Introduction
Neoadjuvant chemoradiotherapy (NA-CRT) consisting of
45–50.4 Gy in 25–28 fractions followed by total mesorectal excision
has been adopted as the standard treatment for locally advanced
rectal cancer (RC) (1–6). The addition of chemotherapy to
neoadjuvant radiotherapy (NA-RT) has been demonstrated to be
feasible, with enhanced antitumor effects (7). Moreover, the use of 5-fluorouracil
(5-FU)-based chemotherapy has gained widespread acceptance for the
treatment of locally advanced rectal adenocarcinoma (1,2).
S-1 is a novel oral anticancer drug composed of
tegafur, 5-chloro-2,4-dihydroxypyridine, oteracil (which was
designed to enhance the oral efficacy of tegafur) and a prodrug of
5-FU. Several clinical studies have demonstrated that NA-CRT
combined with S-1 is associated with mild toxicity and exhibits an
efficacy equivalent to that of other CRT regimens used for RC
(8–12). Clinical studies of irinotecan (CPT-11)
plus S-1 combination therapy have been reported to produce
non-inferiority outcomes for metastatic colorectal cancer. In
addition, several clinical studies have demonstrated that NA-RT
combined with S-1 is associated with mild toxicity and an efficacy
equivalent to that achieved by other systemic chemotherapies
(13).
Diffusion-weighted (DW) magnetic resonance imaging
(MRI) is a non-invasive functional MRI technique that is sensitive
to the mobility of water protons in biological tissues, which is
dependent on a number of factors, such as cell density,
vascularity, the viscosity of the extracellular fluid and cell
membrane integrity (14–16). The apparent diffusion coefficient
(ADC) calculated from DW-MRI measurements may be used to quantify
and express these properties. The majority of the studies have
demonstrated that the pretreatment ADC is negatively correlated
with response to treatment (17–28). It
appears that necrotic areas with high pretreatment ADC values may
be less sensitive to CRT. However, several studies have produced
contradictory results (29,30). Previously published data on the value
of DW-MRI as a predictive tool for anticancer treatment responses
in patients with RC are scarce and conflicting. In addition, there
are no reports on the correlation between MRI and the pathological
response to NA-CRT using CPT-11 and S1.
Therefore, this study was conducted to investigate
the clinical value of DW-MRI as a predictor of tumor response in
patients receiving NA-CRT for RC through the measurement of the
tumor ADC.
Patients and methods
Patients
Patients who were treated with NA-RT (45 Gy in 25
fractions) followed by surgery for primary RC and who underwent
MRI, including DW-MRI, before and after NA-CRT prior to surgery at
the of Hyogo College of Medicine Hospital between July, 2011 and
March, 2014 were included in this study. The study endpoints
included the predictive factors for the pathological response to
NA-CRT for RC using MRI. The patient eligibility criteria were as
follows: Age ≥20 years, histologically confirmed primary
adenocarcinoma of the rectum and no evidence of metastatic disease
in distant organs. A patient with cT2N1M0 RC and a patient with
cT3N2bM1a disease were included in this analysis based on
experienced physicians' decisions. A total of 16 patients were
analyzed in the present study. The patient characteristics are
summarized in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | Values |
|---|
| Total no. of
patients | 16 |
| Age, years [median
(range)] | 62.5 (47–79) |
| Gender, no. |
|
| Male | 13 |
|
Female | 3 |
| Performance status,
no. |
|
| 0 | 16 |
| Histopathology, no.
(%) |
|
| Tub
1 | 8 (50.0) |
| Tub
2 | 8 (50.0) |
| Radiotherapy, no.
(%) |
|
| 45 Gy in
25 fractions (1.8 Gy/fr) | 16 (100.0) |
| Treatment
term, days (range) | 37 (29–51) |
| Chemotherapy, no.
(%) |
|
| S-1 plus
CPT-11 | 16 (100.0) |
| Terms, days
(range) |
|
| Pre-MRI
to start of RT | 21 (3–48) |
|
Completion of RT to
post-MRI | 31.5 (10–45) |
|
Completion of RT to
surgery | 42 (54–69) |
|
Post-MRI to surgery | 24 (11–42) |
| Clinical stage,
no. |
|
| T2 | 1 |
| T3 | 12 |
| T4 | 3 |
| N0 | 6 |
| N1 | 8 |
| N2 | 2 |
| M0 | 15 |
|
M1aa | 1 |
|
IIA | 6 |
|
IIC | 1 |
|
IIIA | 1 |
|
IIIB | 5 |
|
IIIC | 2 |
|
IVA | 1 |
Preoperative clinical staging included clinical
assessment, computed tomography (CT) scans between the chest and
whole pelvis, a pelvic MRI, a full blood analysis and colonoscopy
with biopsy. The study protocol was approved by the Ethics
Committee of Hyogo College of Medicine and all the patients
provided written informed consent prior to NA-CRT.
Treatment protocol
The protocol of NA-CRT applied for the present study
with minor modifications was recently described in detail (13). Briefly, all the patients were placed
in the supine position and helically scanned on an Aquilion LB CT
unit (Toshiba, Otawara-shi, Japan). For each patient, a planning CT
scan of the entire pelvis from the lower abdomen to below the
ischial tuberosities was obtained at 5-mm intervals. The CT dataset
was transferred to the Focus XiO™ treatment-planning system (CMS,
Inc., St Louis, MO, USA) to outline the volumes of interest.
The gross target volume (GTV) included the primary
rectal tumor and nodal metastases. The clinical target volume (CTV)
comprised the GTV with a 1-cm margin, as well as the perirectal,
obturator and internal iliac nodes. The planning target volume
(PTV) was the CTV with a 0.5-cm margin. Furthermore, there was an
additional 7-mm leaf margin to the PTV, in order to cover the PTV
more homogeneously.
RT was performed using a 3D conformal RT technique,
which was typically performed with a 4-field box technique using 10
MV photons. The planned RT was delivered using an Elekta Synergy
device (Elekta, Crawley, UK). The patients were treated with a dose
of 1.8 Gy daily up to a total dose of 45 Gy in 25 fractions. S-1
was administered orally at a dose of 120 (100–140) mg/body/day on
days 1–5, 8–12, 22–26 and 29–33. CPT-11 was delivered at a dose of
60 (60–80) mg/m2/day on days 1, 8, 22 and 29. Surgery
was performed 6–10 weeks after the completion of RT. The removed
specimens were pathologically evaluated.
MRI technique and analysis
An MRI of the pelvis was routinely performed prior
to treatment (pre-CRT) but not after NA-CRT (post-CRT). In the
present study, all the patients had both pre- and post-CRT MRI
data, including DW imaging. Rectal MRI was performed using Magnetom
Avanto (Siemens Medical Solutions, Erlangen, Germany), Intera 1.5T
(Philips Healthcare, Amsterdam, The Netherlands) and Magnetom Skyra
3T (Siemens Medical Solutions, Erlangen, Germany) in 12, 19 and 1
scanning(s), respectively. The MR images were evaluated by a
picture archiving and communication system and analyzed by a single
radiologist with 5 years of clinical experience who was blinded to
the other results and the study design. Without any information
regarding the patient, the sum of the longest diameters (SLD) of
rectal tumors for the tumor sizes and ADC values was calculated by
manually tracing the tumor boundaries on axial post-gadolinium
T1-weighted MR images or axial T2-weighted MR images and by placing
oval-shaped regions of interest (ROI) on axial DW images. The ROIs
were manually drawn within the tumor on axial images with a b value
of 1,000 sec/mm2 on the selected ADC maps. Each ROI was
designed to have ~4.00 mm2 in the rectal tumor. Four
ROIs were generated for each patient and the mean of the ADC values
in the ROIs was considered to be the ADC value of the tumor.
The pre-CRT SLD (baseline SLD) and post-CRT SLD were
compared according to the Response Evaluation Criteria in Solid
Tumors guidelines (31). The %
decrease of each tumor was calculated as follows:
[(SLDpre-CRT-SLDpost-CRT)/SLDpre-CRT]
× 100
In addition, a tumor reduction rate of ≥30% was
considered to be a partial response. The ΔADC value, defined as the
percentage of the difference from the pre-CRT to the post-CRT ADC
was calculated using the formula:
ΔADC (%) =
[(ADCpost-CRT-ADCpre-CRT)/ADCpre-CRT]
× 100
Pathological examination of the
surgical specimens
Pathological staging was conducted according to the
tumor-node-metastasis staging system. The pathological tumor stage
was compared with the clinical stage in each patient. Tumor
downstaging was defined as a lower pathological stage compared with
the clinical stage prior to treatment. The tumor response after CRT
was determined according to the following pathological grading:
Grade 0, not effective; grade 1a, high response in <1/3 cancer
cells; grade 1b, high response in 1/3–2/3 cancer cells; grade 2,
high response in >2/3 cancer cells; grade 3, complete response.
In addition, patients with grade 2 or 3 tumor response were defined
as pathological responders and patients with grade 1a or 1b tumor
response as pathological non-responders (32).
Statistical analysis
The patient data were recorded on standardized
forms, reviewed and expressed as median and range, unless otherwise
indicated. The duration between events was calculated from the day
of surgery to the day of confirmation of an event. Cumulative local
control, disease-free survival and overall survival estimates were
calculated using the Kaplan-Meier method. As regards the
association between the two groups, continuous variables and
incidence of patients, the trend for incidence was assessed using
the Mann-Whitney U test, the Fisher's exact test and Chi-square
test for trend, respectively. The optimal cut-off value was
determined using receiver operating characteristic (ROC) curve
analysis. All the analyses were performed using the GraphPad Prism
6.0b software program (GraphPad Software, Inc., San Diego, CA,
USA). P-values of <0.05 were considered to indicate
statistically significant differences.
Results
ADC values pre- and post-CRT
The baseline SLD was 105.0 mm (range, 59.0–200.0
mm). The percent decrease of SLD and the number of patients who
exhibited PR on MRI was 33.2% (range, 1.7–67.0%) and 9 (56.3%),
respectively. The results of the ADC measurement are shown in
Table II. The ADC values were
significantly increased following CRT (P<0.0001). In addition,
there were no significant differences in the ROIs for the ADC
values between pre- and post-CRT ADCs (P=0.7732). When compared
with the clinical stage, downstaging was achieved in 6 (37.5%), 8
(50.0%) and 8 patients (50.0%) in the T stage, N stage and stage
grouping, respectively. As regards pathological findings, 4, 3, 8
and 1 patient(s) had grade 1a, 1b, 2 and 3 disease, respectively,
and the pathological response rate was 56.3%.
 | Table II.ADC values in MRI findings. |
Table II.
ADC values in MRI findings.
| ADC | Values (range) |
|---|
| Pre-CRT |
|
| Value
(×10−3 mm2/sec) | 0.753
(0.613–0.869) |
| Area of
ROI, mm2 | 4.03
(3.77–4.18) |
| Post-CRT |
|
| Value
(×10−3 mm2/sec) | 1.114
(0.856–1.799) |
| Area of
ROI, mm2 | 4.05
(3.82–4.39) |
| ΔADC, % | 55.8
(3.4–174.2) |
Predictive factors of the pathological
response to NA-CRT
To assess the potential factors that may predict the
pathological response to NA-CRT in RC, we analyzed various factors
between the non-responder and the responder groups. The results of
the analysis are presented in Table
III. The ADC values following CRT were higher compared with
those prior to CRT in all the patients. In addition, the pre-CRT
ADC value was significantly lower in the responder group (P=0.023).
The diagnostic performance of pre-CRT ADC in the prediction of the
response to CRT was evaluated using the ROC curve analysis
(Fig. 1). With a cut-off value of
0.750 mm2/sec, we obtained a sensitivity and specificity
for pathological responders of 77.8 and 85.7%, respectively. In
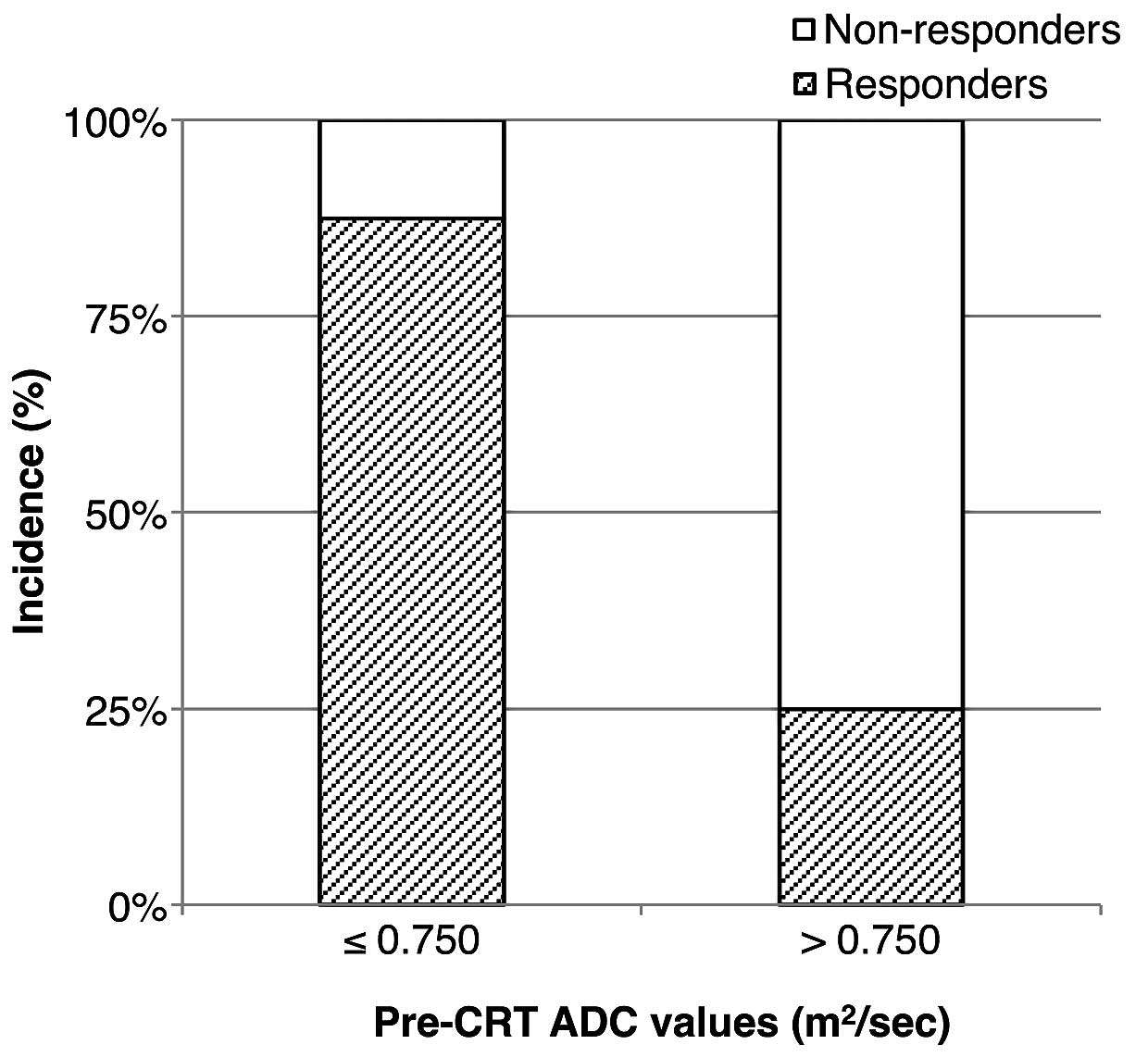
addition, the area under the ROC curve was 84.1%. The patients with
pre-CRT ADC values ≤0.750 mm2/sec included 7 responders
(87.5%); there were significantly more responders with pre-CRT ADC
values ≤0.750 mm2/sec (P=0.041, Fig. 2).
 | Table III.Predictive factors for the
pathological response to NA-CRT. |
Table III.
Predictive factors for the
pathological response to NA-CRT.
| Factors | Non-responders | Responders | P-value |
|---|
| No. of
patients | 7 | 9 |
|
| Gender, no. |
|
| 0.550 |
|
Male | 5 | 8 |
|
|
Female | 2 | 1 |
|
| Clinical T stage,
no. |
|
| 0.897 |
| 2 | 0 | 1 |
|
| 3 | 6 | 6 |
|
| 4 | 1 | 2 |
|
| T downstaging, no.
(%) | 2 (28.6) | 4 (44.4) | 0.633 |
| Baseline sum
diameter, mm (range) | 104 (59–129) | 106 (82–200) | 0.423 |
| Sum diameter
shrinkage, % (range) | 27.5
(1.7–65.9) | 37.7
(14.2–67.0) | 0.585 |
| Pre-CRT ADC,
×10−3 mm2/sec (range) | 0.822
(0.747–0.869) | 0.718
(0.613–0.852) | 0.023 |
| Post-CRT ADC,
×10−3 mm2/sec (range) | 1.153
(0.921–1.799) | 1.031
(0.856–1.680) | 0.598 |
| ΔADC (range) | 0.391
(0.120–1.070) | 0.557
(0.034–1.742) | 0.656 |
Discussion
NA-CRT followed by surgery is currently the standard
treatment for locally advanced RC. However, a proportion of
patients benefit little from NA-CRT. Therefore, the prediction of
the response to NA-CRT is crucial.
DW-MRI is a non-invasive functional MRI technique
that has been shown to predict the response to NA-CRT in RC.
Lambregts et al (28)
previously reported that the quantitative evaluation of the ADC may
be used as a biomarker for the response to treatment. The
diagnostic performance of the ADC with various cut-off values
ranging between 1.2 and 1.4 ×103 mm2/sec in
RC has been reported to be equivalent to ~46–100% sensitivity and
~56–84% specificity (18,19,21,29). The
value of DW-MRI as a predictive tool for assessing response to
NA-CRT in patients with RC is currently poorly understood. In
addition, there is yet no consensus on the true clinical value of
ADC measurement for response assessment in RC.
In this study, we analyzed the pre- and post-CRT ADC
values and the pathological findings in surgically removed
specimens in order to assess the response to treatment. Our
findings demonstrated that the pre-CRT ADC value was strongly
correlated with the response to CRT using S-1 and CPT-11. In
addition, we obtained a cut-off value of 0.750 ×103
mm2/sec that exhibited high sensitivity and specificity.
Moreover, the post-CRT ADC value and the changes in ADC values
following CRT did not significantly affect the pathological
response to NA-CRT. Further investigation with a large number of
patients should be performed to assess the clinical availability of
ADC values and other findings in MRI.
The increase in the ADC value and the tumor
shrinkage following NA-CRT were not found to be correlated with the
pathological response to CRT in the present study. Differences
between previous reported results and our findings are likely
associated with differences in the study design (3,6,14–18).
There were several limitations to the present study.
First, we analyzed a limited number of patients who received
NA-CRT, including S-1 plus CPT-11 therapy, and the ROI size may
exert a considerable effect on the tumor ADC values. Lambregts
et al (28) reported that the
small-sample ROI measurements, similar to our method, exhibited
significantly smaller variance compared with the whole-volume ROIs,
which were generated by freehand. In addition, sampling ROIs in the
tumor may minimize susceptibility artifacts due to air-tissue
interfaces in the rectum, and may simplify the application of
quantitative DW imaging. Therefore, the sample ROI measurement
appears to be straightforward for clinical use, while requiring
less time compared to the whole-volume measurements. The timing of
MRI varied before and after NA-CRT, which may lead to different
results. However, we believe that the pre-CRT ADC value was not
affected by the time variance. Moreover, a phase II clinical trial
is currently ongoing to evaluate the efficacy and safety of NA-CRT
using S-1 and CPT-11. The ADC analysis based on the present study
will therefore be performed with a large number of patients and the
role of MRI on the survival will be also investigated in the near
future.
A relatively high b value of 1,000 sec/m2
may eliminate possible microvascular contamination of the computed
ADC values and may improve the detection of slow-moving water
molecules or small diffusion distances, which are essential in the
early response evaluations. We qualitatively analyzed the ADC
values with a focus on the primary tumor, as it is difficult to
match the histopathological analysis of the lymph nodes site by
site with that of the MRI, mainly due to the depletion of
mesorectal lymph nodes following irradiation on pathological
analysis. We recently reported that the response of the primary
tumor to NA-CRT was correlated with the number of positive nodes on
pathological analysis in patients with RC who received NA-CRT
followed by surgery (33). The
prediction of the pathological response of involved lymph nodes to
NA-CRT is poorly understood. The correlation between the ADC values
and pathological response of metastatic nodes should be further
investigated in order to assess the potential to minimize surgery
with abbreviating lymph node dissection.
Another approach to NA-RT commonly in use for RC is
short-course RT (25 Gy in 5 fractions) followed by surgery within 1
week (2,3). We recently reported the feasibility and
validity of a novel protocol using modified short-course RT
combined with S-1 followed by surgery (10–12).
Further studies are required to standardize a method of
quantitative analysis of DW imaging in order to determine the
definitive clinical application of this technique in patients with
RC treated with short-course RT followed by surgery.
In conclusion, we demonstrated that the ADC value of
MRI in RC may provide valuable information enabling the prediction
of the response to CRT including CPT-11 plus S-1. This method
appears to have the potential to facilitate the physicians'
decision-making process when selecting patients to be treated with
CRT or surgery alone.
References
|
1
|
National Comprehensive Cancer Network, .
Rectal Cancer, Version 3. 2015.http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdfJune
23–2015
|
|
2
|
Glimelius B, Tiret E, Cervantes A and
Arnold D: ESMO Guidelines Working Group: Rectal cancer: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24:(Sul 6). vi81–vi88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marijnen CA and Glimelius B: The role of
radiotherapy in rectal cancer. Eur J Cancer. 38:943–952. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sauer R, Becker H, Hohenberger W, et al:
German Rectal Cancer Study Group: Preoperative versus postoperative
chemoradiotherapy for rectal cancer. N Engl J Med. 351:1731–1740.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagman R, Minsky BD, Cohen AM, Guillem JG
and Paty PP: Sphincter preservation in rectal cancer with
preoperative radiation therapy and coloanal anastomosis: Long term
follow-up. Int J Radiat Oncol Biol Phys. 42:51–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frykholm GJ, Isacsson U, Nygård K, et al:
Preoperative radiotherapy in rectal carcinoma - aspects of acute
adverse effects and radiation technique. Int J Radiat Oncol Biol
Phys. 35:1039–1048. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ceelen W, Fierens K, Van Nieuwenhove Y and
Pattyn P: Preoperative chemoradiation versus radiation alone for
stage II and III resectable rectal cancer: A systematic review and
meta-analysis. Int J Cancer. 124:2966–2972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wada H, Nemoto K, Nomiya T, et al: A phase
I trial of S-1 with concurrent radiotherapy in patients with
locally recurrent rectal cancer. Int J Clin Oncol. 18:273–278.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morimoto S, Shimada M, Kurita N, et al:
Preoperative radiotherapy combined with S-1 for advanced lower
rectal cancer: Phase I trial. Hepatogastroenterology. 59:1428–1432.
2012.PubMed/NCBI
|
|
10
|
Doi H, Beppu N, Odawara S, et al:
Neoadjuvant short-course hyperfractionated accelerated radiotherapy
(SC-HART) combined with S-1 for locally advanced rectal cancer. J
Radiat Res (Tokyo). 54:1118–1124. 2013. View Article : Google Scholar
|
|
11
|
Beppu N, Matsubara N, Noda M, et al: The
timing of surgery after preoperative short-course S-1
chemoradiotherapy with delayed surgery for T3 lower rectal cancer.
Int J Colorectal Dis. 29:1459–1466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beppu N, Matsubara N, Kakuno A, et al:
Feasibility of modified short-course radiotherapy combined with a
chemoradiosensitizer for T3 rectal cancer. Dis Colon Rectum.
58:479–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sato T, Ozawa H, Hatate K, et al: A Phase
II trial of neoadjuvant preoperative chemoradiotherapy with S-1
plus irinotecan and radiation in patients with locally advanced
rectal cancer: Clinical feasibility and response rate. Int J Radiat
Oncol Biol Phys. 79:677–683. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chenevert TL, Stegman LD, Taylor JM, et
al: Diffusion magnetic resonance imaging: An early surrogate marker
of therapeutic efficacy in brain tumors. J Natl Cancer Inst.
92:2029–2036. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamstra DA, Rehemtulla A and Ross BD:
Diffusion magnetic resonance imaging: A biomarker for treatment
response in oncology. J Clin Oncol. 25:4104–4109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DeSouza NM, Riches SF, Vanas NJ, et al:
Diffusion-weighted magnetic resonance imaging: A potential
non-invasive marker of tumour aggressiveness in localized prostate
cancer. Clin Radiol. 63:774–782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SH, Lee JM, Hong SH, et al: Locally
advanced rectal cancer: Added value of diffusion-weighted MR
imaging in the evaluation of tumor response to neoadjuvant chemo-
and radiation therapy. Radiology. 253:116–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Monguzzi L, Ippolito D, Bernasconi DP, et
al: Locally advanced rectal cancer: Value of ADC mapping in
prediction of tumor response to radiochemotherapy. Eur J Radiol.
82:234–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lambregts DM, Maas M, Riedl RG, et al:
Value of ADC measurements for nodal staging after chemoradiation in
locally advanced rectal cancer - a per lesion validation study. Eur
Radiol. 21:265–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Curvo-Semedo L, Lambregts DM, Maas M, et
al: Rectal cancer: Assessment of complete response to preoperative
combined radiation therapy with chemotherapy - conventional MR
volumetry versus diffusion-weighted MR imaging. Radiology.
260:734–743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai G, Xu Y, Zhu J, Gu WL, Zhang S, Ma XJ,
Cai SJ and Zhang Z: Diffusion-weighted magnetic resonance imaging
for predicting the response of rectal cancer to neoadjuvant
concurrent chemoradiation. World J Gastroenterol. 19:5520–5527.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dzik-Jurasz A, Domenig C, George M, et al:
Diffusion MRI for prediction of response of rectal cancer to
chemoradiation. Lancet. 360:307–308. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barbaro B, Vitale R, Valentini V, et al:
Diffusion-weighted magnetic resonance imaging in monitoring rectal
cancer response to neoadjuvant chemoradiotherapy. Int J Radiat
Oncol Biol Phys. 83:594–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ha HI, Kim AY, Yu CS, Park SH and Ha HK:
Locally advanced rectal cancer: Diffusion-weighted MR tumour
volumetry and the apparent diffusion coefficient for evaluating
complete remission after preoperative chemoradiation therapy. Eur
Radiol. 23:3345–3353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lambregts DM, Vandecaveye V, Barbaro B, et
al: Diffusion-weighted MRI for selection of complete responders
after chemoradiation for locally advanced rectal cancer: A
multicenter study. Ann Surg Oncol. 18:2224–2231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SH, Lee JY, Lee JM, Han JK and Choi
BI: Apparent diffusion coefficient for evaluating tumour response
to neoadjuvant chemoradiation therapy for locally advanced rectal
cancer. Eur Radiol. 21:987–995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lambrecht M, Deroose C, Roels S, et al:
The use of FDG-PET/CT and diffusion-weighted magnetic resonance
imaging for response prediction before, during and after
preoperative chemoradiotherapy for rectal cancer. Acta Oncol.
49:956–963. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lambregts DM, Beets GL, Maas M, et al:
Tumour ADC measurements in rectal cancer: Effect of ROI methods on
ADC values and interobserver variability. Eur Radiol. 21:2567–2574.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JC, Lim JS, Keum KC, et al: Comparison
of diffusion-weighted MRI and MR volumetry in the evaluation of
early treatment outcomes after preoperative chemoradiotherapy for
locally advanced rectal cancer. J Magn Reson Imaging. 34:570–576.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DeVries AF, Kremser C, Hein PA, et al:
Tumor microcirculation and diffusion predict therapy outcome for
primary rectal carcinoma. Int J Radiat Oncol Biol Phys. 56:958–965.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: Revised
RECIST guideline (v1.1). Eur J Cancer. 45:228–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Toiyama Y, Inoue Y, Saigusa S, et al:
C-reactive protein as predictor of recurrence in patients with
rectal cancer undergoing chemoradiotherapy followed by surgery.
Anticancer Res. 33:5065–5074. 2013.PubMed/NCBI
|
|
33
|
Beppu N, Matsubara N, Noda M, et al:
Pathologic evaluation of the response of mesorectal positive nodes
to preoperative chemoradiotherapy in patients with rectal cancer.
Surgery. 157:743–751. 2015. View Article : Google Scholar : PubMed/NCBI
|