Introduction
The incidence of brain metastasis strongly depends
on the primary tumor and, in certain types of cancer, also on the
molecular subtype. Approximately 25–30% of all cancer patients will
develop brain metastasis during their life span (1). The incidence of brain metastasis per
cancer type in adult patients has been reported to be as high as
45% (range, 40–50%) for patients with lung cancer, 20% (range,
15–25%) for breast cancer, 15% (range, 5–20%) for malignant
melanoma and 5% (range, 4–6%) for patients with renal cell cancer;
however, it is relatively rare in cancers of the gastrointestinal
tract (2). The incidence of brain
metastasis diagnosed intra vitam is increasing and the
reasons for this are multifactorial: They include a factual
increase in the incidence of cancers associated with tobacco use,
such as lung cancer, but this incidence also increases secondarily
to the prolonged survival of cancer patients with improving
adjuvant therapy techniques. Finally, there is an increase in the
detection rate due to the wider availability and more systematic
utilization of magnetic resonance imaging (MRI) in upfront staging
and during follow-up.
Irrespective of the existence of brain parenchymal
metastasis, leptomeningeal seeding is considered to be a
devastating complication of cancer and conveys a poor prognosis
(3–7).
The incidence of leptomeningeal metastases in patients with
extracranial tumors is strictly correlated with the primary cancer
type and is reportedly ~30% (range, 22–64%) in breast cancer, 16%
(range, 10–26%) in lung cancer, 11% (range, 7–15%) in malignant
melanoma, 6% (range, 4–14%) in gastrointestinal tract cancer and
1–7% in carcinomas of unknown primary origin (8–11). In
addition to metastatic tumors, certain primary brain tumors may
also be associated with leptomeningeal spread, with an incidence
that varies widely from 10 to 32%, and largely depends on tumor
histology and patient age, but also varies with the respective
referral pattern (8–11). Malignant leptomenigeal disease poses a
unique challenge to the health care providers.
Approximately 1–5% of patients with solid brain
tumors or leptomeningeal metastasis suffer from intracranial
hypertension and/or hydrocephalus, with the incidence being even
higher among patients with primary brain tumors (6,12).
Hydrocephalus may result from the obstruction of cerebrospinal
fluid (CSF) pathways by any mass or dissemination of metastatic
cells in the subarachnoid Virchow-Robin space, resulting in CSF
malabsorption. The symptoms of hydrocephalus, such as headache,
nausea, vomiting, gait disturbance, urinary incontinence, visual
decline, cranial nerve palsy and even mental status changes, may be
so disabling as to prevent systemic cancer treatment (6). These symptoms often do not respond well
to conservative medical treatment with corticosteroids and
analgesics or radiation. In fact, the majority of these patients
reportedly present with comparably poor performance status and
their condition in late-stage disease carries a very poor
prognosis, with an overall survival (OS) (with treatment) of only
2–3 months in ~50% of the patients, with only 10% surviving for 1
year (3,13).
Ventriculoperitoneal shunting (VPS) may rapidly
normalize intracranial pressure (ICP) in the setting of
hydrocephalus (6,14–18).
However, despite the fact that it is considered a minor invasive
procedure, it does involve certain risks, such as hemorrhage, shunt
malfunction, or infection (19–21); in
addition, it may rarely result in peritoneal carcinomatosis due to
seeding from the central nervous system (CNS) tumors (22–39).
Nevertheless, not all studies have encountered peritoneal seeding
via VPS (16,30,40).
There are several studies in the literature that
have addressed radiation therapy, systemic chemotherapy and
intrathecal chemotherapy specifically for the treatment for brain
metastasis with/without leptomeningeal seeding (3,41–43). However, the number of published
reports on the palliative surgical management of hydrocephalus
secondary to brain metastasis and/or leptomeningeal seeding from
malignant tumors is currently limited (6,12,17,18).
The aim of this study was to reassess the validity,
safety, and benefits of VPS as symptom-oriented palliative therapy
for oncological patients with advanced-stage cancer. We also aimed
to determine the risk/benefit ratio and investigate the outcomes of
patients in whom previous medical management for hydrocephalic
symptoms had failed and who were then selected for VPS.
Patients and methods
Patients
Data were prospectively collected from 59
consecutive adult patients diagnosed with hydrocephalus in the
setting of brain metastasis or primary brain tumors, who underwent
de novo VP shunt placement at the Beth Israel Deaconess
Medical Center (BIDMC; Boston, USA) between April 2004 and July
2012. Patients who had previously undergone VPS and presented with
shunt failure requiring shunt revision, as well as those with
non-oncological indications for shunting, were excluded.
This retrospective cohort analysis was approved by
our Institutional Review Board (no. 2011P-000101/4).
Medical record review
All pertinent medical records and related imaging
studies were reviewed, including initial clinical and neurological
presentation, primary tumor histology, Karnofsky performance status
(KPS), recursive partitioning analysis (RPA) class status and
radiographic imaging studies, such as pre- and postoperative
computed tomography (CT)/MRI. Clinical symptom severity was
assessed prior to and following VP placement. The durability
(longevity) of the implanted VPS system was also examined via
longitudinal follow-up appointments with clinical and imaging
examinations, in order to detect possible occurrence, time and
cause of shunt failure.
CNS metastasis and hydrocephalus
CNS metastasis was diagnosed based on available
histopathology from systemic disease status and evaluation of
cranial MRI findings. The presence of leptomenigeal metastasis at
the time of VPS was diagnosed by the clinical status of the patient
and the presence of characteristic leptomenigeal enhancing lesions
on MRI, or by the presence of malignant cells found in a spinal tap
sample of CSF. Hydrocephalus was classified as communicating
hydrocephalus with leptomeningeal enhancement by MRI and high CSF
protein content or positive CSF cytology, or as obstructive
hydrocephalus due to a parenchymal or intraventricular mass.
Indications for VPS and procedure
The indications for placement of a VP shunt were: i)
Clinical deterioration due to an increase in ICP despite aggressive
medical ICP management, or ii) progressive ventriculomegaly in the
setting of progressively developing neurological deficits.
All VP placements were performed under general
anesthesia with endotracheal intubation. Preoperative MRI or CT
imaging of the head was used for surgical planning. The proximal
shunt catheter was passed into the lateral ventricle using a Ghajar
guide or by the free-hand technique, depending on the surgeon's
preference. Preset Medtronic Delta® valves (Medtronic, Dublin,
Republic of Ireland), performance level 1.5, were used in all
cases. The distal catheter was tunneled subcutaneously and its end
was placed into the peritoneal cavity under direct visualization,
either through a mini-laparotomy or via laparoscopic assistance.
For intrathecal drug delivery and to provide access for CSF
sampling, certain patients with suitable leptomeningeal disease
(e.g., breast cancer) also had an Ommaya reservoir placed during
the same operation via a separate incision. Postoperative CT scans
of the head were performed on all patients to confirm the
appropriate positioning of the proximal catheter and to rule out
any procedural complications.
Follow-up
The most recent patient radiographic assessment
(clinical visit or hospital discharge) was defined as the end-point
of the follow-up period. For the purpose of this study and based on
local referral patterns in our commonwealth, we hypothesized that
patients who were not referred back to our hospital or office for
any shunt-related problems, retained a functioning shunt for the
entire study period. The end-points of the study were any
occurrence of shunt revision, timing of shunt revision or shunt
removal, and patient death. A Kaplan-Meier analysis was performed
to evaluate the rate of shunt survival at 3 months and 1 year.
Results
Patient characteristics
A total of 59 adult patients with hydrocephalus from
CNS tumors or cranial metastasis underwent VPS at the BIDMC between
2005 and 2012. The mean age of the patients was 57.2 years (range,
22.0–81.6) years; our cohort included 23 (39%) men and 36 (61%)
women. A total of 40 patients (68%) had CNS metastasis from
extracranial tumors, among those 13/59 (22%) from lung cancer,
11/59 (18%) from breast cancer, 6/59 (10%) from melanoma, 3/59 (5%)
from renal cell cancer, 3/59 (5%) from colorectal cancer, 2/59 (4%)
from ovarian cancer and 2/59 (4%) from lymphoma; of the entire
cohort of 59 patients, 19 (32%) patients had a primary CNS
tumor.
A total of 19 (32.2%) patients in this study group
had obstructive hydrocephalus, whereas 40 (67.8%) patients had
communicating hydrocephalus; significant intracranial hypertension
(ICP>20) was documented in 12/59 patients (20.3%) at the time of
VPS. At the time of palliative VP shunt placement, 20/59 (33.8%) of
this patient cohort had leptomeningeal metastasis, whereas the
remaining 39 (66%) had parenchymal metastasis (Table I).
 | Table I.Characteristics of the patient
cohort. |
Table I.
Characteristics of the patient
cohort.
|
Characteristics | All shunts
(n=59) |
|---|
| Mean age, years
(median, range) | 57.2 (61.9,
22.0–81.6) |
| Gender, no.
(%) |
|
|
Male |
23
(39.0) |
|
Female |
36
(61.0) |
| Indication for
surgery, no. (%) |
|
|
Lung |
13
(22.0) |
|
Breast |
11
(18.0) |
|
Melanoma |
6
(10.0) |
|
Renal |
3
(5.0) |
|
Colorectal |
3
(5.0) |
|
Ovarian |
2
(4.0) |
|
Lymphoma |
2
(4.0) |
| CNS
tumors |
19
(32.0) |
| Type of
hydrocephalus, no. (%) |
|
|
Obstructive |
19
(32.2) |
|
Communicating |
40
(67.8) |
| Presence of
LMS |
|
|
Yes |
20
(34.0) |
| No |
39
(66.0) |
| Mean Karnofsky
score (range) | 65
(30–100) |
| RPA class, no.
(%) |
|
| I |
10
(17.0) |
| II |
16
(27.0) |
|
III |
33
(56.0) |
| Progressive
systemic disease, no. (%) |
|
|
Yes |
49
(83.0) |
| No |
10
(17.0) |
Symptoms
The majority of the patients presented with a
variety of neurological symptoms, the most common being headache,
nausea and vomiting (60%), followed by gait disturbances (40%),
cognitive dysfunction such as memory impairment (30%), seizures
(3%) and urinary incontinence (2%) (Table II).
 | Table II.Presenting symptoms and improvement
following VP shunt placement. |
Table II.
Presenting symptoms and improvement
following VP shunt placement.
| Presenting
symptom | No. of patients
(%) | Clinical
improvement after VPS (%) |
|---|
| Headache, nausea,
vomiting | 36 (60.0) | 32 (89.0) |
| Gait
disturbance | 24 (40.0) | 12 (5.0) |
| Cognitive
dysfunction | 18 (30.0) | 8
(440) |
| Seizure | 2 (3.0) | 2
(100.0) |
| Urinary
incontinence | 1 (2.0) | 1
(1000) |
Preoperative assessment
The preoperative mean KPS score was found to be 65
(range, 30–100). The patients were also categorized according to
the RPA class and the results revealed class I status in 10 (17%),
II in 16 (27%), and III in 33 (56%) patients. At the time of VP
placement, there was documented progression of systemic disease in
49 (83%) patients (Table I).
Outcome and complications
The vast majority of the patients (55/59; 93.2%)
from our cohort experienced an overall improvement in the
neurological symptoms after surgery, but 4 (6.8%) patients did not
demonstrate a clear benefit from the procedure. The majority of the
patients exhibited a significant clinical improvement within the
first 3 or 4 days after surgery. Improvement was most commonly
observed in terms of the headache, nausea, vomiting and level of
alertness. Cognitive function and gait disturbance did not respond
as well to VPS, mainly reflecting the location of metastatic
disease (Table II).
A total of 7 (11.8%) postoperative complications
were encountered (Table III). One
patient developed proximal catheter obstruction and presented with
changes in mental status requiring catheter replacement. Two
patients suffered small intracranial hemorrhage from catheter
placement, but were monitored by a series of CT scans and neither
required shunt revision. One patient, who developed peritonitis
from methicillin-resistant Staphylococcus aureus, was
initially treated with antibiotics (vancomycin), but later
underwent shunt removal. Another patient developed a proximal shunt
infection (Staphylococcus aureus); this shunt was
temporarily changed to an external ventricular drainage (EVD) prior
to successful reinsertion, without any complications. Finally, 1
patient developed cellulitis at the abdominal incision, which was
successfully treated with antibiotics alone, and 1 patient had a
valve dysfunction that required revision. No further complications
were encountered during the observation period (Table III).
 | Table III.Postoperative complications. |
Table III.
Postoperative complications.
| Complications | No. of patients
(%) |
|---|
| Overall
postoperative complications | 7 (11.8) |
| Shunt
obstruction |
|
|
Proximal | 1 |
|
Distal | 0 |
| Intracerebral
bleeding | 2 |
| Infection |
|
|
Proximal shunt | 1 |
| Distal
shunt | 1 |
|
Wound | 1 |
| Valve revision | 1 |
Of note, there were no cases of clinically relevant
intraperitoneal tumor dissemination via the inserted VP shunt,
although the possibility of clinically silent intraperitoneal
seeding cannot be excluded, as routine abdominal reimaging was not
performed in all cases.
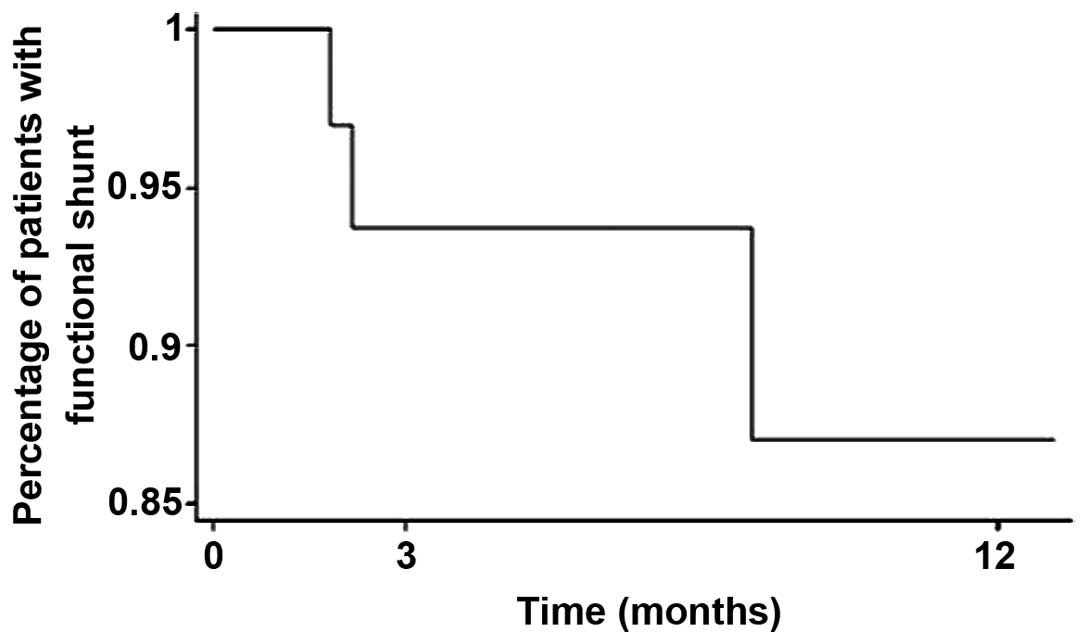
The mean overall shunt survival was 6.4 months
(range, 1.0 day-76.0 months) from the placement of the VP shunt. At
3 months after VPS, 93.5% of patient were alive with functioning
shunts and at 1 year 87% of the shunts remained functional
(Fig. 1). We hypothesized that the
shunts remained functional as long as the patients survived, and no
deaths were recorded as a direct cosequence of early procedural or
delayed shunt complications.
Discussion
Hydrocephalus in patients with cerebral metastases
or primary brain tumors may severely compromise the patient's
quality of life and poses a considerable challenge to the health
care providers, since hydrocephalic CNS symptoms in terminally ill
cancer patients are often medically uncontrollable. This leads to
the difficult choice of either offering further surgical treatment,
or transitioning the patient to pre-final hospice care. The
available literature on this aspect of late-stage cancer patient
care remains scarce. This issue is further complicated by the fact
that a number of late- and end-stage brain cancer patients are in
an immunocompromised state, due to leukopenia from myelosuppression
following prior chemotherapy, or secondary to steroid treatment for
edema control, which may lead to additional complications. Such
frail patients are also at increased risk from anesthesia and may
be at an increased risk of surgery- or shunt-related complications
(18,40,44,45).
The intracranial tumor burden in this setting may
cause obstructive or malresorptive hydrocephalus, or lead to
intracranial hypertension with altered CSF flow. Beyond general
clinical symptoms that are amenable to medical treatment in this
setting, intrathecal chemotherapy is not generally considered to be
a suitable treatment option, since CSF compartmentalization
prohibits effective drug delivery and tissue penetration and also
increases the risk of neurotoxicity (3,25,46,47). The
experience with major surgical intervention in terms of any
substantial benefit is limited in this setting, and quality of life
for the remaining lifespan of such patients should be one of the
goals of any health care provider.
In this study, we assessed a symptom-oriented
approach and reported on the outcomes of 59 patients with primary
extracranial or primary brain tumors who experienced clinical
symptoms from their intracranial tumor burden causing hydrocephalus
and/or intracranial hypertension. The patients analyzed in this
cohort had been refractory to medical treatment of their symptoms
despite aggressive management, and were therefore selected to
undergo palliative VPS to restore CSF flow and treat intracranial
hypertension.
In our cohort, the vast majority of the patients
(55/59; 93.2%) experienced immediate postoperative symptom relief.
Global symptoms, such as headache, nausea and vomiting exhibited
the most significant improvement following VPS, which compares
favorably with the results from other studies on postoperative
symptom relief in adult patients with metastatic CNS disease, which
reportedly ranges between 70 and 80% (12,48,49).
Lee et al (18)
reported their findings from a cohort of 50 patients with
hydrocephalus from cerebral metastasis; in their experience, 80% of
the patients improved after surgery, particularly with respect to
preoperative headaches. Another study by Omuro et al
(6) from the Memorial Sloan Kettering
Cancer Center reported the outcome of 37 patients with
leptomeningeal metastasis requiring VPS; in that particular cohort,
27/37 (77%) patients exhibited some overall improvement following
surgery, which translated into a substantial improvement in the
quality of life of terminally ill patients. Several other studies
and case reports in the literature have confirmed the efficacy of
VPS as palliative therapy in patients with CNS metastasis or
primary brain tumors (14–17).
In our cohort, the vast majority of the patients
reported that headache, nausea and vomiting were the symptoms that
improved the most following VPS, resolving in 89% of the cases;
however, gait disturbances and cognitive dysfunction only improved
in 50 and 40% of the patients, respectively, which may best be
explained by the more local effects of the underlying disease. Of
the 59 patients in our cohort, 2 presented with delayed new-onset
seizures, which may not be associated with the VPS procedure per
se (since seizures are a well-known occurrence in patients with
CNS tumors), whereas 1 patient presented with new-onset urinary
incontinence 3 months after VPS, which may be explained as
secondary to systemic disease progression, since his other
preceding CNS symptoms improved following VP shunt placement. All
these patients experienced improvement following surgery, without
any recurrent CNS symptoms.
Of our 59 patients, 7 (11.8%) experienced some form
of postoperative complications, 4 of whom required shunt
replacement with EVD prior to definitive care via shunt
reinsertion; the remaining 3 cases were monitored only and did not
require shunt revision or removal. The literature reports a rate of
postoperative complications between 8 and 30% (6,12,48–50) in an
unselected collective of VPS patients; therefore, our cohort is
favorably at the lower end of that spectrum.
The mean OS of the patients in our cohort was 6.4
months, which is not necessarily a meaningful number, given the
mixed underlying primary pathologies included in this study cohort;
however, this number is in line with other studies (3,5,7,12,17,18,43,47,51)
and corroborates the observation that VPS in such patients is a
durable form of therapy for symptom relief, particularly when high
shunt patency rates are ensured. We did not observe any significant
differences in post-VPS survival time according to primary
malignancy type, which is in accordance with the existing
literature (12,18). This observation may be further
explained by the fact that metastatic and, particularly,
leptomeningeal CNS disease, is encountered at the very late stages
of cancer, when the life expectancy of the patient is not
associated with the therapy form assessed here, and remains very
short. At 3 months after VPS placement, 93.5% of the shunts in our
study group were found to be functioning, and at 1 year 87% of the
shunts remained functional (Fig.
1).
Our results demonstrated that VPS in symptomatic
metastatic patients frequently leads to marked improvement in
hydrocephalic symptoms, even in the setting of leptomeningeal tumor
spread. Based on our results and following a comprehensive review
of the literature, we confirm the important role of VPS as
palliation in end-stage patients suffering from either metastatic
disease or primary brain tumors, with a poor prognosis. Based on
our observations, these shunts show excellent durability that often
exceeds the expected survival time and significantly improves the
quality of the remaining lifetime, even when the overall prognosis
is very poor.
In our cohort, we did not observe any clinical cases
of peritoneal carcinomatosis secondary to seeding through the
peritoneal catheter, although routine abdominal imaging was not
performed. Another explanation may be that the patients had
asymptomatic tumor deposits that remained clinically silent during
the short OS period following VP shunt placement. Only an autopsy
study may address this question in a definite way.
Further research should be focused on improving the
diagnostic and therapeutic tools for patients with late-stage CNS
malignancies, in order to identify patients who may benefit from
this therapy earlier. More sensitive diagnostic tools are required
to overcome the high false-negative rates of the currently
available imaging modalities, which often fail to demonstrate
leptomenigeal seeding (39), and the
problem of negative cytology findings from CSF samples in this
patient population. Beyond this, novel therapeutic modalities for
targeted leptomenigeal disease are required, employing
high-affinity agents that may be effectively applied despite CSF
flow stagnation. Another useful tool would be to develop reliable,
non-static, but rather dynamic VPS valve systems. The latter may
allow for a timed ‘switched-off’ mode, thus enabling intermittent
intrathecal chemotherapy, as well as long-term CSF shunting.
There were several limitations to this study. i) A
major limitation lies with the fact that it is a retrospective
study with a treatment paradigm that comes from a single
institution and, thus, patient selection and their treatment
pathways are subject to some selection bias; ii) another limitation
of this study is the relatively small sample size; iii) long-term
postoperative neurological evaluation was not performed in all
patients, once the shunt was functional; and iv) not all patients
were restaged to assess their clinical status regarding progression
of systemic disease.
A prospective study with a larger sample size and
more elaborate neurological scales to evaluate the postoperative
condition over time for each patient, with accurate restaging for
systemic disease, may add valuable information to this field of
research.
In conclusion, although the prognosis of patients
with CNS metastasis or malignant primary brain tumors in the
clinical setting of hydrocephalus is very poor, VPS offers an
effective, safe and valid palliative option for symptom relief and
improvement of quality of life, even in patients with very poor
overall prognosis. In addition to the improvement in neurological
symptoms, VPS also allows for relatively simple and efficient
delivery of intrathecal chemotherapy in those patients.
References
|
1
|
Kehrli P: Epidemiology of brain
metastases. Neurochirurgie. 45:357–363. 1999.((In French)).
PubMed/NCBI
|
|
2
|
Fidler IJ: The role of the organ
microenvironment in brain metastasis. Semin Cancer Biol.
21:107–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herrlinger U, Förschler H, Küker W,
Meyermann R, Bamberg M, Dichgans J and Weller M: Leptomeningeal
metastasis: Survival and prognostic factors in 155 patients. J
Neurol Sci. 223:167–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miralbell R, Tolnay M, Bieri S, Probst A,
Sappino AP, Berchtold W, Pepper MS and Pizzolato G: Pediatric
medulloblastoma: Prognostic value of p53, bcl-2, Mib-1 and
microvessel density. J Neurooncol. 45:103–110. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oechsle K, Lange-Brock V, Kruell A,
Bokemeyer C and de Wit M: Prognostic factors and treatment options
in patients with leptomeningeal metastases of different primary
tumors: A retrospective analysis. J Cancer Res Clin Oncol.
136:1729–1735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Omuro AM, Lallana EC, Bilsky MH and
DeAngelis LM: Ventriculoperitoneal shunt in patients with
leptomeningeal metastasis. Neurology. 64:1625–1627. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taillibert S and Hildebrand J: Treatment
of central nervous system metastases: Parenchymal, epidural and
leptomeningeal. Curr Opin Oncol. 18:637–643. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaplan JG, DeSouza TG, Farkash A, Shafran
B, Pack D, Rehman F, Fuks J and Portenoy R: Leptomeningeal
metastases: Comparison of clinical features and laboratory data of
solid tumors, lymphomas and leukemias. J Neurooncol. 9:225–229.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pedersen PH, Rucklidge GJ, Mørk SJ, Terzis
AJ, Engebraaten O, Lund-Johansen M, Backlund EO, Laerum OD and
Bjerkvig R: Leptomeningeal tissue: A barrier against brain tumor
cell invasion. J Natl Cancer Inst. 86:1593–1599. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shapiro WR, Posner JB, Ushio Y, Chemik NL
and Young DF: Treatment of meningeal neoplasms. Cancer Treat Rep.
61:733–743. 1977.PubMed/NCBI
|
|
11
|
Wasserstrom WR, Glass JP and Posner JB:
Diagnosis and treatment of leptomeningeal metastases from solid
tumors: Experience with 90 patients. Cancer. 49:759–772. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CC, Kasper E and Warnke P: Palliative
stereotactic-endoscopic third ventriculostomy for the treatment of
obstructive hydrocephalus from cerebral metastasis. Surg Neurol
Int. 2:762011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Glantz MJ, Jaeckle KA, Chamberlain MC,
Phuphanich S, Recht L, Swinnen LJ, Maria B, LaFollette S, Schumann
GB, Cole BF, et al: A randomized controlled trial comparing
intrathecal sustained-release cytarabine (DepoCyt) to intrathecal
methotrexate in patients with neoplastic meningitis from solid
tumors. Clin Cancer Res. 5:3394–3402. 1999.PubMed/NCBI
|
|
14
|
Gonda DD, Kim TE, Warnke PC, Kasper EM,
Carter BS and Chen CC: Ventriculoperitoneal shunting versus
endoscopic third ventriculostomy in the treatment of patients with
hydrocephalus related to metastasis. Surg Neurol Int. 3:972012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schiff D, Kline C, Meltzer H and Auger J:
Palliative ventriculoperitoneal shunt in a pediatric patient with
recurrent metastatic medulloblastoma. J Palliat Med. 12:391–393.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inamasu J, Nakamura Y, Saito R, Kuroshima
Y, Mayanagi K, Orii M and Ichikizaki K: Postoperative communicating
hydrocephalus in patients with supratentorial malignant glioma.
Clin Neurol Neurosurg. 106:9–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lokich J, Levine H and Nasser I:
Malignancy-related hydrocephalus: Clinical features and results of
ventricular peritoneal shunt procedure in three patients. Am J Clin
Oncol. 21:366–368. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SH, Kong DS, Seol HJ, Nam DH and Lee
JI: Ventriculoperitoneal shunt for hydrocephalus caused by central
nervous system metastasis. J Neurooncol. 104:545–551. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Handler MH and Callahan B: Laparoscopic
placement of distal ventriculoperitoneal shunt catheters. J
Neurosurg Pediatr. 2:282–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naftel RP, Argo JL, Shannon CN, Taylor TH,
Tubbs RS, Clements RH and Harrigan MR: Laparoscopic versus open
insertion of the peritoneal catheter in ventriculoperitoneal shunt
placement: Review of 810 consecutive cases. J Neurosurg.
115:151–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roth J, Sagie B, Szold A and Elran H:
Laparoscopic versus non-laparoscopic-assisted ventriculoperitoneal
shunt placement in adults. A retrospective analysis. Surg Neurol.
68:177–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brust JC, Moiel RH and Rosenberg RN: Glial
tumor metastases through a ventriculo-pleural shunt. Resultant
massive pleural effusion. Arch Neurol. 18:649–653. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duffner PK and Cohen ME: Extraneural
metastases in childhood brain tumors. Ann Neurol. 10:261–265. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eralp Y, Saip P, Aydin Z, Berkman S and
Topuz E: Leptomeningeal dissemination of ovarian carcinoma through
a ventriculoperitoneal shunt. Gynecol Oncol. 108:248–250. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Glantz MJ, Cole BF, Recht L, Akerley W,
Mills P, Saris S, Hochberg F, Calabresi P and Egorin MJ: High-dose
intravenous methotrexate for patients with nonleukemic
leptomeningeal cancer: Is intrathecal chemotherapy necessary? J
Clin Oncol. 16:1561–1567. 1998.PubMed/NCBI
|
|
26
|
Hoffman HJ, Hendrick EB and Humphreys RP:
Metastasis via ventriculoperitoneal shunt in patients with
medulloblastoma. J Neurosurg. 44:562–566. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoffman HJ and Duffner PK: Extraneural
metastases of central nervous system tumors. Cancer. 56(Suppl 7):
1778–1782. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ingold B, Moschopulos M, Hutter G, Seeger
H, Röthlisberger B, Landolt H, Yonekawa Y, Jochum W and Heppner FL:
Abdominal seeding of an atypical teratoid/rhabdoid tumor of the
pineal gland along a ventriculoperitoneal shunt catheter. Acta
Neuropathol. 111:56–59. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Korones DN, Meyers SP, Rubio A, Torres C
and Constine LSA: A 4-year-old girl with a ventriculoperitoneal
shunt metastasis of a central nervous system atypical
teratoid/rhabdoid tumor. Med Pediatr Oncol. 32:389–391. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Belongia M and Jogal S: Extraneural
metastasis of a nongerminomatous germ cell tumor of the central
nervous system in a pediatric patient with a ventriculoperitoneal
shunt: A case report and review of the literature. J Pediatr
Hematol Oncol. 34:e12–e16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oberbauer RW, Tritthart H, Ascher PW,
Walter GF and Becker H: Shunt metastases in posterior fossa tumors.
Neuropadiatrie. 10:296–300. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paul MJ, Summers Y, Calvert AH, Rustin G,
Brampton MH, Thatcher N and Middleton MR: Effect of temozolomide on
central nervous system relapse in patients with advanced melanoma.
Melanoma Res. 12:175–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rickert CH: Extraneural metastases of
paediatric brain tumours. Acta Neuropathol. 105:309–327.
2003.PubMed/NCBI
|
|
34
|
Rubery ED and Wheeler TK: Metastases
outside the central nervous system from a presumed pineal
germinoma. Case report. J Neurosurg. 53:562–565. 1980.PubMed/NCBI
|
|
35
|
Russell DS and Rubinstein LJ: Pathology of
tumors of the nervous system. (4th). (Arnold, London). 206–207.
1977.
|
|
36
|
Sakata K, Yamada H, Sakai N, Hosono Y,
Kawasako T and Sasaoka I: Extraneural metastasis of pineal tumor.
Surg Neurol. 3:49–54. 1975.PubMed/NCBI
|
|
37
|
Wakamatsu T, Matsuo T, Kawano S, Teramoto
S and Matsumura H: Glioblastoma with extracranial metastasis
through ventriculopleural shunt. Case report. J Neurosurg.
34:697–701. 1971.PubMed/NCBI
|
|
38
|
Triolo PJ and Schulz EE: Metastatic
germinoma (pinealoma) via a ventriculoperitoneal shunt. AJR Am J
Roentgenol. 135:854–855. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han YP, Zhao Y, He XG and Ma J: Peritoneal
metastasis of third ventricular atypical teratoid/rhabdoid tumor
after VP shunt implantation for unexplained hydrocephalus. World J
Pediatr. 8:367–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berger MS, Baumeister B, Geyer JR,
Milstein J, Kanev PM and LeRoux PD: The risks of metastases from
shunting in children with primary central nervous system tumors. J
Neurosurg. 74:872–877. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Berg SL and Chamberlain MC: Systemic
chemotherapy, intrathecal chemotherapy, and symptom management in
the treatment of leptomeningeal metastasis. Curr Oncol Rep.
5:29–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boogerd W, van den Bent MJ, Koehler PJ,
Heimans JJ, van der Sande JJ, Aaronson NK, Hart AA, Benraadt J and
Vecht CHJ: The relevance of intraventricular chemotherapy for
leptomeningeal metastasis in breast cancer: A randomised study. Eur
J Cancer. 40:2726–2733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
DeAngelis LM and Boutros D: Leptomeningeal
metastasis. Cancer Invest. 23:145–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lobotesis K, U-King-Im JM, Cross JJ,
Gillard JH and Antoun NM: Gliomatosis peritonei associated with a
ventriculo-peritoneal shunt. Clin Radiol. 64:95–99. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zemack G and Romner B: Seven years of
clinical experience with the programmable Codman Hakim valve: A
retrospective study of 583 patients. J Neurosurg. 92:941–948. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lassman AB, Abrey LE, Shah GD, Panageas
KS, Begemann M, Malkin MG and Raizer JJ: Systemic high-dose
intravenous methotrexate for central nervous system metastases. J
Neurooncol. 78:255–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pentheroudakis G and Pavlidis N:
Management of leptomeningeal malignancy. Expert Opin Pharmacother.
6:1115–1125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Farahmand D, Hilmarsson H, Högfeldt M and
Tisell M: Perioperative risk factors for short term shunt revisions
in adult hydrocephalus patients. J Neurol Neurosurg Psychiatry.
80:1248–1253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hoh BL, Lang SS, Ortiz MV, Chi YY, Lewis
SB and Pincus DW: Lower incidence of reoperation with longer shunt
survival with adult ventriculoperitoneal shunts placed for
hemorrhage-related hydrocephalus. Neurosurgery. 63:70–74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Reddy GK, Bollam P, Caldito G, Willis B,
Guthikonda B and Nanda A: Ventriculoperitoneal shunt complications
in hydrocephalus patients with intracranial tumors: An analysis of
relevant risk factors. J Neurooncol. 103:333–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin N, Dunn IF, Glantz M, Allison DL,
Jensen R, Johnson MD, Friedlander RM and Kesari S: Benefit of
ventriculoperitoneal cerebrospinal fluid shunting and intrathecal
chemotherapy in neoplastic meningitis: A retrospective,
case-controlled study. J Neurosurg. 115:730–736. 2011. View Article : Google Scholar : PubMed/NCBI
|