Introduction
Cervical cancer is the second most common
gynecological malignancy (1).
Concurrent chemoradiation therapy (CCRT) with platinum-based
chemotherapy has been established as the standard of care for
patients with stage IB2-IVA cervical cancer [International
Federation of Gynecology and Obstetrics (FIGO) classification]
(2,3).
However, the recurrence rate of cervical cancer varies between 11
and 22% in FIGO stage IB-IIA and between 28 and 64% in FIGO stage
IIB-IVA (4).
Inflammation has been shown to enable various cancer
characteristics, thus affecting prognosis. Recent evidence has
indicated that relative differences in neutrophil, platelet and
lymphocyte counts, and neutrophil-lymphocyte ratio (NLR) and
platelet-lymphocyte ratio (PLR) are systemic indicators of
prognosis (5). C-reactive protein
(CRP) also increases under inflammation in cancer (6). Pretreatment measurements of peripheral
blood NLR, PLR and CRP are independent predictors of a poor
prognosis in various types of cancer (7–12).
However, their predictive role in recurrent cervical cancer is
unknown. In the present study, the associations among NLR, PLR and
CRP and the clinical characteristics and prognoses of patients with
recurrent cervical cancer following CCRT were investigated.
Materials and methods
Study subjects
The clinicopathological characteristics of 32
patients with recurrent cervical cancer who had been treated in the
Department of Obstetrics and Gynecology of Okayama University
Hospital (Okayama, Japan) between April 2005 and June 2014 were
retrospectively analyzed. The primary treatment of these patients
was CCRT. All the patients were treated with a combination of
external irradiation (50 Gy administered in 25 fractions over 5
weeks; 4-field box technique) and high-dose intracavitary
brachytherapy (24 Gy/4 times); and concurrent chemotherapy of
either cisplatin (40 mg/m2 infusion weekly for 6 cycles)
or nedaplatin (30 mg/m2 infusion weekly for 8 cycles).
Following the primary treatment, the patients underwent follow-up
examinations approximately every 1–2 months for the first 6 months,
and subsequently, every 3 months for the next 2 years. The study
protocol was approved by the Institutional Review Board of Okayama
University Hospital. Informed consent was obtained from all the
patients.
Chemotherapy
The policy of the Department of Obstetrics and
Gynecology requires a performance score ≤2 (World Health
Organization criteria) prior to initiating second-line
chemotherapy, involving 60 mg/m2 TC-paclitaxel weekly
and carboplatin [area under the plasma-concentration curve (AUC),
1.5] (Bristol-Myers Squibb, New York, NY, USA). Chemotherapy for
recurrent disease was continued until complete response or
progressive disease was observed. Third-line chemotherapy consisted
of single-agent irinotecan (CPT-11; 70 mg/m2 weekly for
3 weeks followed by 1 week off; Yakult, Tokyo, Japan); and
fourth-line chemotherapy was single-agent gemcitabine (700
mg/m2 weekly for 3 weeks followed by 1 week off; Eli
Lilly and Co., Indiana, IN, USA). These patient objective responses
were principally evaluated by the Response Evaluation Criteria in
Solid Tumors (version 1.1).
NLR, PLR and CRP
Each subject had a complete blood cell count and
differential white blood cell counts recorded within 7 days prior
to chemotherapy. NLR was defined as the absolute neutrophil count
divided by the absolute lymphocyte count. PLR was defined as the
absolute platelet count divided by the lymphocyte count (Bayer
HealthCare, Diagnostics Division, Tarrytown, NY, USA). Serum CRP
was measured by latex nephelometry (LT Auto Wako, Osaka,
Japan).
Statistical analysis
Statistical analyses were performed using the
Mann-Whitney U test for comparisons with controls and one-factor
analysis of variance followed by Fisher's protected least
significant difference test for all the pairwise comparisons.
Receiver operating characteristic (ROC) curves were generated for
pretreatment NLR, PLR and CRP to determine cut-off values that
predicted survival for <200 days (i.e., approximately the median
survival period of 198 days for patients in the present study) that
yielded optimal sensitivity and specificity; patients were
subsequently grouped by these cut-off values. Univariate and
multivariate analyses were performed using Cox's proportional
hazards model to identify biomarkers that predict survival
following adjustment for the effects of known prognostic factors.
Analyses used SPSS software version 20.0 (IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics and
treatments
Histological type, duration of recurrence-free
survival and recurrence sites are listed in Table I. Tumors that were simultaneously
found inside and outside the previously irradiated area were
treated as extra-irradiation areas. Among the patients with
recurrent disease, 32 (100%), 15 (46.9%) and 4 (12.5%) patients
received second-, third- and fourth-line chemotherapy, respectively
(Fig. 1A). Responses of patients who
received second- to fourth-line chemotherapy are shown in Fig. 1B. Tumor response rate (RR) and disease
control rate were 12.5 and 43.7% for second-line chemotherapy, 6.7
and 20.0% for third-line chemotherapy, and 0.0 and 25.0% for
fourth-line chemotherapy, respectively. The median numbers of
cycles of second-, third- and fourth-line chemotherapy received
were 12 (range, 3–24), 8 (3–18) and 6 (2–12),
respectively. Median survival time was 198 days (range, 42–1,022
days). Final chemotherapy regimens for the 32 patients were
second-line for 17 patients (53.1%), third-line for 11 (34.4%) and
fourth-line for 4 (12.5%). Their mean survival periods were 178.9,
483.0 and 493.5 days, respectively; thus patients whose final
treatment was a second-line regimen had a significantly shorter
survival time compared with those who reached third- and
fourth-line regimens (P=0.001 and P=0.001) (Fig. 1C).
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
| Baseline
characteristics | All patients |
|---|
| Age at diagnosis,
mean years (range) | 52.6 (25–78) |
| Histology, n (%) |
|
| SCC | 27 (84.3) |
| AD | 3 (9.4) |
| ADSQ | 2 (6.3) |
| TFI, n (%) |
|
| ≤6
months | 10 (31.2) |
| 7–12
months | 18 (56.3) |
| ≥13
months | 4 (12.5) |
| Recurrent site, n
(%) |
|
|
Prior-irradiation area | 18 (56.3) |
|
Extra-irradiation area | 11 (34.3) |
| Extra +
prior-irradiation area | 3 (9.4) |
NLR, PLR and CRP
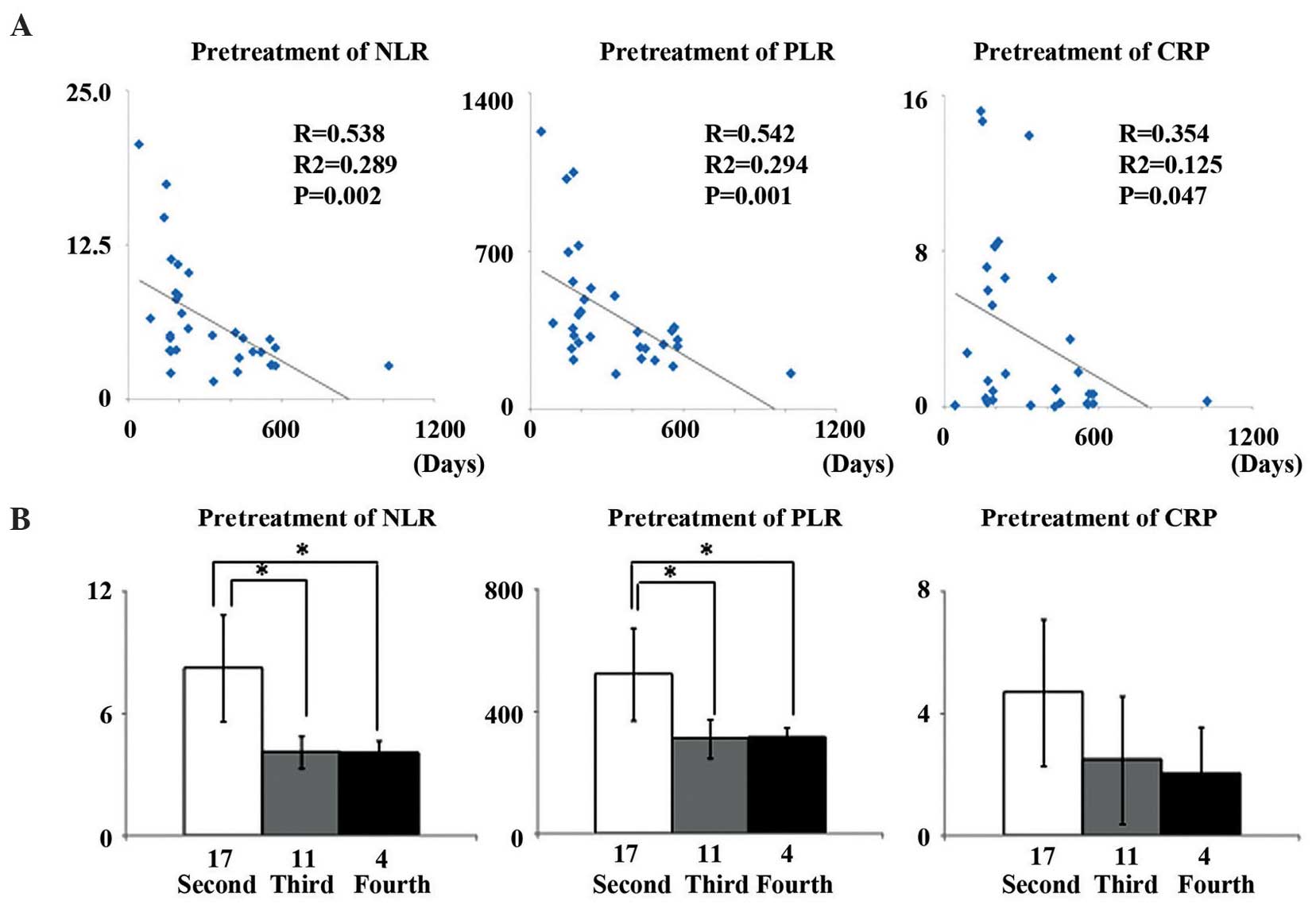
Median pretreatment NLR, PLR and CRP in these
patients were NLR, 6.36 (range, 1.44–20.63); PLR, 426.06 (range,
154.77–1,227.6); and CRP, 3.64 mg/dl (range, 0.01–15.2 mg/dl).
Patient pretreatment NLR (R=-0.538, R2=-0.289, P=0.002)
and PLR (R=-0.542, R2=-0.294, P=0.001) were
significantly and inversely correlated with their survival time
(Fig. 2A). Pretreatment NLR and PLR
for patients whose final regimen was second-line were significantly
higher compared with for patients who survived to third- and
fourth-line regimens (P=0.006, P=0.007, P=0.019 and P=0.019,
respectively). However, CRP concentration showed no association
with any line of chemotherapy (Fig.
2B).
Whether the cancer recurrence sites were correlated
with NLR, PLR, CRP, treatment-free interval (TFI), final-line
chemotherapy or survival period were examined. Recurrences in
extra-irradiation areas were associated with lower final-line
chemotherapy number (P=0.011) and shorter survival time (P=0.021).
However, none of the inflammatory markers were correlated with the
recurrence site (Fig. 3).
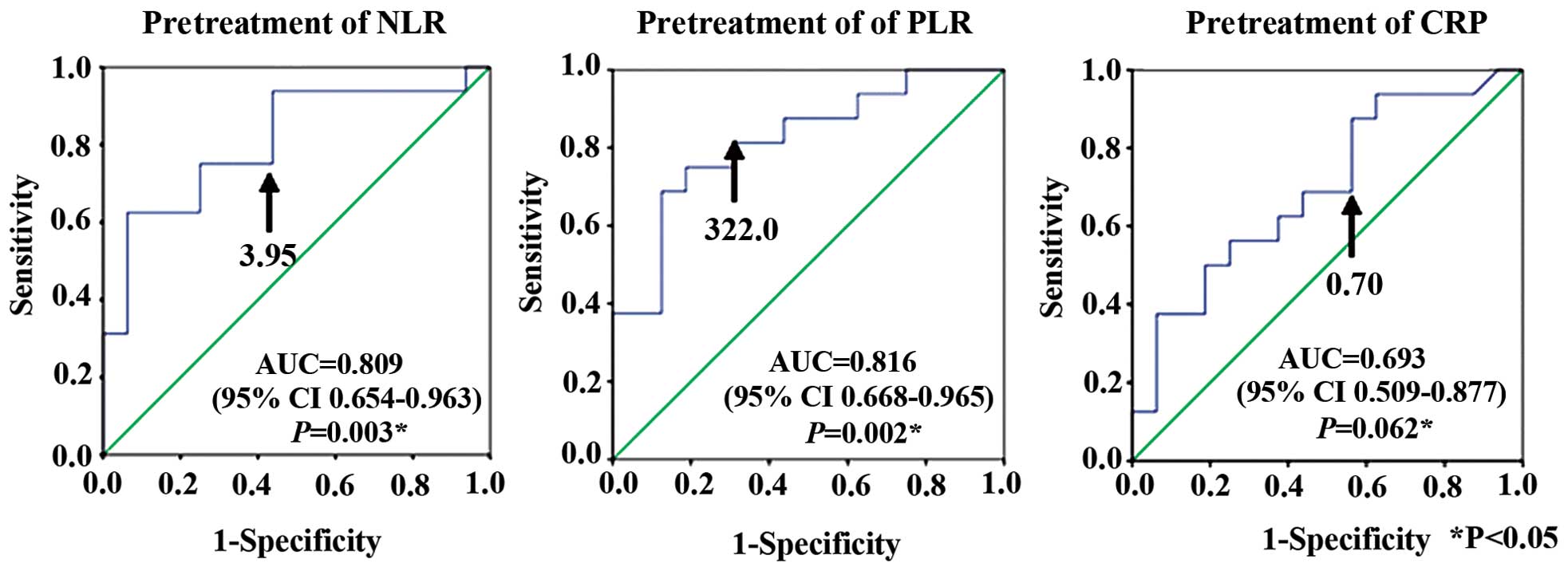
ROC curve analyses determined cut-off values for
pretreatment NLR, PLR and CRP that predicted survival <200 days
at NLR, 3.95 (AUC, 0.809; sensitivity 81.3%; specificity 56.2%);
PLR, 322.0 (AUC, 0.816; sensitivity 81.3%; specificity 68.7%); and
CRP, 0.7 mg/dl (AUC, 0.693; sensitivity 68.8%; specificity 56.2%)
(Fig. 4).
Survival time analysis
Whether clinical factors correlated with survival
<200 days was assessed by univariate and multivariate analyses.
Univariate analyses were significantly associated with
extra-irradiation area (P=0.009), second-line chemotherapy as
final-line chemotherapy (P=0.003) and pretreatment PLR >322.0
(P=0.015) with survival <200 days. Multivariate analyses showed
that patients with high pretreatment PLR had a significantly higher
hazard ratio (4.204) for survival <200 days compared to the
patients with lower PLR (P=0.029) (Table
II).
 | Table II.Prognostic factors for survival within
200 days according to Coxs univariate and multivariate
analysis. |
Table II.
Prognostic factors for survival within
200 days according to Coxs univariate and multivariate
analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Histology
(non-SCC) | 1.202 | 0.339–4.264 | 0.776 |
|
|
|
| TFI (≤6
months) | 3.339 | 0.423–26.376 | 0.253 |
|
|
|
| Extra-irradiation
area | 4.187 | 1.431–12.248 | 0.009a | 2.712 | 0.800–9.192 |
0.109 |
| Second-line
chemotherapy as final line chemotherapy | 5.875 | 1.858–18.581 | 0.003a | 3.071 | 0.840–11.229 | 0.09 |
| Pretreatment-NLR
(>3.95) | 2.564 | 0.826–7.961 | 0.103 |
|
|
|
| Pretreatment-PLR
(>322.0) | 4.814 | 1.364–16.988 | 0.015a | 4.204 | 1.158–15.268 |
0.029a |
| CRP (>0.7
mg/dl) | 1.858 | 0.644–5.357 | 0.252 |
|
|
|
Discussion
Although treatment of recurrent cervical cancer
depends on the site or extent of recurrence, disease-free interval
and patient performance status (13,14), this
condition has no standard chemotherapy regimen. The previous RR and
median overall survival (OS) for second-line monotherapy regimens
were cisplatin (50–100 mg/m2): 17–38%, 6.1–7.1 months
(15,16); carboplatin (400 mg/m2 every
4 weeks): 15–28%, 6.75 months (17,18);
gemcitabine (800 mg/m2, days 1, 8 and 15 every 4 weeks):
4.5–8%, 4.9–6.5 (19,20); paclitaxel (250 mg/m2 every
3 weeks): 21–26%, 7.3 months (21,22); and
irinotecan (125 mg/m2/week for 4 weeks every 6 weeks or
350 mg/m2 every 3 weeks): 16–21%, 6.4–8.2 months
(23,24). The previous RR and median OS for
combination regimens were cisplatin (75 mg/m2) plus
paclitaxel (135–175 mg/m2): 45–47%, 7–10 months, more
efficient than either of them used as single agents (25–27); and
CBDCA (AUC, 5) plus paclitaxel (135–175 mg/m2): 40–78%,
9.6–13.0 months (28,29). As carboplatin plus paclitaxel versus
cisplatin plus paclitaxel regimens do not differ in terms of RR and
OS (30), the side effects and
anticancer agent continuations were considered in selecting weekly
carboplatin plus paclitaxel for second-line regimens, irinotecan
for third-line regimens, and gemcitabine for fourth-line regimens.
In the present study, RR was 12.5% for second-line chemotherapy,
6.7% for third-line and 0.0% for fourth-line. Median survival was
198 days for the cohort, and 178.9, 483.0 and 493.5 days for
patients whose final regimens were second-, third- and fourth-line
chemotherapy, respectively, i.e., significantly shorter for
patients whose final therapy was a second-line regimen.
Systemic inflammatory processes have been examined
as possible predictors of prognosis in various types of cancer.
Neutrophils release inflammatory cytokines and leukocytic and other
phagocytic mediators that would induce damage to cellular DNA,
inhibit apoptosis and promote angiogenesis (31,32).
Platelets can release potent mitogens or adhesive glycoprotein such
as platelet-derived growth factor, transforming growth factor-β and
vascular endothelial growth factor (VEGF). Lymphocytes, such as
cluster of differentiation 3+ T cells and natural killer
cells, can affect growth and metastasis (33,34). CRP
may be released as a result of producing inflammation-related
cytokines, such as VEGF and interleukin-6 (35,36). Thus,
NLR and PLR have attracted the interest of investigators as
possible markers of systemic inflammation, and therefore of
prognosis. High pretreatment NLR and PLR have been reportedly
associated with mortality in various types of cancer type (7–11). High
pretreatment NLR is an independent indicator of poor prognosis in
patients with cervical cancer (37,38); and
CRP levels are also a prognostic parameter in patients with
cervical cancer (39).
However, to the best of our knowledge, no studies
have previously reported the correlations between pretreatment NLR,
PLR and CRP and the survival of patients with recurrent cervical
cancer following first-line CCRT. In the present study,
pretreatment NLR and PLR were significantly and inversely
correlated with survival time in these patients. Furthermore,
patients whose final regimen was second-line chemotherapy had
significantly higher pretreatment NLR and PLR compared with those
who survived to undergo third- and fourth-line chemotherapy. NLR,
PLR, CRP, TFI, final-line chemotherapy and survival against
recurrence sites were examined, and the patients whose recurrence
spread to extra-irradiation areas had a significantly shorter
survival rate compared with those whose tumors recurred in the
prior-irradiation area. Higher inflammatory markers (NLR, PLR and
CRP) were associated, but not significantly so, with recurrence
sites.
One of the aims of the present study was to confirm
whether pretreatment NLR, PLR or CRP could predict survival <200
days. ROC curve analyses showed that the optimal cut-off
pretreatment values were NLR, 3.95; PLR, 322.0; and CRP, 0.70
mg/dl. Univariate and multivariate analyses were significantly
associated with pretreatment PLR >322.0 with survival <200
days.
The study has certain limitations; including
relatively few patients, followed up over a relatively short
period. Further prospective studies with more patients and longer
follow-up periods would verify the present findings and clarify
their significance.
In conclusion, the present findings suggest that
pretreatment PLR is an important predictor of prognosis in patients
with recurrent cervical cancer following CCRT.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chemoradiotherapy for Cervical Cancer
Meta-Analysis Collaboration, . Reducing uncertainties about the
effects of chemoradiotherapy for cervical cancer: A systematic
review and meta-analysis of individual patient data from 18
randomized trials. J Clin Oncol. 26:5802–5812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Green JA, Kirwan JM, Tierney JF, Symonds
P, Fresco L, Collingwood M and Williams CJ: Survival and recurrence
after concomitant chemotherapy and radiotherapy for cancer of the
uterine cervix: A systematic review and meta-analysis. Lancet.
358:781–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quinn MA, Benedet JL, Odicino F,
Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY and
Pecorelli S: Carcinoma of the cervix uteri. Int J Gynaecol Obstet.
95 (Suppl 1):S43–S103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee Y, Kim SH, Han JY, Kim HT, Yun T and
Lee JS: Early neutrophil-to-lymphocyte ratio reduction as a
surrogate marker of prognosis in never smokers with advanced lung
adenocarcinoma receiving gefitinib or standard chemotherapy as
first-line therapy. J Cancer Res Clin Oncol. 138:2009–2016. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aliustaoglu M, Bilici A, Seker M, Dane F,
Gocun M, Konya V, Ustaalioglu BB and Gumus M: The association of
pre-treatment peripheral blood markers with survival in patients
with pancreatic cancer. Hepatogastroenterology. 57:640–645.
2010.PubMed/NCBI
|
|
8
|
Azab B, Bhatt VR, Phookan J, Murukutla S,
Kohn N, Terjanian T and Widmann WD: Usefulness of the
neutrophil-to-lymphocyte ratio in predicting short- and long-term
mortality in breast cancer patients. Ann Surg Oncol. 19:217–224.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kishi Y, Kopetz S, Chun YS, Palavecino M,
Abdalla EK and Vauthey JN: Blood neutrophil-to-lymphocyte ratio
predicts survival in patients with colorectal liver metastases
treated with systemic chemotherapy. Ann Surg Oncol. 16:614–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarraf KM, Belcher E, Raevsky E, Nicholson
AG, Goldstraw P and Lim E: Neutrophil/lymphocyte ratio and its
association with survival after complete resection in non-small
cell lung cancer. J Thorac Cardiovasc Surg. 137:425–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamanaka T, Matsumoto S, Teramukai S,
Ishiwata R, Nagai Y and Fukushima M: The baseline ratio of
neutrophils to lymphocytes is associated with patient prognosis in
advanced gastric cancer. Oncology. 73:215–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang CS and Sun CF: C-reactive protein and
malignancy: Clinico-pathological association and therapeutic
implication. Chang Gung Med J. 32:471–482. 2009.PubMed/NCBI
|
|
13
|
Kesic V: Management of cervical cancer.
Eur J Surg Oncol. 32:832–837. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cadron I, Van Gorp T, Amant F, Leunen K,
Neven P and Vergote I: Chemotherapy for recurrent cervical cancer.
Gynecol Oncol. 107 (Suppl 1):S113–S118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonomi P, Blessing JA, Stehman FB, DiSaia
PJ, Walton L and Major FJ: Randomized trial of three cisplatin dose
schedules in squamous-cell carcinoma of the cervix: A Gynecologic
Oncology Group study. J Clin Oncol. 3:1079–1085. 1985.PubMed/NCBI
|
|
16
|
Thigpen JT, Blessing JA, Fowler WC Jr and
Hatch K: Phase II trials of cisplatin and piperazinedione as single
agents in the treatment of advanced or recurrent non-squamous cell
carcinoma of the cervix: A Gynecologic Oncology Group Study. Cancer
Treat Rep. 70:1097–1100. 1986.PubMed/NCBI
|
|
17
|
Arseneau J, Blessing JA, Stehman FB and
McGehee R: A phase II study of carboplatin in advanced squamous
cell carcinoma of the cervix (a Gynecologic Oncology Group Study).
Invest New Drugs. 4:187–191. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McGuire WP III, Arseneau J, Blessing JA,
DiSaia PJ, Hatch KD, Given FT Jr, Teng NN and Creasman WT: A
randomized comparative trial of carboplatin and iproplatin in
advanced squamous carcinoma of the uterine cervix: A Gynecologic
Oncology Group study. J Clin Oncol. 7:1462–1468. 1989.PubMed/NCBI
|
|
19
|
Schilder RJ, Blessing JA, Morgan M, Mangan
CE and Rader JS: Evaluation of gemcitabine in patients with
squamous cell carcinoma of the cervix: A Phase II study of the
gynecologic oncology group. Gynecol Oncol. 76:204–207. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schilder RJ, Blessing J and Cohn DE:
Evaluation of gemcitabine in previously treated patients with
non-squamous cell carcinoma of the cervix: A phase II study of the
Gynecologic Oncology Group. Gynecol Oncol. 96:103–107. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kudelka AP, Winn R, Edwards CL, Downey G,
Greenberg H, Dakhil SR, Freedman RS, Loyer E, Rusinkiewicz J,
Gacrama P, et al: Activity of paclitaxel in advanced or recurrent
squamous cell cancer of the cervix. Clin Cancer Res. 2:1285–1288.
1996.PubMed/NCBI
|
|
22
|
Kudelka AP, Winn R, Edwards CL, Downey G,
Greenberg H, Dakhil SR, Freedman RS, LoCoco S, Umbreit J, Delmore
JE, et al: An update of a phase II study of paclitaxel in advanced
or recurrent squamous cell cancer of the cervix. Anticancer Drugs.
8:657–661. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verschraegen CF, Levy T, Kudelka AP,
Llerena E, Ende K, Freedman RS, Edwards CL, Hord M, Steger M,
Kaplan AL, et al: Phase II study of irinotecan in prior
chemotherapy-treated squamous cell carcinoma of the cervix. J Clin
Oncol. 15:625–631. 1997.PubMed/NCBI
|
|
24
|
Lhommé C, Fumoleau P, Fargeot P, Krakowski
Y, Dieras V, Chauvergne J, Vennin P, Rebattu P, Roche H, Misset JL,
et al: Results of a European Organization for Research and
Treatment of Cancer/Early Clinical Studies Group phase II trial of
first-line irinotecan in patients with advanced or recurrent
squamous cell carcinoma of the cervix. J Clin Oncol. 17:3136–3142.
1999.PubMed/NCBI
|
|
25
|
Rose PG, Blessing JA, Gershenson DM and
McGehee R: Paclitaxel and cisplatin as first-line therapy in
recurrent or advanced squamous cell carcinoma of the cervix: A
gynecologic oncology group study. J Clin Oncol. 17:2676–2680.
1999.PubMed/NCBI
|
|
26
|
Papadimitriou CA, Sarris K, Moulopoulos
LA, Fountzilas G, Anagnostopoulos A, Voulgaris Z, Gika D,
Giannakoulis N, Diakomanolis E and Dimopoulos MA: Phase II trial of
paclitaxel and cisplatin in metastatic and recurrent carcinoma of
the uterine cervix. J Clin Oncol. 17:761–766. 1999.PubMed/NCBI
|
|
27
|
Piver MS, Ghamande SA, Eltabbakh GH and
O'Neill-Coppola C: First-line chemotherapy with paclitaxel and
platinum for advanced and recurrent cancer of the cervix - a phase
II study. Gynecol Oncol. 75:334–337. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tinker AV, Bhagat K, Swenerton KD and
Hoskins PJ: Carboplatin and paclitaxel for advanced and recurrent
cervical carcinoma: The British Columbia Cancer Agency experience.
Gynecol Oncol. 98:54–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pectasides D, Fountzilas G, Papaxoinis G,
Pectasides E, Xiros N, Sykiotis C, Koumarianou A, Psyrri A,
Panayiotides J and Economopoulos T: Carboplatin and paclitaxel in
metastatic or recurrent cervical cancer. Int J Gynecol Cancer.
19:777–781. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saito I, Kitagawa R, Fukuda H, Shibata T,
Katsumata N, Konishi I, Yoshikawa H and Kamura T: A phase III trial
of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in
stage IVB, persistent or recurrent cervical cancer: Gynecologic
Cancer Study Group/Japan Clinical Oncology Group Study (JCOG0505).
Jpn J Clin Oncol. 40:90–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jackson JR, Seed MP, Kircher CH,
Willoughby DA and Winkler JD: The codependence of angiogenesis and
chronic inflammation. FASEB J. 11:457–465. 1997.PubMed/NCBI
|
|
32
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Assoian RK and Sporn MB: Type beta
transforming growth factor in human platelets: Release during
platelet degranulation and action on vascular smooth muscle cells.
J Cell Biol. 102:1217–1223. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaplan KL, Broekman MJ, Chernoff A,
Lesznik GR and Drillings M: Platelet alpha-granule proteins:
Studies on release and subcellular localization. Blood. 53:604–618.
1979.PubMed/NCBI
|
|
35
|
Hefler LA, Zeillinger R, Grimm C, Sood AK,
Cheng WF, Gadducci A, Tempfer CB and Reinthaller A: Preoperative
serum vascular endothelial growth factor as a prognostic parameter
in ovarian cancer. Gynecol Oncol. 103:512–517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tempfer C, Zeisler H, Sliutz G, Haeusler
G, Hanzal E and Kainz C: Serum evaluation of interleukin 6 in
ovarian cancer patients. Gynecol Oncol. 66:27–30. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee YY, Choi CH, Kim HJ, Kim TJ, Lee JW,
Lee JH, Bae DS and Kim BG: Pretreatment neutrophil:lymphocyte ratio
as a prognostic factor in cervical carcinoma. Anticancer Res.
32:1555–1561. 2012.PubMed/NCBI
|
|
38
|
Zhang Y, Wang L, Liu Y, Wang S, Shang P,
Gao Y and Chen X: Preoperative neutrophil-lymphocyte ratio before
platelet-lymphocyte ratio predicts clinical outcome in patients
with cervical cancer treated with initial radical surgery. Int J
Gynecol Cancer. 24:1319–1325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Polterauer S, Grimm C, Tempfer C, Sliutz
G, Speiser P, Reinthaller A and Hefler LA: C-reactive protein is a
prognostic parameter in patients with cervical cancer. Gynecol
Oncol. 107:114–117. 2007. View Article : Google Scholar : PubMed/NCBI
|