Introduction
Krukenberg tumor is a rare metastatic signet ring
cell tumor of the ovary, accounting for 1–2% of all ovarian tumors.
The stomach is the primary site in the majority of Krukenberg tumor
cases, followed by carcinomas of the colon, appendix and breast,
particularly invasive lobular carcinoma (1). The eponym was attributed to this tumor
following the description of 5 cases by Friedrich Krukenberg
(1871–1946) in 1896, who described it as being common among young
women, presenting with ascites, an uneven knobby ovarian surface
and lymphatic involvement (2). These
tumors are characterised by uncertain pathogenesis, challenging
etiological diagnosis and poorer prognosis compared with their
primaries. Previously, any metastatic ovarian cancer was referred
to as Krukenberg tumor; however, Novak and Gray (3) created new diagnostic criteria to
eliminate any confusion. Accordingly, a mucin-secreting signet ring
cell carcinoma in the dense fibroblastic stroma of the ovary is
referred to as Krukenberg tumor. The diagnosis of Krukenberg tumor
is currently based on the diagnostic criteria of the World Health
Organization based on the pathological description by Serov and
Scully (4). The presence of the
following characteristics is required for diagnosis: Stromal
involvement, mucin-producing neoplastic signet ring cells and
ovarian stromal sarcomatoid proliferation.
Krukenberg tumor is considered as a late-stage
disease with poor prognosis and may account for 30–40% of
metastatic cancers to the ovaries (5). The treatment approach to these
metastatic ovarian tumors remains controversial. To date, treatment
mainly consists of ovarian metastasectomy, chemotherapy or
radiotherapy; however, the optimal treatment has not yet been
established. As the biological behavior and clinical outcome of
Krukenberg tumors are rarely summarized, the aim of this
retrospective study was to analyze the characteristics and outcome
of all patients with Krukenberg tumors over a 20-year period,
evaluate the clinical characteristics of such tumors and
investigate the prognostic factors.
Patients and methods
Patient characteristics
Patients who were diagnosed with Krukenberg tumor
between January, 1990 and December, 2010, were retrospectively
identified from the database of the Department of Oncology, the
First Affiliated Hospital of Xi'an Jiaotong University School of
Medicine, Tumor Hospital of Shaanxi and the Department of Oncology,
People's Hospital of Shaanxi. The data were obtained from the
patients' medical records and pathology reports. Clinical and
pathological variables included age, menopausal status, size of
ovarian metastasis of pathologic gross specimen, pathology reports,
primary tumor site and subsequent therapy. The quality of the
cancer registry database was reviewed and approved by the Ethics
Committee of the First Affiliated Hospital of Xi'an Jiaotong
University School of Medicine. Overall survival (OS) was calculated
from the date of diagnosis of the primary tumor or ovarian
metastasis to the date of death or last follow-up. If the time
interval between the diagnosis of the primary tumor and that of the
ovarian metastasis exceeded 6 months, the metastasis was defined as
metachronous in the present study.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 17.0 (SPSS Inc., Chicago, IL, USA). A two-sided
P<0.05 was considered to indicate a statistically significant
difference. The Kaplan-Meier method was used to calculate survival.
Clinically relevant variables were analyzed univariately for their
association with OS using the log-rank test. The independent
prognostic significance of variables in terms of survival was
determined in a multivariate analysis using the Cox proportional
hazards regression model and the estimates are presented as hazard
ratio (HR) with 95% confidence interval (CI).
Results
Clinical characteristics
A total of 128 patients were enrolled in this study.
The baseline patient characteristics are listed in Table I. The median age at diagnosis of
Krukenberg tumor was 48 years (range, 27–65 years). The most common
primary tumors were located in the colorectum (58, 45.31%) and the
stomach (41, 32.03%). Krukenberg tumors were more common in
premenopausal women (75.78%) rather than in postmenopausal women.
Of the 128 patients, 92 (71.87%) had metachronous ovarian
metastasis. The treatment options for Krukenberg tumor include
metastasectomy and chemotherapy: Metastasectomy was performed in
114 patients (89.06%), whereas 14 patients (10.94%) did not undergo
surgery due to additional metastatic lesions; chemotherapy was
administered to 89 patients (69.53%). The surgical procedures
included unilateral or bilateral adnexectomy and hysterectomy with
bilateral adnexectomy. The main chemotherapeutic drugs included
cisplatin, carboplatin, oxaliplatin, docetaxel and 5-fluorouracil.
The majority of the patients received 2- or 3-drug combinations,
usually for 4–6 cycles. The majority of the cases (98, 76.56%)
exhibited bilateral ovarian involvement. The median ovarian tumor
size was 9.6 cm (range, 4–18 cm), with 58.60% of the patients
having tumors sized ≥10 cm. A total of 63 patients (49.22%)
presented with ascites, while 65 patients (50.78%) had no apparent
ascites at diagnosis. A total of 71 patients (55.47%) had ovarian
metastasis, while 57 patients (45.53%) had combined metastases
outside the ovaries, including the pelvis, bone, lung and other
distant organs.
 | Table I.Baseline patient characteristics
(n=128). |
Table I.
Baseline patient characteristics
(n=128).
| Characteristics | Patients, no.
(%) |
|---|
| Age, years |
|
| Median
(range) | 48
(27–65) |
| Menopausal
status |
|
|
Premenopausal | 97
(75.78) |
|
Postmenopausal | 31
(24.22) |
| Primary site |
|
|
Stomach | 41
(32.03) |
| Colon and
rectum | 58
(45.31) |
|
Breast | 8
(6.25) |
| Small
intestine | 5
(3.92) |
|
Gallbladder | 4
(3.12) |
| Vermiform
appendix | 4
(3.12) |
|
Unknown | 8
(6.25) |
| Ovarian
involvement |
|
|
Bilateral | 98
(76.56) |
|
Unilateral | 30
(23.44) |
| Tumor diameter,
cm |
|
| Median
(range) | 9.6
(4–18) |
| ≤5 | 11 (8.59) |
| 5–10 | 42
(32.81) |
| ≥10 | 75
(58.60) |
| Chronology |
|
|
Synchronous | 36
(28.13) |
|
Metachronous | 92
(71.87) |
| Extent of
disease |
|
|
Ovary | 71
(55.47) |
|
Pelvis | 34
(26.56) |
| Beyond
pelvis | 23
(17.97) |
| Chemotherapy |
|
| Yes | 89
(69.53) |
| No | 39
(30.47) |
| Ascites |
|
| Yes | 63
(49.22) |
| No | 65
(50.78) |
| Metastasectomy |
|
| Yes | 114 (89.06) |
| No | 14
(10.94) |
Prognosis of Krukenberg tumor
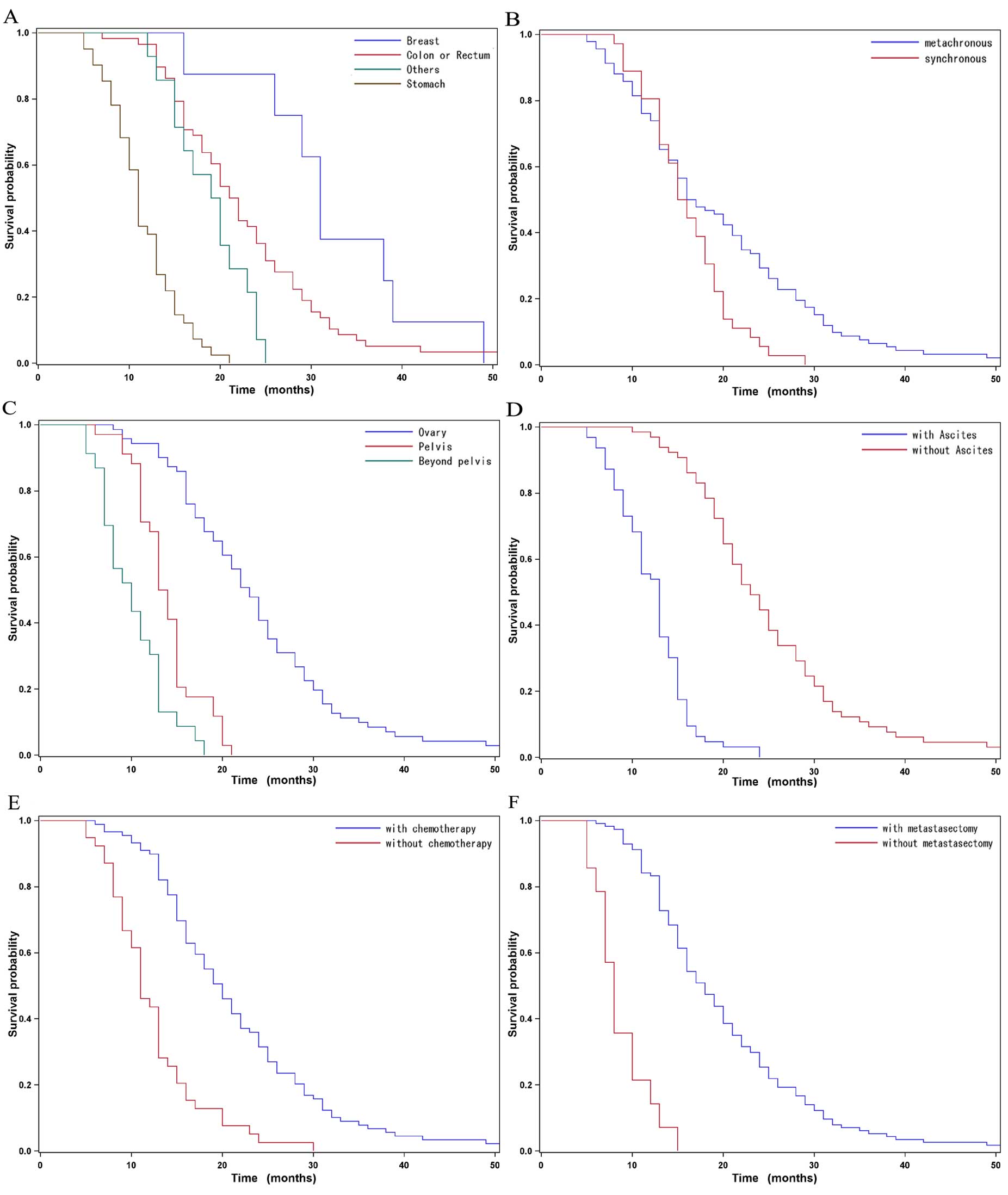
The OS of the 128 patients ranged between 5 and 52
months. The median OS was 16 months (95% CI: 15–19 months)
(Fig. 1). Among all patients, the
mean OS for tumors originating in the breast was longer compared
with that for tumors of gastric origin (31 vs. 11 months,
respectively; P<0.0001). Among all patients, those with
metachronous cancer exhibited a longer mean survival time compared
with those who exhibited synchronous metastases (P=0.0113). The
patients with metastatic disease confined to the ovaries had a
median survival time of 23 months compared with 13.5 months for
those with more extensive metastasis (P<0.0001) (Table II). By contrast, no correlation was
observed between patient age and survival. Menopausal status,
bilaterality and size of ovarian metastases were also included in
the univariate analysis; however, these factors were not identified
as significant prognostic indicators for OS (P>0.05) (Table II).
 | Table II.Prognostic factors for Krukenberg
tumor. |
Table II.
Prognostic factors for Krukenberg
tumor.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≤50 vs. >50
years) | 1.121
(0.607–1.310) |
0.5599 | - | - |
| Menopausal status
(pre- vs. postmenopausal) | 0.844
(0.786–1.787) |
0.4171 | - | - |
| Primary site (stomach
vs. colon and rectum) | 0.124
(0.074–0.208) | <0.0001 | 0.252
(0.135–0.469) | <0.0001 |
| Primary site (stomach
vs. breast) | 0.059
(0.025–0.141) | <0.0001 | 0.125
(0.046–0.339) | <0.0001 |
| Primary site (stomach
vs. others) | 0.249
(0.131–0.474) | <0.0001 | 0.389
(0.182–0.833) |
0.0151 |
| Ovarian involvement
(bilateral vs. unilateral) | 1.316
(0.503–1.148) |
0.1916 | - | - |
| Tumor diameter (≥10
vs. <10 cm) | 0.905
(.0464–1.767) |
0.7708 | - | - |
| Chronology
(metachronous vs. synchronous) | 1.701
(1.127–2.564) |
0.0113 | 1.898
(1.182–3.049) |
0.0080 |
| Extent of disease
(ovary vs. pelvis) | 5.486
(3.272–9.198) | <0.0001 | 2.156
(1.170–3.974) |
0.0138 |
| Extent of disease
(ovary vs. beyond pelvis) | 12.702
(6.965–23.166) | <0.0001 | 0.856
(0.307–2.387) |
0.7666 |
| Chemotherapy (no vs.
yes) | 0.293
(0.195–0.440) | <0.0001 | 0.626
(0.371–1.057) |
0.0796 |
| Ascites (no vs.
yes) | 7.816
(4.913–12.436) | <0.0001 | 4.820
(2.537–9.157) | <0.0001 |
| Metastasectomy (yes
vs. no) | 9.346
(4.950–17.544) | <0.0001 | 4.878
(1.572–15.15) |
0.0060 |
The multivariate Cox regression analysis
demonstrated that synchronous metastasis (HR=1.898, 95% CI:
1.182–3.049, P=0.008), pelvic invasion (HR=2.156, 95% CI:
1.170–3.974, P=0.0138), ascites (HR=4.820, 95% CI: 2.537–9.157,
P<0.0001) and no metastasectomy (HR=4.878, 95% CI: 1.572–15.15,
P=0.0060) were independent factors for predicting an unfavorable OS
(Table II and Fig. 2).
Discussion
The outcome and prognosis of the 128 patients with
Krukenberg tumor included in our study reflects the complexity and
challenges in the management of this patient population. The
prognosis of Krukenberg tumor is dismal and the benefit of ovarian
metastasectomy remains to be elucidated.
Although Krukenberg tumors may be induced by complex
mechanisms, lymph node metastasis is considered to be the most
significant risk factor for recurrence. It was reported that
patients with Krukenberg tumor were younger compared with those who
had primary ovarian cancer, whereas the functioning ovary was prone
to metastatic disease due to the rich ovarian blood supply
predisposing to hematogenous metastasis (6). Several mechanisms have been suggested to
explain the progression and recurrence pathway of gastric cancer,
such as lymphatic spread, hematogenous spread, direct invasion and
peritoneal seeding. Among these, the incidence of hematogenous
recurrence is the highest (7,8). The exact mechanism of the spread of
breast cancer to the ovaries had not been elucidated, but the risk
of primary ovarian cancer is increased in women with breast-ovarian
cancer syndrome, which is caused by BRCA1/2 mutations (9).
The most common primary tumor site in patients with
Krukenberg tumor is reportedly the stomach (1,10,11). However, recent observations have
reported a higher incidence of colorectal rather than gastric
origin and, in particular, more frequently from the colon rather
than the rectum (12), which was
consistent with the findings in our study, as over half of our
patients exhibited primary colorectal cancer. Radiotherapy for
rectal cancer with lymphovascular invasion may contribute to
reducing the risk of ovarian spread.
The survival of patients with Krukenberg tumor is
associated with the primary tumor site. In our study, patients with
tumors originating in the breast exhibited the longest median OS of
31 months, followed by those with cancer of colorectum, with a
median survival time of 21.5 months; the prognosis of patients with
a gastric origin was the poorest (median OS, 11 months), which was
comparable with previous data (13).
The possible explanations are as follows: i) The prognosis of
advanced gastric cancer is worse compared with that of advanced
colorectal cancer; and ii) patients with Krukenberg tumor of
gastric origin usually exhibit a lower performance status score and
severe anemia. Furthermore, breast cancer is generally associated
with a better prognosis compared with tumors of the
gastrointestinal tract.
In the present study, we identified synchronous
ovarian metastasis as an independent risk factor associated with
poor survival. It was previously reported that synchronous ovarian
metastasis was an unfavorable factor correlated with poor survival
(14), which was consistent with our
study, suggesting that the metastasis-free interval was shortened
between primary tumor diagnosis and ovarian metastasis.
The survival of patients without ascites was
significantly longer compared with that of patients with ascites
(median OS, 23 vs. 13 months, respectively; P<0.0001) and
ascites was found to be an independent risk factor associated with
poor survival. These results were consistent with those of other
studies (15,16). In Krukenberg tumor patients, ascites
may be caused by tumor invasion of the peritoneum or malnutrition,
and it is usually associated with dissemination to the abdominal or
pelvic cavity. Peritoneal dissemination was reported as an adverse
factor affecting survival (17,18). In
our analysis, high incidence of pelvic invasion or extension beyond
the pelvis were also determined as poor prognostic factors.
Additionally, the fact that there was no survival difference
according to tumor size or bilaterality indicates that the
development of ovarian metastases is a sign of more aggressive
disease and ovarian metastases are diagnosed late during cancer
progression.
Several studies have investigated the options of
metastasectomy and cytoreductive surgery for Krukenberg tumor
(13,19,20). The
role of metastasectomy for Krukenberg tumor was assessed between
different primary cancer types, different types of surgery or
extent of residual disease. Bilateral oophorectomy for Krukenberg
tumor have been shown to positively affect OS in isolated ovarian
metastasis patients in an Italian study (21). Cheong et al (22) reported on 54 patients with Krukenberg
tumors who experienced disease relapse following curative surgery
of primary gastric cancer. Of the 54 patients who underwent
resection of the Krukenberg tumor, the 33 who underwent
metastasectomy exhibited a significantly longer median OS compared
with those who did not undergo metastasectomy (17 vs. 3 months,
respectively). In our study, metastasectomy was also a beneficial
prognostic factor in terms of OS. Therefore, resection of
metastatic ovarian tumors and cytoreductive surgery as part of the
treatment for Krukenberg tumor play a pivotal role in prolonging
the survival time of the patients, provided that there is no
distant metastasis. In addition to surgical treatment, chemotherapy
is also an option. Palliative radiotherapy may be applied for
unresectable or distant metastatic Krukenberg tumors. In order to
improve survival, there is a need to investigate the optimal
management of Krukenberg tumors. For patients with gastric cancer,
a Korean study suggested that debulking or gastrectomy plus
metastasectomy may achieve survival benefits for patients with
distant metastases who were receiving systemic chemotherapy
(23). In our study, over two-thirds
of the patients received chemotherapy. The majority of the
chemotherapeutic regimens included a platinum agent (cisplatin,
carboplatin or oxaliplatin) plus 5-fluorouracil, whereas other
patients received docetaxel and paclitaxel, with distinct survival
benefits.
In conclusion, in our study, Krukenberg tumors more
commonly appeared to originate from primary gastrointestinal tract
tumors. The prognosis of Krukenberg tumor is dismal and patients
may benefit from ovarian metastasectomy. Metastasis outside the
ovaries, ascites and no metastasectomy were independent factors for
predicting an unfavorable OS. As Krukenberg tumors are rather rare,
a national registry should be created to collect information on
these patients, with the aim to improve diagnosis and treatment
outcome. The identification of the primary tumor is crucial for
designing an effective treatment regimen for this group of
patients, whereas imaging examinations and gastrointestinal
endoscopy are recommended prior to ovarian metastasectomy.
Acknowledgements
The authors would like to thank the Department of
Oncology, the First Affiliated Hospital of Xi'an Jiaotong
University School of Medicine, Tumor Hospital of Shaanxi and the
Department of Oncology, People's Hospital of Shaanxi, for their
assistance with stastistical analysis. The authors also acknowledge
the efforts of the dedicated study volunteers.
References
|
1
|
Al-Agha OM and Nicastri AD: An in-depth
look at Krukenberg tumor: An overview. Arch Pathol Lab Med.
130:1725–1730. 2006.PubMed/NCBI
|
|
2
|
Young RH: From Krukenberg to today: The
ever present problems posed by metastatic tumors in the ovary: Part
I. Historical perspective, general principles, mucinous tumors
including the Krukenberg tumor. Adv Anat Pathol. 13:205–227.
2006.PubMed/NCBI
|
|
3
|
Novak C and Gray LA: Krukenberg tumor of
the ovary: Clinical and pathological study of four cases. Surg
Gynecol Obstet. 66:157–165. 1938.
|
|
4
|
Serov SF and Scully RE: Histological
typing of ovarian tumors. International Histological Classification
of Tumours. 9:(Geneva). WHO. 1973.
|
|
5
|
Lu W, Yuan L, Liu X and Guo SW:
Identification of prognostic factors for Krukenberg tumor. GMIT.
2:52–56. 2013.
|
|
6
|
La Fianza A, Alberici E, Pistorio A and
Generoso P: Differential diagnosis of Krukenberg tumors using
multivariate analysis. Tumori. 88:284–287. 2002.PubMed/NCBI
|
|
7
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin DW, Hyung WJ, Noh SH and Min JS: Risk
factors for recurrence after curative surgery for early gastric
cancer. J Korean Gastric Cancer Assoc. 1:106–112. 2001.
|
|
9
|
Kauff ND and Barakat RR: Risk-reducing
salpingo-oophorectomy in patients with germline mutations in BRCA1
or BRCA2. J Clin Oncol. 25:2921–2927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Januszewska M, Emerich J, Dibniak J,
Sliwinski W and Stukan M: Clinical analysis of patients with
Krukenberg tumor of the ovary. Ginekol Pol. 77:203–208. 2006.((In
Polish)). PubMed/NCBI
|
|
11
|
Kiyokawa T, Young RH and Scully RE:
Krukenberg tumors of the ovary: A clinicopathologic analysis of 120
cases with emphasis on their variable pathologic manifestations. Am
J Surg Pathol. 30:277–299. 2006.PubMed/NCBI
|
|
12
|
Moore RG, Chung M, Granai CO, Gajewski W
and Steinhoff MM: Incidence of metastasis to the ovaries from
nongenital tract primary tumors. Gynecol Oncol. 93:87–91. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang R, Tang J, Cheng X and Zang RY:
Surgical treatment for patients with different origins of
Krukenberg tumors: Outcomes and prognostic factors. Eur J Surg
Oncol. 35:92–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xue Z, Yan H, Li J, Liang S, Cai X, Chen
X, Wu Q, Gao L, Wu K, Nie Y, et al: Identification of cancer stem
cells in vincristine preconditioned SGC7901 gastric cancer cell
line. J Cell Biochem. 113:302–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Wang H, Wang JLVF, Zhu X and Wang Z:
Ovarian metastases resection from extragenital primary sites:
Outcome and prognostic factor analysis of 147 patients. BMC Cancer.
12:2782012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng W, Hua RX, Jiang R, Ren C, Jia YN, Li
J and Guo WJ: Surgical treatment for patients with Krukenberg tumor
of stomach origin: Clinical outcome and prognostic factors
analysis. PLoS One. 8:e682272013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HK, Heo DS, Bang YJ and Kim NK:
Prognostic factors of Krukenberg's tumor. Gynecol Oncol.
82:105–109. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yonemura Y, Bandou E, Kinoshita K,
Kawamura T, Takahashi S, Endou Y and Sasaki T: Effective therapy
for peritoneal dissemination in gastric cancer. Surg Oncol Clin N
Am. 12:635–648. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ayhan A, Guvenal T, Salman MC, Ozyuncu O,
Sakinci M and Basaran M: The role of cytoreductive surgery in
nongenital cancers metastatic to the ovaries. Gynecol Oncol.
98:235–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim WY, Kim TJ, Kim SE, Lee JW, Lee JH,
Kim BG and Bae DS: The role of cytoreductive surgery for
non-genital tract metastatic tumors to the ovaries. Eur J Obstet
Gynecol Reprod Biol. 149:97–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Erroi F, Scarpa M, Angriman I, Cecchetto
A, Pasetto L, Mollica E, Bettiol M, Ruffolo C, Polese L, Cillo U,
et al: Ovarian metastasis from colorectal cancer: Prognostic value
of radical oophorectomy. J Surg Oncol. 96:113–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheong JH, Hyung WJ, Chen J, Kim J, Choi
SH and Noh SH: Survival benefit of metastasectomy for Krukenberg
tumors from gastric cancer. Gynecol Oncol. 94:477–482. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim KH, Lee KW, Baek SK, Chang HJ, Kim YJ,
Park J, Kim JH, Kim HH and Lee JS: Survival benefit of gastrectomy
± metastasectomy in patients with metastatic gastric cancer
receiving chemotherapy. Gastric Cancer. 14:130–138. 2011.
View Article : Google Scholar : PubMed/NCBI
|