Hertzberg BS, Intenzo CM, Jeffrey RB, Langer
JE, Larsen PR, Mandel SJ, Middleton WD, Reading CC, Sherman SI and
Tessler FN: Management of thyroid nodules detected at US: Society
of radiologists in ultrasound consensus conference statement.
Radiology. 237:794–800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartolotta TV, Midiri M, Galia M, Runza G,
Attard M, Savoia G, Lagalla R and Cardinale AE: Qualitative and
quantitative evaluation of solitary thyroid nodules with
contrast-enhanced ultrasound: initial results. Eur Radiol.
16:2234–2241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nemec U, Nemec SF, Novotny C, Weber M,
Czerny C and Krestan CR: Quantitative evaluation of
contrast-enhanced ultrasound after intravenous administration of a
microbubble contrast agent for differentiation of benign and
malignant thyroid nodules: assessment of diagnostic accuracy. Eur
Radiol. 22:1357–1365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hornung M, Jung EM, Georgieva M, Schlitt
HJ, Stroszczynski C and Agha A: Detection of microvascularization
of thyroid carcinomas using linear high resolution
contrast-enhanced ultrasonography (CEUS). Clin Hemorheol Microcirc.
52:197–203. 2012.PubMed/NCBI
|
|
8
|
Zhang B, Jiang YX, Liu JB, Yang M, Dai Q,
Zhu QL and Gao P: Utility of contrast-enhanced ultrasound for
evaluation of thyroid nodules. Thyroid. 20:51–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Friedrich-Rust M, Sperber A, Holzer K,
Diener J, Grünwald F, Badenhoop K, Weber S, Kriener S, Herrmann E,
Bechstein WO, Zeuzem S and Bojunga J: Real-time elastography and
contrast-enhanced ultrasound for the assessment of thyroid nodules.
Exp Clin Endocrinol Diabetes. 118:602–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu HX: Contrast-enhanced ultrasound: The
evolving applications. World J Radiol. 1:15–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agha A, Hornung M, Rennert J, Uller W,
Lighvani H, Schlitt HJ and Jung EM: Contrast-enhanced
ultrasonography for localization of pathologic glands in patients
with primary hyperparathyroidism. Surgery. 151:580–586. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giusti M, Orlandi D, Melle G, Massa B,
Silvestri E, Minuto F and Turtulici G: Is there a real diagnostic
impact of elastosonography and contrast-enhanced ultrasonography in
the management of thyroid nodules? J Zhejiang Univ Sci B.
14:195–206. 2013.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng XJ, Zhang YK, Zhao CY, Liang JR, LE
HB, Jiang JF, Wang H, Zou SD and Chen YF: Enhancement pattern of
thyroid carcinoma with contrast-enhanced ultrasound. Zhonghua YiXue
Za Zhi. 90:42–45. 2010.(In Chinese).
|
|
14
|
Averkious M, Powers J, Skyba D, Bruce M
and Jensen S: Ultrasound contrast imaging research. ultrasound Q.
19:27–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|