Introduction
Acute kidney injury (AKI) is defined as an absolute
increase in serum creatinine level by either >0.3 mg/dl, an
increase of ≥50% from baseline, a reduction of the glomerular
filtration rate (GFR) ≥25% from the baseline or a reduction of the
urine output by <0.5 ml/kg for >8 h (1). The requirement to describe AKI precisely
and sensitively has caused the development of a multidimensional
AKI classification system, proposed by the Acute Dialysis Quality
Initiative group and expanded by the Acute Kidney Injury Network
that brought the Risk, Injury, Failure, Loss and End-Stage renal
disease (RIFLE) criteria and staging into position as the standard
definition and diagnosis of this syndrome (2,3). The
advantages of using RIFLE criteria are that a diagnosis can be
established at the stage of preventable renal dysfunction. The
etiology of AKI over the past decades has shifted from primary
renal disease to multifactorial causes, particularly in
hospitalized children (4).
Solid tumors represent >40% of all childhood
malignancies. Examples of solid tumors are neuroblastoma, Wilms
tumor, hepatoblastoma, rhabdomyosarcoma, brain tumors and sarcomas
(5). Renal complications in children
with malignancies primarily arise from renal parenchymal tumors,
tumor lysis syndrome, malignant infiltration or obstruction of the
urinary tract, or it may be secondary to a variety of treatment
modalities, such as chemotherapy, surgery or radiotherapy; and
finally, it can result from supportive measures (6). Chemotherapy can cause nephrotoxicity,
while renal impairment can result in altered excretion and
metabolism of chemotherapeutic agents. Renal dysfunction is a
problematic adverse effect that can hinder the continued
administration of anticancer treatment and the optimal use of
ancillary and supportive measures (7).
There is an urgent requirement for early predictive
biomarkers of AKI, as early intervention can significantly improve
the prognosis. AKI is largely asymptomatic, and establishing the
diagnosis in this increasingly common disorder currently depends on
functional biomarkers, such as serial serum creatinine
measurements. However, serum creatinine is a delayed and unreliable
indicator of AKI due to various reasons (8). Neutrophil gelatinase-associated
lipocalin (NGAL) is emerging as a biomarker in the urine and
plasma, for the early prediction of AKI and for the prognosis of
AKI in several common clinical scenarios (9). These include cardiopulmonary bypass
(10), kidney transplantation
(11,12), diarrhea-associated hemolytic uremic
syndrome (13), contrast nephropathy
(14) and Henoch-Schonlein purpura
nephritis (15). However, the
expression of NGAL in the urine of children administered
chemotherapy for solid tumors has yet to be elucidated.
The aim of the present study was to compare the
predictability of urinary NGAL (uNGAL) as a biomarker of AKI with
creatinine as a traditional biomarker in children with solid tumors
under chemotherapy.
Patients and methods
Subjects
The cross sectional study was conducted in the
Pediatric Oncology Unit, Zagazig University Hospital (Zagazig,
Egypt) during the period between March 2011 and March 2013. The
patient group included 30 male and female patients, aged from one
month to 18 years, with different solid tumors diagnosed at the
Pediatric Oncology Unit, Zagazig University Hospital using the
routine methods of diagnosis of solid tumors (history, clinical
examination and investigations including biopsy and radiology).
There was no renal impairment at the beginning of the study, as
guided by the serum creatinine level. All the patients completed
their protocol of therapy. The control group included 20 age- and
gender-matched controls with no renal impairment, as guided by
history, clinical examination and serum creatinine.
Methods
All the participants in the study were subjected to
a full history and thorough clinical examination. Kidney function
tests, including serum creatinine and blood urea nitrogen (BUN),
were performed three times. The first sample was the baseline
before the beginning of the protocol. The second sample was taken
one week after the beginning of chemotherapy. The third sample was
obtained at the end of the treatment protocol. Serum creatinine and
BUN were measured using a Dimension RxL autoanalyzer (Siemens
Healthcare Diagnostics Inc., Newark, DE, USA). The uNGAL ELISA
assay was analyzed at the same time points with serum
creatinine.
Sampling and principle of the uNGAL
assay
Morning urine samples were aseptically collected and
voided directly into a sterile container. The samples were
centrifuged to remove particulate matter, aliquoted and stored at
−20°C until the measurement using the commercial uNGAL ELISA kit
(Quantikine; R&D Systems, Minneapolis, MN, USA) in accordance
with the manufacturer's instructions. The uNGAL cut-off level of 25
ng/ml was used for the definition of AKI, as based on the studies
by Cho et al (16) and de Geus
et al (17).
Statistical analysis
Statistical analyses were performed using SPSS 10.0
statistical software (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation for quantitative
variables and as numbers and percentages for qualitative variables.
The independent t-test value, χ2 test and correlation
coefficient were used when appropriate. P<0.05 was considered to
indicate a statistically significant difference. The performance
characteristics of the uNGAL levels and serum creatinine in
detecting the acute renal injury were described using the area
under a receiver operator characteristic (ROC) curve. Confidence
intervals (CI) refer to 95% boundaries.
Ethics
The study was conducted in accordance with the
Helsinki Declaration 1964 as revised in 2000 (18), and was approved by the Ethics
Committee of the Faculty of Medicine, Zagazig University. Informed
consent was obtained from all the study parents or guardians.
Results
Patient characteristics and changes
between the three samples
The patients were 50% female and 50% male, and the
mean age was 4.9±3.1 years. The age and gender data of the studied
groups are represented in Table I.
There were 12 cases with Wilm's tumor (40.0%), 10 with
neuroblastoma (33.3%), four with medulloblastoma (13.3%), three
with rhabdomyosarcoma (10.0%), and one case of Ewing sarcoma
(3.3%). There was no significant difference in serum creatinine
level between cases and controls in the baseline samples. The mean
of serum creatinine was 0.4±0.1 in patients vs. 0.36±0.13 mg/dl in
controls (P>0.05). However, there was a highly significant
difference in the serum creatinine level between the cases and
controls in the second and third samples, where the means of serum
creatinine in cases were 0.5±0.1 and 0.6±0.12 mg/dl, respectively
vs. 0.36±0.13 and 0.4±0.15 mg/dl in controls (P<0.001). The
percentage of change in mean creatinine in patients in the second
and third results compared to the baseline was 26.1 and 54.1%,
respectively. Although these two percentages were statistically
significant (P<0.003 and P<0.001, respectively), a percentage
of 26.1% in the second sample is not enough to diagnose AKI in
these patients. Regarding uNGAL, there was a significant difference
between cases and controls in the initial baseline results, in the
second and third samples following chemotherapy initiation and at
the end of the protocol, respectively. The mean of uNGAL in the
first, second and third samples in order was 7.5±4.2, 31.3±17.3 and
49±16.2 ng/ml in cases vs. 3.6±1.2, 3.2±1.3 and 3.5±1.6 ng/ml in
controls (P<0.001). The percentage of change in mean uNGAL in
the second and third samples compared to the baseline was 376.8 and
698.2%, respectively, which is highly significant (P<0.001).
Table II illustrates serum
creatinine and uNGAL values in cases and controls in the three
samples. The percentage of change in serum creatinine and uNGAL in
the three samples of the cases is illustrated in Tables III and IV, respectively, and the correlation
between the two is represented in Table
V. There was a highly statistically significant difference
between the percentage of patients diagnosed with AKI based on
uNGAL and serum creatinine in the second sample (one week after
chemotherapy initiation). AKI cases based on uNGAL were 10 out of
30 (33.3%) vs. 0 out of 30 (0.0%) when based on serum creatinine
(P=0.002). However, in the third sample (after the end of the
chemotherapy protocol), there was no significant difference between
the percentage of patients with AKI based on uNGAL and serum
creatinine. AKI cases based on uNGAL were 14 out of 30 (46.7%) vs.
12 out of 30 (40.0%) when based on serum creatinine (P=0.068).
Table VI illustrates the AKI cases
based on uNGAL and serum creatinine in patients. When comparing the
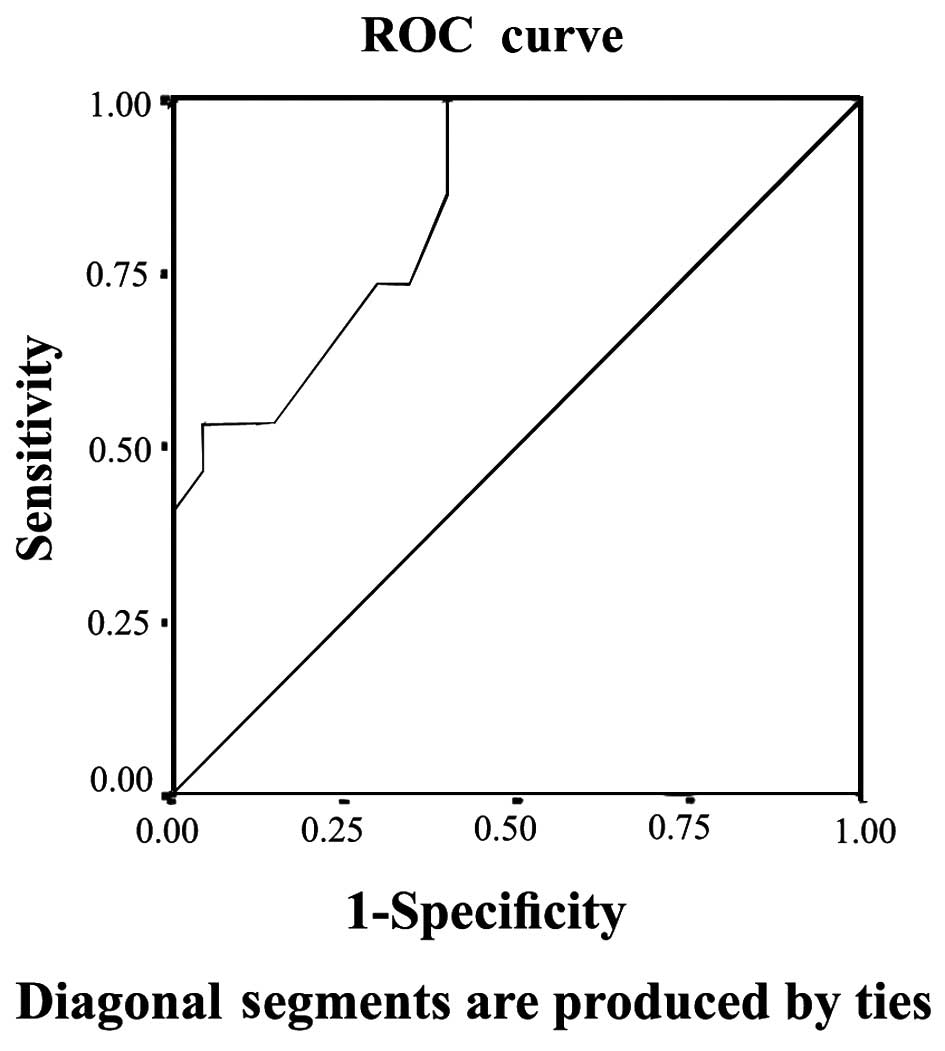
predictive value of serum creatinine for AKI depending on the ROC
curve with that of uNGAL, the area under the curve (AUC) for
creatinine was 0.60 with a standard error (SE) of 0.086 and 95%
confidence interval (CI) between 0.432 and 0.768, while that of
uNGAL was highly predictive with AUC 0.847, SE 0.55 and 95% CI
between 0.739 and 0.955. The ROC curves of serum creatinine and
uNGAL are illustrated in Figs 1 and
2, respectively.
 | Table I.Age and gender data of cases and
controls. |
Table I.
Age and gender data of cases and
controls.
|
Characteristics | Cases | Controls | t-test value | P-value |
|---|
| Gender, n (%) |
|
|
|
|
|
Males | 15 (50) | 13 (65) |
|
|
|
Females | 15 (50) | 7
(35) |
χ2=1.1 | 0.360 |
|
Total | 30
(100) | 20
(100) |
|
|
| Age, years | 4.9±3.1 | 6.6±3.1 | t=1.85 | 0.183 |
 | Table II.Serum creatinine and uNGAL values in
cases and controls in the three samples. |
Table II.
Serum creatinine and uNGAL values in
cases and controls in the three samples.
| Time points | Cases (n=30) | Controls
(n=20) | t-test value | P-value |
|---|
| First sample |
|
|
|
|
| S.
creatinine, mg/dl (range) |
0.41±0.1
(0.3–0.6) |
0.35±0.1
(0.2–0.5) | 1.24 | 0.168 |
| uNGAL,
ng/ml (range) |
7.5±4.2
(4–22) |
3.6±1.2
(1.1–6.2) |
3.147 | <0.003 |
| Second sample |
|
|
|
|
| S.
creatinine, mg/dl (range) |
0.5±0.1
(0.3–0.8) |
0.36±0.13
(0.18–0.45) | 3.58 | <0.001 |
| uNGAL,
ng/ml (range) |
31.3±17.3
(10–70) |
3.2±1.3
(1.4–5.2) |
5.122 | <0.001 |
| Third sample |
|
|
|
|
| S.
creatinine, mg/dl (range) |
0.6±0.12
(0.5–0.8) |
0.4±0.15
(0.2–0.7) | 5.4 | <0.001 |
| uNGAL,
ng/ml (range) |
49.0±16.2
(16–82) |
3.5±1.6
(1.3–7) |
12.6 | <0.001 |
 | Table III.Serum creatinine level in patients
and the percentages of change in the three samples. |
Table III.
Serum creatinine level in patients
and the percentages of change in the three samples.
| Time points | Mean + SD | Range | Change, % | Paired t-test | P-value |
|---|
| First sample |
0.4±0.1 | 0.3–0.6 |
|
|
|
| Second sample |
0.5±0.1 | 0.3–0.8 | 26.1 | 3.95 | <0.003 |
| Third sample |
0.6±0.1 | 0.5–0.8 | 54.1 | 2.73 | <0.001 |
 | Table IV.uNGAL in patients and the percentages
of change in the three samples. |
Table IV.
uNGAL in patients and the percentages
of change in the three samples.
| Time points | Mean ± SD | Range | Change, % | Paired t-test | P-value |
|---|
| First sample |
7.5±4.2 | 4–22 |
|
|
|
| Second sample |
31.3±17.3 | 10–70 | 376.8 | 8.13 | <0.001 |
| Third sample |
49.0±16.2 | 16–82 | 698.2 | 14.40 | <0.001 |
 | Table V.Correlation between uNGAL and serum
creatinine levels in patients. |
Table V.
Correlation between uNGAL and serum
creatinine levels in patients.
| Variables | Correlation
coefficient, r | P-value |
|---|
| First sample | >0.05 | 0.30 |
| Second sample | <0.001 | 0.33 |
| Third sample | <0.001 | 0.63 |
 | Table VI.AKI in patients based on uNGAL and
serum creatinine. |
Table VI.
AKI in patients based on uNGAL and
serum creatinine.
| Time points | AKI based on
uNGAL | AKI based on S.
creatinine | χ2
value | P-value |
|---|
| First sample | 0 (0.0%) | 0 (0.0%) |
|
|
| Second sample | 10 (33.3%) | 0 (0.0%) | 9.720 | 0.002 |
| Third sample | 14 (46.7%) | 12 (40.0%) | 0.795 | 0.0680 |
Discussion
In total, 5–10% of all hospitalized patients and
<40% of critically ill patients are estimated to experience an
episode of AKI during the course of their illness (19,20). The
AKI complications include increased susceptibility to infections,
extra-renal organ damage, chronic kidney disease and an increased
rate of hospital readmission (21,22).
Acute renal failure (ARF) is a serious complication
of malignancies that causes substantial morbidity and mortality.
Among critically ill cancer patients (CICPs), 12–49% experience ARF
and 9–32% require renal replacement therapy during their time at an
intensive care unit (ICU) (23–27). The
ARF risk appears to be higher in CICPs compared to other critically
ill patients (24,28). In CICPs, acute renal dysfunction
usually occurs in the context of multiple organ dysfunctions and is
associated with mortality rates ranging from 72–85% when renal
replacement therapy is required (23,24).
NGAL was identified by microarray analysis as one of
the earliest and strongly-induced proteins in the kidney following
ischemic or nephrotoxic injury in animals and humans, where it was
easily detected in the blood and urine soon after AKI (29).
Serum and uNGAL have been heavily studied in
different clinical scenarios for early prediction and prognosis of
AKI (9). However, to the best of our
knowledge there are no studies concerning the role of NGAL as an
AKI marker in children with solid tumors undergoing
chemotherapy.
In the present study, there was no significant
difference in the serum creatinine between cases and controls in
baseline samples; however, there was a highly significant
difference between the two groups one week after the initiation of
therapy and at the end of the chemotherapy protocol. The percentage
of change in the creatinine level at the second and third
time-points was 26.1 and 54.1%; however, although highly
significant in comparison to the baseline, a 26.1% rise in serum
creatinine is not sufficient for diagnosis of AKI according to the
finding published by Mehta et al (30), which stated that AKI is defined as a
change in creatinine level by >50% of the baseline. Therefore,
it is clear that the serum creatinine level in the present study
only rises >50% in the third sample, meaning that depending only
on the creatinine level for adequately detecting the AKI markedly
delays the diagnosis. This result is in agreement with de Geus
et al (17) and Waikar et
al (31) who confirmed the poor
predictive value of creatinine for AKI detection, particularly in
the early stage of renal injury. Regarding uNGAL, there was a
significant difference between cases and controls in the first
baseline results, in the second and third samples after
chemotherapy initiation and at the end of the protocol. The
percentage of change was 376.8 and 698%, respectively, which is
highly significant.
There was a highly statistically significant
difference between the percentage of patients diagnosed with AKI
based on uNGAL and serum creatinine in the second sample one week
after chemotherapy initiation. AKI cases based on uNGAL were 10 out
of 30 (33.3%) vs. 0 out of 30 (0.0%) when based on serum creatinine
(P=0.002). However, in the third sample after the end of the
chemotherapy protocol, there was no significant difference between
the percentage of patients with AKI based on uNGAL and serum
creatinine. AKI cases based on uNGAL were 14 out of 30 (46.7%) vs.
12 out of 30 (40.0%) when based on serum creatinine (P=0.068).
These data are confirmed when comparing the predictive value of
serum creatinine for AKI detection depending on the ROC curve with
that of uNGAL, which showed that uNGAL is a good predictor of AKI
with an AUC of 0.847. These data agree with those published by
Haase et al (32), which is,
to the best of our knowledge, the only published meta-analysis for
the accuracy of uNGAL as a predictive parameter of AKI. The data
also agree with Haase-Fielitz et al (33) who strongly supported the use of NGAL
as a predictor of AKI, as well as subsequent initiation of renal
replacement therapy with high sensitivity and specificity. Numerous
studies have tested the probability of uNGAL as a predictor of AKI
in pediatrics, such as the study conducted by Mishra et al
(34) who studied 71 children
undergoing cardiac surgery and identified AKI (RIFLE stage R or
worse) in 20 patients. The study concluded that NGAL (with a
cut-off value, >50 ng/ml) is an early predictor of AKI with an
AUC of 0.99, 100% sensitivity and 98% specificity. Parikh et
al (35) also conducted a study
on 311 children undergoing cardiac surgery and identified AKI
(renal replacement therapy or doubling in creatinine) in 53
patients, and concluded that uNGAL (with a cut-off value, >70
ng/ml) is an early predictor of AKI with an AUC of 0.71, 42%
sensitivity and 85% specificity. uNGAL has also been investigated
by Zappitelli et al (36) who
studied 140 critically ill patients; 106 developed AKI and it was
identified that uNGAL (cut-off value >1.5 µg/mg urine
creatinine) was a predictor of AKI with an AUC of 0.78, 54%
sensitivity and 97% specificity. Du et al (37) performed a study on 250 critically ill
children, in which 18 developed AKI (pediatric RIFLE), and uNGAL
was identified as a predictor of AKI with an AUC of 0.8. Wheeler
et al (38) studied serum NGAL
as a marker of AKI in intensive care children and had comparable
results. According to de Geus et al (17), NGAL measured at ICU admission
predicted the development of severe AKI similarly to
serum-creatinine-derived estimated GFR (eGFR). However, when
patients with an eGFR <60 ml/min/1.73 m2 at ICU
admission were excluded, plasma (AUC 0.75) and urine NGAL (AUC
0.79) exhibited improved diagnostics over serum creatinine (AUC
0.65) and eGFR (AUC 0.67) for predicting AKI. Martensson et
al (39) studied the impact of
inflammation on NGAL concentrations in plasma and urine in patients
with systemic inflammatory response syndrome, severe sepsis and
septic shock with and without AKI, and concluded that plasma (AUC
0.85) and urine NGAL (AUC 0.86) were good predictors of AKI
developing within the subsequent 12 h. However, the ability of
plasma NGAL to predict AKI in patients with septic shock was less
powerful (AUC 0.67) compared to urine NGAL (AUC 0.86). In a recent
multi-centre study investigating five urinary biomarkers in 1,635
emergency department patients at the time of hospital admission,
NGAL was the most useful biomarker (81% specificity, 68%
sensitivity at a 104 ng/ml cut-off) and predictor of the severity
and duration of AKI (40). Rahimzadeh
et al (41) studied 27
children post-transplant, and measured NGAL 6–12 h after kidney
transplantation (cut-off value, >175 ng/ml), which showed an
overall superior performance (AUC 0.95, 100% sensitivity and 95.5%
specificity) for the prediction of delayed graft rejection.
By contrast, Royakkers et al (42) investigated systemic NGAL and uNGAL in
140 critically ill adults, defined with AKI by RIFLE stage R or
worse, and concluded that systemic NGAL and uNGAL are poor
predictors of AKI in unselected critically ill patients with an AUC
of 0.45 for systemic NGAL and 0.45 for uNGAL. In the study by Endre
et al (43), among the general
adult ICU patients, 82 subjects developed AKI within 48 h of
admission and the predictive performance for NGAL, corrected for
urinary creatinine concentration, yielded an AUC of 0.55. Metzger
et al (44) compared the
classification performance of urinary proteome analysis with
classical markers. For uNGAL, the ROC analysis revealed a low
classification accuracy with an AUC of 0.54.
Regardless of the large number of studies concerning
the use of NGAL as a predictor of AKI in different situations,
except for the study by Mishra et al (29) on a murine model for cisplatin
nephrotoxicity which revealed that NGAL represents an early and
quantitative urinary biomarker for cisplatin nephrotoxicity when
compared to serum creatinine and urinary
N-acetyl-β-D-glucosaminidase, the present study is the first to
evaluate NGAL in patients with solid tumors. However, numerous
studies have been performed to assess renal toxicity in Wilm's
tumor, such as Daw et al (45)
and Bailey et al (46), but
these depend on other parameters not including uNGAL.
In conclusion, uNGAL is a novel marker for the
detection of early kidney injury in children with solid tumors
under chemotherapy. Larger studies are required to support the
conclusions drawn from the present study.
References
|
1
|
Akcan-Arikan A, Zappitelli M, Loftis LL,
Washburn KK, Jefferson LS and Goldstein SL: Modified RIFLE criteria
in critically ill children with acute kidney injury. Kidney Int.
71:1028–1035. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Srisawat N, Hoste EE and Kellum JA: Modern
classification of acute kidney injury. Blood Purif. 29:300–307.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adiyanti SS and Loho T: Acute Kidney
Injury (AKI) biomarker. Acta Med Indones. 44:246–255.
2012.PubMed/NCBI
|
|
4
|
Andreoli SP: Acute kidney injury in
children. Pediatr Nephrol. 24:253–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Memon F, Rathi SL and Memon MH: Pattern of
solid paediatric malignant neoplasm at Lumhs, Jamshoro, Pakistan. J
Ayub Med Coll Abbottabad. 19:55–57. 2007.PubMed/NCBI
|
|
6
|
Launay-Vacher V, Izzedine H, Rey JB, Rixe
O, Chapalain S, Nourdine S, Paci A, Bourget P and Deray G:
Incidence of renal insufficiency in cancer patients and evaluation
of information available on the use of anticancer drugs in renally
impaired patients. Med Sci Monit. 10:CR209–CR212. 2004.PubMed/NCBI
|
|
7
|
Kintzel PE: Anticancer drug-induced kidney
disorders. Drug Saf. 24:19–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murray PT, Devarajan P, Levey AS, Eckardt
KU, Bonventre JV, Lombardi R, Herget-Rosenthal S and Levin A: A
framework and key research questions in AKI diagnosis and staging
in different environments. Clin J Am Soc Nephrol. 3:864–868. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Devarajan P: Biomarkers for the early
detection of acute kidney injury. Curr Opin Pediatr. 23:194–200.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dent CL, Ma Q, Dastrala S, Bennett M,
Mitsnefes MM, Barasch J and Devarajan P: Plasma NGAL predicts acute
kidney injury, morbidity and mortality after pediatric cardiac
surgery: A prospective uncontrolled cohort study. Crit Care.
11:R1272007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori
K, Barasch J and Devarajan P: Kidney NGAL is a novel marker of
acute injury following transplantation. Pediatr Nephrol.
21:856–863. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parikh CR, Jani A, Mishra J, Ma Q, Kelly
C, Barasch J, Edelstein CL and Devarajan P: Urine NGAL and IL-18
are predictive biomarkers for delayed graft function following
kidney transplantation. Am J Transplant. 6:1639–1645. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trachtman H, Christen E, Cnaan A, Patrick
J, Mai V, Mishra J, Jain A, Bullington N and Devarajan P: And
Investigators of the HUS-SYNSORB Pk Multicenter Clinical Trial:
Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: A
novel marker of renal injury. Pediatr Nephrol. 21:989–994. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirsch R, Dent C, Pfriem H, et al: NGAL is
an early predictive biomarker of contrast-induced nephropathy in
children. Pediatr Nephrol. 22:2089–2095. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du Y, Hou L, Guo J, Sun T, Wang X and Wu
Y: Renal neutrophil gelatinase-associated lipocalin and kidney
injury molecule-1 expression in children with acute kidney injury
and Henoch-Schönlein purpura nephritis. Exp Ther Med. 7:1130–1134.
2014.PubMed/NCBI
|
|
16
|
Cho E, Yang HN, Jo SK, Cho WY and Kim HK:
The role of urinary liver-type fatty acid-binding protein in
critically ill patients. J Korean Med Sci. 28:100–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Geus HR, Bakker J, Lesaffre EM and le
Noble JL: Neutrophil gelatinase-associated lipocalin at ICU
admission predicts for acute kidney injury in adult patients. Am J
Respir Crit Care Med. 183:907–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Christie B: Doctors revise declaration of
Helsinki. BMJ. 321:9132000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costa e Silva VT, de Castro I, Liaño F,
Muriel A, Rodríguez-Palomares JR and Yu L: Sequential evaluation of
prognostic models in the early diagnosis of acute kidney injury in
the intensive care unit. Kidney Int. 75:982–986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palsson R and Niles JL: Regional citrate
anticoagulation in continuous venovenous hemofiltration in
critically ill patients with a high risk of bleeding. Kidney Int.
55:1991–1997. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haase M and Haase-Fielitz A: Neutrophil
gelatinase-associated lipocalin for acute kidney injury - the renal
troponin. J Lab Med. 165:28–32. 2010.
|
|
22
|
Feltes CM, Van Eyk J and Rabb H:
Distant-organ changes after acute kidney injury. Nephron, Physiol.
109:80–84. 2008. View Article : Google Scholar
|
|
23
|
Lanore JJ, Brunet F, Pochard F, et al:
Hemodialysis for acute renal failure in patients with hematologic
malignancies. Crit Care Med. 19:346–351. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benoit DD, Hoste EA, Depuydt PO, Offner
FC, Lameire NH, Vandewoude KH, Dhondt AW, Noens LA and Decruyenaere
JM: Outcome in critically ill medical patients treated with renal
replacement therapy for acute renal failure: Comparison between
patients with and those without haematological malignancies.
Nephrol Dial Transplant. 20:552–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Azoulay E, Recher C, Alberti C, Soufir L,
Leleu G, Le Gall JR, Fermand JP and Schlemmer B: Changing use of
intensive care for hematological patients: The example of multiple
myeloma. Intensive Care Med. 25:1395–1401. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azoulay E, Moreau D, Alberti C, Leleu G,
Adrie C, Barboteu M, Cottu P, Levy V, Le Gall JR and Schlemmer B:
Predictors of short-term mortality in critically ill patients with
solid malignancies. Intensive Care Med. 26:1817–1823. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Darmon M, Thiery G, Ciroldi M, de Miranda
S, Galicier L, Raffoux E, Le Gall JR, Schlemmer B and Azoulay E:
Intensive care in patients with newly diagnosed malignancies and a
need for cancer chemotherapy. Crit Care Med. 33:2488–2493. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bagshaw SM, Laupland KB, Doig CJ, Mortis
G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW and Rosenal T:
Prognosis for long-term survival and renal recovery in critically
ill patients with severe acute renal failure: A population-based
study. Crit Care. 9:R700–R709. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mishra J, Mori K, Ma Q, Kelly C, Barasch J
and Devarajan P: Neutrophil gelatinase-associated lipocalin: A
novel early urinary biomarker for cisplatin nephrotoxicity. Am J
Nephrol. 24:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mehta RL, Kellum JA, Shah SV, Molitoris
BA, Ronco C, Warnock DG and Levin A: Acute Kidney Injury Network:
Acute Kidney Injury Network: Report of an initiative to improve
outcomes in acute kidney injury. Crit Care. 11:R312007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Waikar SS and Bonventre JV: Biomarkers for
the diagnosis of acute kidney injury. Nephron Clin Pract.
109:c192–c197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haase M, Bellomo R, Devarajan P and
Schlattmann P: Haase-Fielitz A and NGAL Meta-analysis Investigator
Group: Accuracy of neutrophil gelatinase-associated lipocalin
(NGAL) in diagnosis and prognosis in acute kidney injury: A
systematic review and meta-analysis. Am J Kidney Dis. 54:1012–1024.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haase-Fielitz A, Haase M and Devarajan P:
Neutrophil gelatinase-associated lipocalin as a biomarker of acute
kidney injury: A critical evaluation of current status. Ann Clin
Biochem. 51:335–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mishra J, Dent C, Tarabishi R, et al:
Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker
for acute renal injury after cardiac surgery. Lancet.
365:1231–1238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parikh CR, Devarajan P, Zappitelli M, et
al: Postoperative biomarkers predict acute kidney injury and poor
outcomes after pediatric cardiac surgery. J Am Soc Nephrol.
22:1737–1747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zappitelli M, Washburn KK, Arikan AA,
Loftis L, Ma Q, Devarajan P, Parikh CR and Goldstein SL: Urine NGAL
is an early marker of acute kidney injury in critically ill
children. Crit Care. 11:R842007. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Du Y, Zappitelli M, Mian A, Bennett M, Ma
Q, Devarajan P, Mehta R and Goldstein SL: Urinary biomarkers to
detect acute kidney injury in the pediatric emergency center.
Pediatr Nephrol. 26:267–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wheeler DS, Devarajan P, Ma Q, Harmon K,
Monaco M, Cvijanovich N and Wong HR: Serum neutrophil
gelatinase-associated lipocalin (NGAL) as a marker of acute kidney
injury in critically ill children with septic shock. Crit Care Med.
36:1297–1303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martensson J, Bell M, Oldner A, Xu S,
Venge P and Martling CR: Neutrophil gelatinase-associated lipocalin
in adult septic patients with and without acute kidney injury.
Intensiv Care Med. 36:1333–1340. 2010. View Article : Google Scholar
|
|
40
|
Nickolas TL, Schmidt-Ott KM, Canetta P, et
al: Diagnostic and prognostic stratification in the emergency
department using urinary biomarkers of nephron damage: A
multicenter prospective cohort study. J Am Coll Cardiol.
59:246–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rahimzadeh N, Otukesh H, Hoseini R, Sorkhi
H, Otukesh M, Hoseini S and Torkzaban M: Are serum and urine
neutrophil gelatinase-associated lipocalin predictive of renal
graft function in short term. Pediatr Transplant. 16:796–802. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Royakkers AA, Bouman CS, Stassen PM,
Korevaar JC, Binnekade JM, van de Hoek W, Kuiper MA, Spronk PE and
Schultz MJ: Systemic and urinary neutrophil gelatinase-associated
lipocalins are poor predictors of acute kidney injury in unselected
critically ill patients. Crit Care Res Pract.
2012:7126952012.PubMed/NCBI
|
|
43
|
Endre ZH, Pickering JW, Walker RJ, et al:
Improved performance of urinary biomarkers of acute kidney injury
in the critically ill by stratification for injury duration and
baseline renal function. Kidney Int. 79:1119–1130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Metzger J, Kirsch T, Schiffer E, Ulger P,
Mentes E, Brand K, Weissinger EM, Haubitz M, Mischak H and
Herget-Rosenthal S: Urinary excretion of twenty peptides forms an
early and accurate diagnostic pattern of acute kidney injury.
Kidney Int. 78:1252–1262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Daw NC, Gregornik D, Rodman J, Marina N,
Wu J, Kun LE, Jenkins JJ, McPherson V, Wilimas J and Jones DP:
Renal function after ifosfamide, carboplatin and etoposide (ICE)
chemotherapy, nephrectomy and radiotherapy in children with Wilms
tumour. Eur J Cancer. 45:99–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bailey S, Roberts A, Brock C, Price L,
Craft AW, Kilkarni R, Lee RE, Skillen AW and Skinner R:
Nephrotoxicity in survivors of Wilms' tumours in the North of
England. Br J Cancer. 87:1092–1098. 2002. View Article : Google Scholar : PubMed/NCBI
|