Introduction
Prostate cancer is one of the most common cancers
among men in China. The morbidity and mortality rates of prostate
cancer were reported to be 2.74 and 2.26% in 2013; however, these
rates have decreased over the last 10 years due to
prostate-specific antigen (PSA) screening. The standard first-line
treatment of metastatic prostate cancer is androgen-deprivation
therapy (ADT) (1). However, the
majority of the patients develop progressive disease in 14–30
months, which is resistant to ADT and referred to as
castration-resistant prostate cancer (CRPC).
The optimal therapy for patients with CRPC is
considered to be chemotherapy with taxane-based regimens,
abiraterone and sipuleucel-T (2–4). However,
patients aged ≥70 years may not be suitable for these therapies and
treatment selection should be carefully considered due to the
different patient status. A phase II clinical study demonstrated
that the combination of granulocyte-macrophage colony-stimulating
factor (GM-CSF) and thalidomide may decrease the level of PSA and
exert antitumor effects (5).
To the best of our knowledge, our study was the
first to evaluate the efficacy and determine the mechanism of
action of GM-CSF plus thalidomide in the treatment of CRPC patients
aged ≥70 years in China.
Patients and methods
Patient selection
Patients aged ≥70 years with a life expectancy of
>1 year, with histologically confirmed adenocarcinoma of the
prostate, radiographic evidence of metastases, failed previous ADT
and evidence of disease progression demonstrated by at least 3
consecutive increases in PSA, were considered eligible for this
study. Additional requirements included an Eastern Cooperative
Oncology Group (ECOG) performance status of 0 or 1 (Table I), adequate hematological function
(absolute peripheral granulocyte count ≥1,500/mm3 and
platelet count ≥100,000/mm3), adequate renal function
(serum creatinine ≤2.0 mg/ml) adequate hepatic function (bilirubin
≤1.5 mg/dl and alanine aminotransferase (ALT) ≤2 times the upper
limit of normal). All the patients signed an informed consent
approved by the Institutional Review Board of the 101th Hospital of
The People's Liberation Army, Wuxi, China.
 | Table I.Patient characteristics (n=11). |
Table I.
Patient characteristics (n=11).
| Characteristics | Values |
|---|
| Median age,
years | 77 |
| Median Gleason score
at diagnosis | 7 |
| ECOG performance
status |
|
| 0 | 7 |
| 1 | 4 |
| Sites of metastatic
disease, no. |
|
| Bone | 10 |
|
Extraosseous disease | 1 |
Treatment plan
The patients received 300 µg GM-CSF subcutaneously 3
times per week (on days 1, 3 and 6 of weeks 1 and 2); thalidomide
was initiated at a dose of 50 mg at bed time, with a 50-mg
increment over 5 days to reach the study dose of 100 mg/day. One
cycle was defined as 4 weeks of treament. The patients were treated
continuously for 4 cycles or until disease progression, patient
non-compliance or refusal to continue therapy, or development of
unpredictable, irreversible, or grade 4 toxicity.
Every 4 weeks, complete blood counts, differentials
and platelet counts were measured; electrolytes, blood urea
nitrogen and creatinine were measured and liver function tests
(aspartate aminotransferase, ALT and lactate dehydrogenase) were
performed; serum PSA levels were measured on days 1 and 8.
Considerations
The major objectives were to asses PSA response to
treatment with GM-CSF plus thalidomide and to determine whether
this combination is worthy of further investigation. A decrease in
PSA level of ≥50% from the baseline was considered to be of
clinical interest.
Results
Patient characteristics
A total of 11 CRPC patients were enrolled in this
study. All the patients were aged ≥70 years (median, 77 years), had
an ECOG performance status score of 0 or 1 and had metastatic
disease. The patient characteristics are summarized in Table I.
PSA response to GM-CSF plus
thalidomide by treatment cycle
In cycle 1, all the patients exhibited a decrease in
PSA levels, with 3 patients (27.2%) exhibiting a PSA decrease of
>50% (Table II), which indicated
that the patients responded well to this treatment. In cycle 2, 8
patients exhibited continuously decreasing PSA levels. However, 3
patients (27.2%) had a PSA rebound and in 1 patient the PSA rebound
was >50% (Table III). In cycle
3, 10 patients exhibited continuously decreasing PSA levels and in
2 patients (18.2%) the PSA decrease was >50%. In addition, 1
patient (27.2%) had a PSA rebound, although it was <50%
(Table IV). In cycle 4, 9 patients
exhibited continuously decreasing PSA levels, whereas 2 patients
(18.2%) had a PSA rebound of >50% (Table V). All 11 patients exhibited a PSA
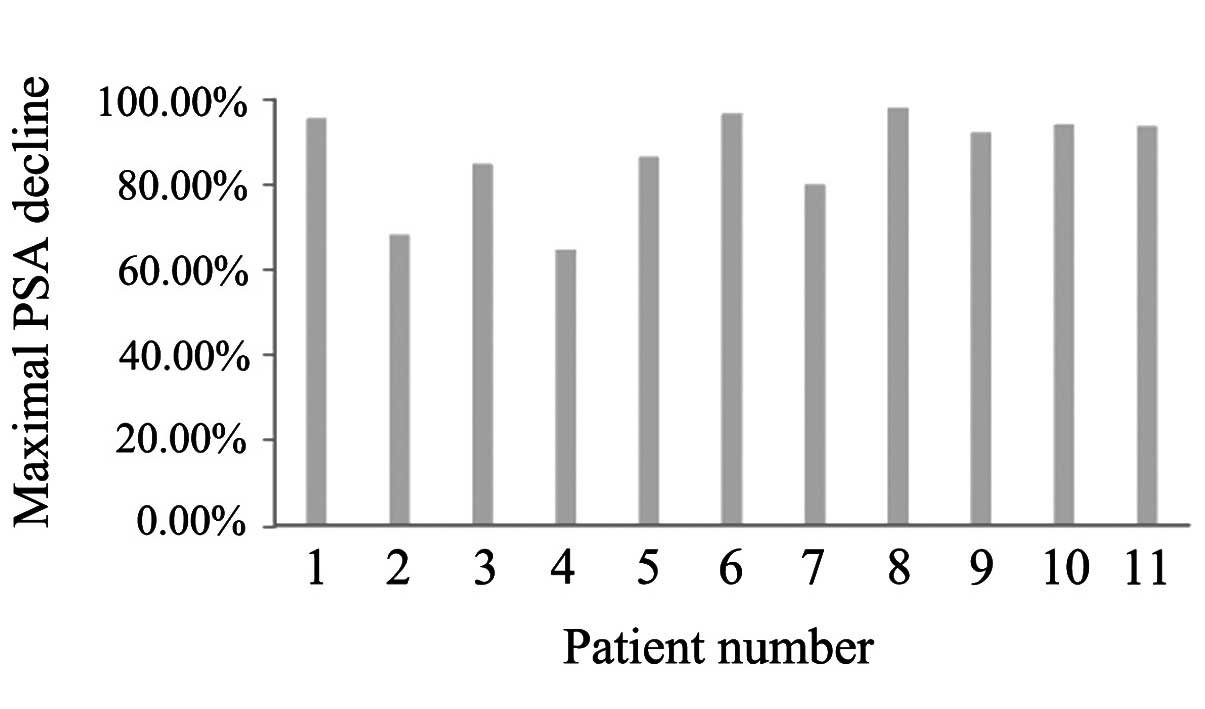
decrease in this study, with a median decrease of 92.2% (Fig. 1).
 | Table II.Prostate-specific antigen (PSA) levels
and percentage decrease in cycle 1. |
Table II.
Prostate-specific antigen (PSA) levels
and percentage decrease in cycle 1.
|
|
Patients |
|---|
|
|
|
|---|
| PSA (µg/l) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|
| Day 1 | 95.09 | 98.32 | 4,667.1 | 494.8 | 32.07 | 47.08 | 19.31 | 11.04 | 11.72 | 28.80 | 511.10 |
| Day 8 | 34.74 | 91.03 | 3,878 | 488.9 | 16.75 | 24.21 | 14.46 | 2.71 | 10.88 | 13.75 | 295.50 |
| Decrease (%) | 63.50 | 7.40 | 16.90 | 1.2 | 47.80 | 48.60 | 25.10 | 75.50 | 7.20 | 52.40 | 42.20 |
 | Table III.Prostate-specific antigen (PSA) levels
and percentage decrease in cycle 2. |
Table III.
Prostate-specific antigen (PSA) levels
and percentage decrease in cycle 2.
|
|
Patients |
|---|
|
|
|
|---|
| PSA (µg/l) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|
| Day 1 | 19.81 | 56.29 | 3,378 | 494.1 | 15.78 | 16.02 | 9.34 | 1.12 | 7.46 | 6.93 | 152.3 |
| Day 8 | 30.91 | 54.26 | 2,138 | 449.7 | 18.03 | 19.83 | 9.15 | 0.79 | 7.39 | 6.48 | 136.4 |
| Decrease (%) | −56.00 | 3.60 | 36.7 | 9.0 | −14.30 | −23.80 | 2.00 | 29.50 | 0.90 | 6.50 | 10.4 |
 | Table IV.Prostate-specific antigen (PSA) levels
and percentage decrease in cycle 3. |
Table IV.
Prostate-specific antigen (PSA) levels
and percentage decrease in cycle 3.
|
|
Patients |
|---|
|
|
|
|---|
| PSA (µg/l) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|
| Day 1 | 13.90 | 52.25 | 2,118 | 295.7 | 14.30 | 7.80 | 6.71 | 0.36 | 3.87 | 3.20 | 126.40 |
| Day 8 | 9.19 | 35.95 | 1,613 | 317.3 | 10.69 | 5.29 | 6.09 | 0.13 | 2.08 | 1.38 | 80.94 |
| Decrease (%) | 33.90 | 31.20 | 23.8 | −7.3 | 25.20 | 32.20 | 9.20 | 63.90 | 46.30 | 56.90 | 36.00 |
 | Table V.Prostate-specific antigen (PSA) levels
and percentage decrease in cycle 4. |
Table V.
Prostate-specific antigen (PSA) levels
and percentage decrease in cycle 4.
|
|
Patients |
|---|
|
|
|
|---|
| PSA (µg/l) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|
| Day 1 | 6.04 | 43.69 | 832.6 | 286.2 | 6.01 | 3.02 | 5.47 | 0.11 | 1.55 | 1.11 | 38.64 |
| Day 8 | 4.11 | 31.30 | 705.4 | 224.1 | 4.32 | 1.62 | 3.87 | 0.21 | 0.91 | 1.68 | 31.84 |
| Decrease (%) | 32.00 | 28.40 | 15.3 | 21.7 | 28.10 | 46.40 | 29.30 | −90.90 | 42.30 | −51.40 | 18.00 |
Treatment-related toxicity
The most frequent grade 1–2 toxicities were fever,
nausea, constipation, neuropathy and fatigue, whereas 1 patient
developed grade 1 deep venous thrombosis (Table VI).
 | Table VI.Toxicities associated with
granulocyte-macrophage colony-stimulating factor plus thalidomide
in patients with castration-resistant prostate cancer. |
Table VI.
Toxicities associated with
granulocyte-macrophage colony-stimulating factor plus thalidomide
in patients with castration-resistant prostate cancer.
| Toxicities | Grade 1 | Grade 2 | Grade 3 |
|---|
| Fever | 4 | 0 | 0 |
| Nausea | 4 | 0 | 0 |
| Tinnitus | 1 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 |
| Constipation | 7 | 0 | 0 |
| Neuropathy | 2 | 1 | 0 |
| Fatigue | 2 | 1 | 0 |
| Skin rash | 0 | 1 | 0 |
| Deep venous
thrombosis | 1 | 0 | 0 |
Discussion
The recommended therapy for patients with CRPC is
chemotherapy with taxane-based regimens, abiraterone and
sipuleucel-T (2–4). However, elderly patients may not be
suitable for these therapies and treatment should be carefully
selected. A phase II clinical study demonstrated that the
combination of GM-CSF and thalidomide effectively decreased the
level of PSA and exhibited antitumor activity (5).
The antitumor effect of thalidomide is attributed to
the inhibition of tumor angiogenic activity, induction of apoptosis
in vitro and reduction of the high levels of angiogenic
factors, such as vascular endothelial growth factor and basic
fibroblast growth factor in patients with prostate cancer (6–8).
Thalidomide was primarily assessed by a decline in PSA expression
at doses of 100–200 mg/day (8). A
phase 2 study reported that low-dose thalidomide (100 mg daily for
≤6 months) was effective in reducing PSA levels: 37.5% of the
treated patients exhibited a median decrease in PSA levels of 48%,
whereas a decrease of 50% was sustained throughout treatment for
15% of the patients. Another phase 2 trial compared low-dose (200
mg/day) and high-dose (≤1,200 mg/day) thalidomide in patients with
metastatic androgen-independent prostate cancer (AIPC). Serum PSA
decreases of 50% were observed in 18% of the patients in the
low-dose arm. Patients in the high-dose arm exhibited no PSA
decrease, although the side effects limited dose escalation to
>200 mg/day in 30% of the cases. A total of 27% of the patients
exhibited a PSA decrease of 40%, often associated with improvement
of the clinical symptoms (8,9).
Prostate cancer cells kill mature dendritic cells
(DCs), inhibit DC proliferation, differentiation and maturation,
thereby reducing the number of DCs and inhibiting their function.
DC deficiency and dysfunction is a major cause of prostate cancer
immune evasion. Small et al (10) demonstrated the biological activity of
GM-CSF (PSA decrement) in a series of androgen-independent and
hormonal therapy-naive prostate cancer patients. Rini et al
(11,12) investigated the biological effect of
GM-CSF (250 µg/m2/day), as measured by PSA kinetics, in
30 patients with increasing PSA levels following radical
prostatectomy or radiotherapy. Of the 29 evaluable patients, 3
(10%) achieved a 50% reduction in PSA [95% confidence interval (CI):
2–27%]. In the 26 patients whose pre-treatment PSA doubling time
(PSADT) was calculated, the median PSADT increased from 8.4 to 15
months (P﹤0.001) and the median slope of the PSA vs. time curve
decreased with treatment (P﹤0.004). Of the 29 evaluable patients, 7
(24%) remained free of disease progression after a median of 4.4
years (range, 4.0–4.8 years) of GM-CSF treatment.
Dreicer et al (5) conducted a phase 2 trial of thalidomide
plus GM-CSF in 22 patients with metastatic AIPC. GM-CSF (250 µg)
was administered 3 times per week, with thalidomide titrated to 200
mg/day, for 6 months. All 22 patients exhibited decreased PSA
levels 2 weeks after treatment. A total of 5 patients exhibited a
50% decrease in PSA from baseline (median, 58 ng/ml), verified at 4
weeks after best response. The observed response rate was 23% (95%
CI: 8–45%).
Amato et al (13) performed another phase 2 trial of
thalidomide plus GM-CSF in 21 patients with hormone-naïve prostate
cancer. GM-CSF (250 µg/m2; maximum, 500 µg) was
administered 3 times per week, with thalidomide titrated to 100
mg/day (maximum, 400 mg). In the 18 patients who responded to this
treatment, the median PSA reduction was 59% (range, 26–89%) and 12
patients (67%) exhibited a reduction of ≥50%.
In our study, the median age of the 11 patients was
77 years and, as determined by the NCCN guidelines, the patients
were not suitable for chemotherapy with taxane-based regimens. In
addition, abiraterone and sipuleucel-T have not been approved for
use in China. After obtaining permission from the patients and
their families, GM-CSF plus thalidomide was selected as the
treatment regimen. The results indicated that this combination was
clinically effective in older (aged ≥70 years, with a life
expectancy of >1 year) patients with CRPC (Fig. 1). In our study, 3 patients (27.2%) had
a PSA rebound, with 1 patient exhibiting a PSA rebound of >50%
(Table III) in cycle 2; 1 patient
(27.2%) had a PSA rebound of <50% (Table IV) in cycle 3; and 2 patients (18.2%)
had PSA rebounds of >50% (Table V)
in cycle 4. PSA rebound was attributed to extensive tumor cell
necrosis caused by the treatment, which may exhibit significant
individual differences; therefore, the changing trend of PSA during
treatment should be the main focus rather than the PSA level. In
responders, the overall median decrease in PSA was 92.2%, although
several patients exhibited a PSA rebound in different cycles. The
regimen was well tolerated, with mild (grade 1–2) toxicities. The
most frequent grade 1–2 toxicities were fever, nausea,
constipation, neuropathy and fatigue, whereas 1 patient developed
grade 1 deep venous thrombosis.
In conclusion, our study demonstrated that the
combination of GM-CSF plus thalidomide was clinically effective and
well tolerated and may be considered an optimal treatment choice
for elderly patients with CRPC.
References
|
1
|
Loblaw DA, Virgo KS, Nam R, et al: Initial
hormonal management of androgen-sensitive metastatic, recurrent, or
progressive prostate cancer. J Clin Oncol. 25:1596–1605. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tannock IF, de Wit R, Berry WR, et al:
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bissery MC, Vrignaud P and Bayssas M:
Preclinical in vivo activity of docetaxel containing combinations.
Proc Am Soc Clin Oncol. 14:4891995.
|
|
4
|
Kantoff PW, Higano CS, Shore ND, et al:
Sipuleucel-T immunotherapy for castration-resistant prostate
cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dreicer R, Klein EA, Elson P, et al: Phase
II trial of GM-CSF thalidomide in patients with
androgen-independent metastatic prostate cancer. Urol Oncol.
23:82–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Franks ME, Macpherson GR and Figg WD:
Thalidomide. Lancet. 363:1802–1811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Amato RJ, Loughnan MS, Flynn E, et al:
Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci
USA. 91:4082–4085. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Drake MJ, Robson W, Mehta P, et al: An
open-label phase II study of low-dose thalidomide in
androgen-independent prostate cancer. Br J Cancer. 88:822–827.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Figg WD, Dahut W, Duray P, et al: A
randomized phase II trial of thalidomide, an angiogenesis
inhibitor, in patients with androgen-independent prostate cancer.
Clin Cancer Res. 7:1888–1893. 2001.PubMed/NCBI
|
|
10
|
Small EJ, Reese DM, Um B, et al: Therapy
of advanced prostate cancer with granulocyte macrophage
colony-stimulating factor. Clin Can Res. 5:1738–1744. 1999.
|
|
11
|
Rini B, Weinberg V, Bok R and Small EJ:
Prostate-specific antigen kinetics as a measure of the biologic
effect of granulocyte-macrophage colony-stimulating factor in
patients with serologic progression of prostate cancer. J Clin
Oncol. 21:99–105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rini BI, Fong L, Weinberg V, Kavanaugh B
and Small EJ: Clinical and immunological characteristics of
patients with serologic progression of prostate cancer achieving
long-term disease control with granulocyte-macrophage
colony-stimulating factor. J Urol. 75:2087–2091. 2006. View Article : Google Scholar
|
|
13
|
Amato RJ, Hernandez-McClain J and Henary
H: Phase 2 study of granulocyte-macrophage colony-stimulating
factor plus thalidomide in patients with hormone-naïve
adenocarcinoma of the prostate. Urol Oncol. 27:8–13. 2009.
View Article : Google Scholar : PubMed/NCBI
|