Introduction
Capecitabine is an oral fluorouracil (FU) prodrug
commonly used in patients with colorectal cancer, particularly as
adjuvant and palliative chemotherapy in colon cancer (1). The most common gastrointestinal adverse
events include nausea, vomiting and diarrhea. Capecitabine may also
cause serious gastrointestinal adverse events, such as intestinal
perforation or obstruction (1).
We treated 2 patients with ileitis associated with
capecitabine administration. In one of the patients, ileitis
occurred during palliative second-line combination chemotherapy for
metastatic colon cancer, despite good tolerance to capecitabine
during previous chemotherapy. A literature search identified no
previous reported cases among Chinese adults who had received
capecitabine as second-line chemotherapy. The other patient
developed ileitis while on single-agent adjuvant therapy with
capecitabine for sigmoid colon cancer. The purpose of this study
was to present the case histories, pathophysiology, imaging
findings and literature review for this uncommon condition.
Case reports
Case 1
A 61-year-old Chinese woman underwent laparoscopic
right hemicolectomy for cancer of the ascending colon. On
pathological examination, the tumor was diagnosed as moderately
differentiated adenocarcinoma, stage pT4aN1aM0, KRAS wild-type. The
patient completed 6 months of adjuvant capecitabine and oxaliplatin
treatment, with good tolerance.
The carcinoembryonic antigen (CEA) levels increased,
and a positron emission tomography/computed tomography (PET/CT)
scan at 4 months after treatment revealed lung and liver
metastases. The patient was treated with capecitabine and
irinotecan (CAPIRI) and cetuximab (every 2 weeks). After 5 cycles,
treatment was changed to CAPIRI and bevacizumab (every 2 weeks), as
a follow-up PET/CT scan revealed disease progression. The patient
exhibited a good clinical response, with a decrease in abdominal
pain; the CEA level decreased from 82.1 to 18.4 ng/ml. However, on
day 9 of cycle 4 of CAPIRI and bevacizumab, the patient was
admitted with right lower quadrant abdominal pain, watery diarrhea
and vomiting. The patient also developed fever and the blood tests
revealed neutropenia (nadir neutrophil count, 0.3×109/1)
and hypokalemia. The liver function tests were within normal
limits. The differential diagnosis included infectious
gastroenteritis aggravated by neutropenia and bevacizumab-related
bowel perforation. The patient was treated conservatively with
intravenous fluids, broad-spectrum antibiotics and granulocyte
colony-stimulating factor (G-CSF). The fever subsided 1 day later
and the neutrophil count increased to >0.5×109/1.
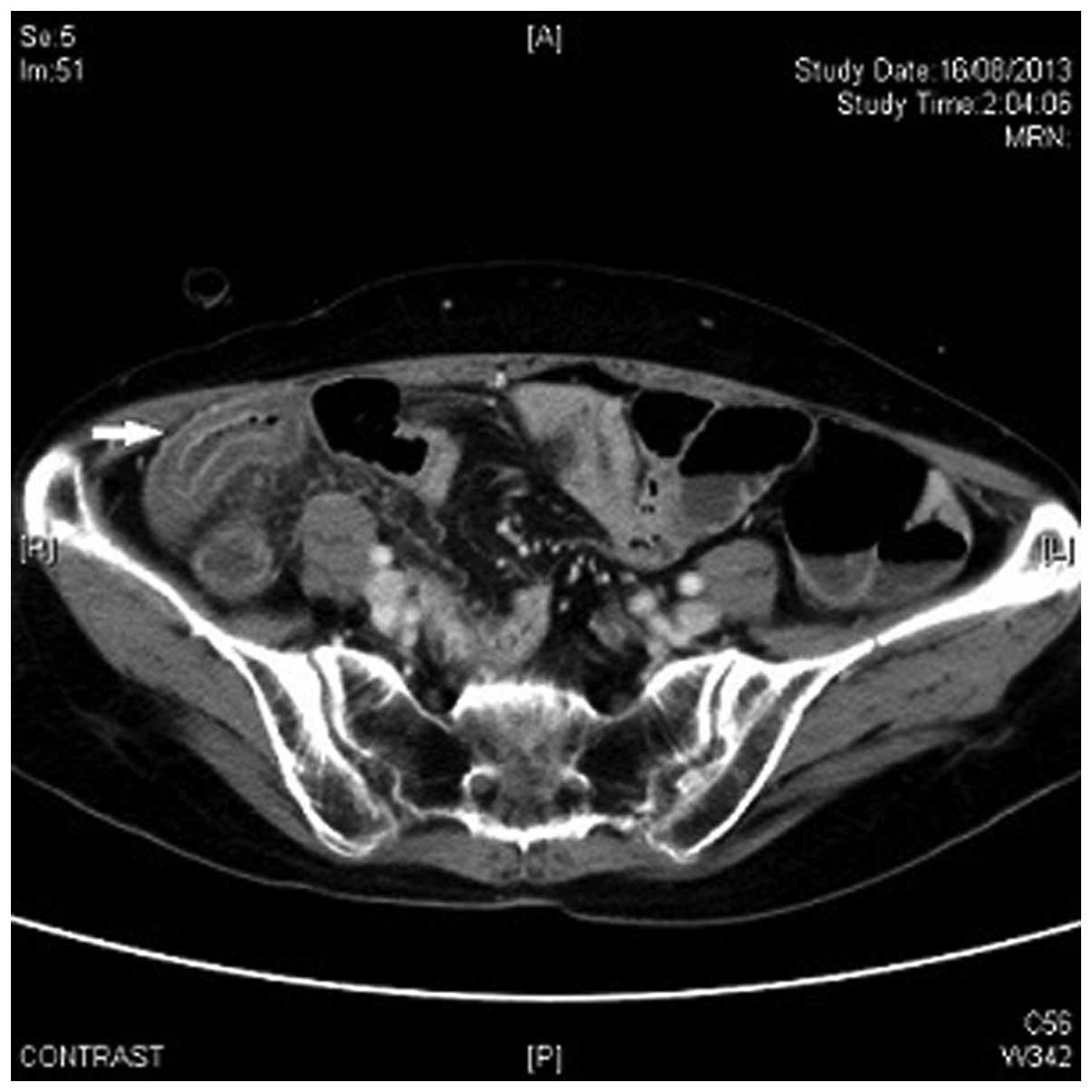
A CT scan of the abdomen and pelvis revealed
extensive submucosal edema at the terminal and middle part of the
ileum, associated with adjacent increase in fat stranding (Fig. 1). There was no pneumoperitoneum or
bowel dilation. Evaluation was negative for sepsis, including
blood, stool and urine cultures.
Hypokalemia persisted for 1 week and was managed
with intravenous fluids and dietary modifications. The patient's
condition gradually improved with conservative treatment, with
improvement in oral intake and decreased vomiting and diarrhea. The
patient remained in the hospital for a total of 12 days. The final
diagnosis was terminal ileitis associated with capecitabine.
Chemotherapy with irinotecan and bevacizumab was
resumed 1 week after hospital discharge, with no recurrence of
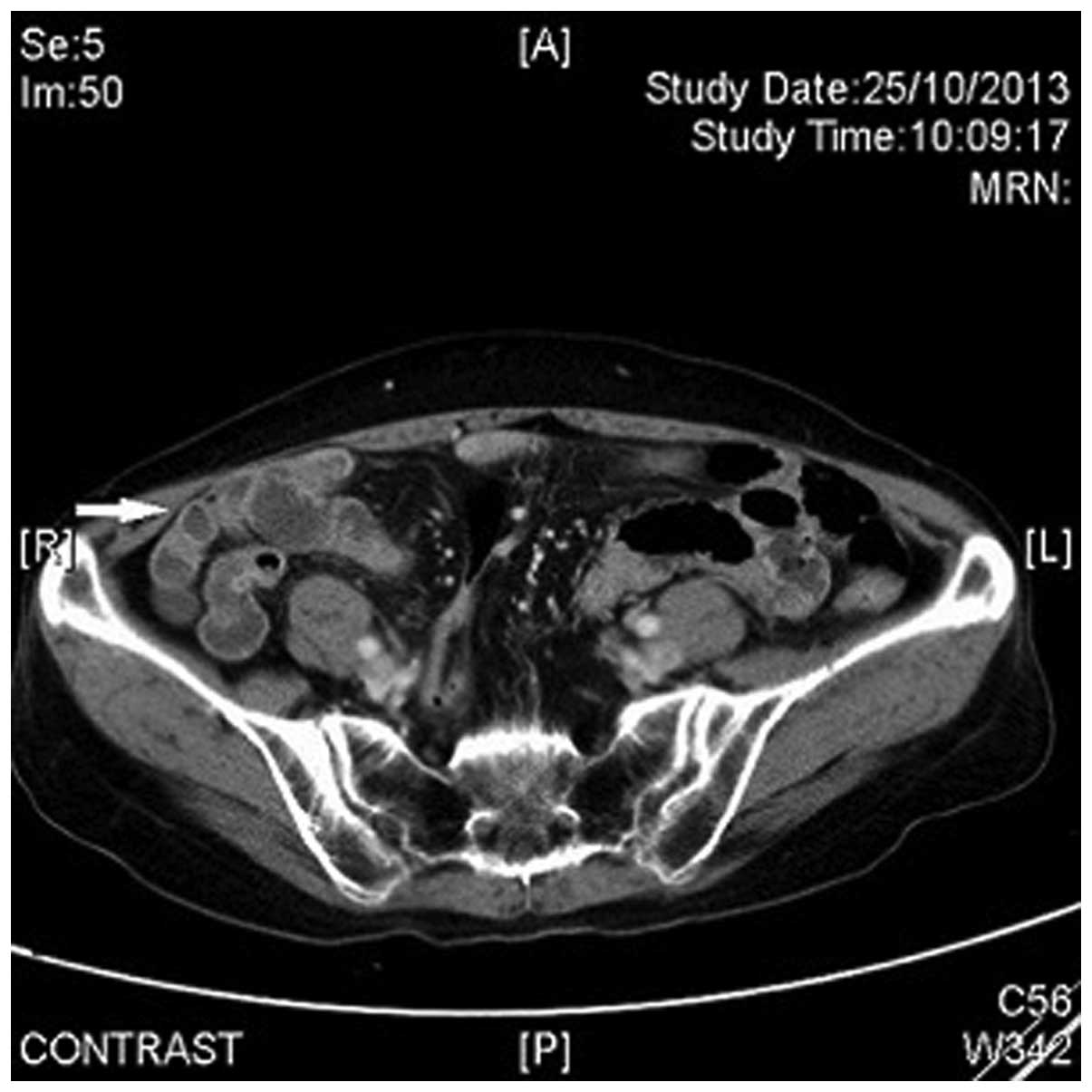
ileitis. A CT scan performed 2 months later revealed complete
resolution of the small bowel wall submucosal edema (Fig. 2).
Case 2
A 59-year-old Chinese woman underwent laparoscopic
sigmoidectomy for sigmoid colon cancer. On pathological
examination, the tumor was diagnosed as pT3N0 adenocarcinoma with
extramural vascular invasion. The patient was started on adjuvant
capecitabine (2,500 mg/m2/day orally on days 1–14 every
3 weeks) and exhibited good tolerance to chemotherapy during the
first 2 cycles; however, she developed grade 1 diarrhea,
hand-foot-skin reaction and stomatitis.
After day 14 of cycle 3, the patient exhibited
worsening diarrhea, and was admitted to the hospital on day 19
after chemotherapy initiation; she had grade 3 mucositis,
hand-foot-skin reaction, grade 4 diarrhea and severe generalized
abdominal pain. A contrast-enhanced CT scan of the abdomen revealed
diffuse submucosal edema with surrounding fat stranding, suggestive
of ileitis in a long segment of the distal ileum to the terminal
ileum. Multiple small gas bubbles were observed along the wall of
the edematous distal ileum, consistent with pneumatosis
intestinalis (Figs. 3 and 4).
The patient was admitted to the intensive care unit
for close monitoring and received total parenteral nutrition due to
diarrhea, hypoalbuminemia and decreased oral intake. Inotropic
support and electrolyte replacement were given to maintain
hemodynamic stability and avoid electrolyte disturbances.
The patient exhibited a decreased neutrophil count
(minimum, 0.7×109/1 on day 20) and developed fever on
day 21. Evaluation was negative for sepsis, including blood, stool
and urine cultures. The patient was treated with broad-spectrum
antibiotics for a total of 10 days and G-CSF. The neutrophil count
increased to 1.39×109/1 after 2 days.
The patient's condition gradually improved and
diarrhea subsided on day 29 after chemotherapy initiation. The
mucositis and skin reaction resolved and parenteral nutrition was
discontinued after 16 days.
Discussion
The two patients developed ileitis associated with
capecitabine. Previously reported cases of capecitabine-associated
ileitis included 1 patient who had received adjuvant capecitabine
monotherapy for colon cancer, 1 patient who had received
capecitabine, oxaliplatin and bevacizumab for metastatic rectal
cancer, and 1 patient who had received capecitabine and oxaliplatin
for metastatic rectal cancer (2–4). In these
previous cases, the ileitis dveloped early, between the first and
third cycle of chemotherapy, and none of the patients in these
reports had previous exposure to capecitabine. Case 1 highlights
the fact that small bowel complications may occur during later
cycles of chemotherapy, despite previous good tolerance to
capecitabine.
In both presented cases, it was difficult to confirm
the diagnosis, as the initial clinical findings were non-specific,
the patients were on combination chemotherapy plus targeted
therapy, and this type of toxicity is rare. The CT scan was an
important tool for diagnosis and monitoring. The radiographic
findings were comparable in the two cases, and ileitis was observed
as diffuse submucosal edema of the small bowel wall, which was
consistent with previous case reports and confirmed the
diagnosis.
It was important to closely monitor serum
electrolyte levels during the acute phase of the ileitis. Adequate
hydration was also important, as capecitabine is excreted primarily
by the kidneys.
Capecitabine is administered as an oral prodrug; it
is metabolized in successive enzymatic steps to 5-FU, which is
metabolized into two active metabolites that exert the cytotoxic
effect. The step of conversion to 5-FU preferentially occurs in
tumor tissues (1). 5-FU may cause
vasospasm in the coronary vessels and cardiotoxicity (5). Furthermore, the vascular endothelium is
susceptible to 5-FU via the generation of free radicals. The
endothelial damage may cause a procoagulant state and thrombosis
(6,7).
These vascular effects of 5-FU may also affect intestinal vessels.
A previous case of 5-FU-associated small bowel vasculitis due to
arterial ischemia has been reported (8). Another study demonstrated that
proinflammatory cytokines may contribute to 5-FU-associated
intestinal mucosal injury (9).
In our cases, the contribution of bevacizumab and
irinotecan to ileitis was unlikely. According to a previous case
series, the most common clinical findings in bevacizumab-associated
bowel perforation include abdominal pain and tenderness, vomiting,
fever and leukocytosis. Diarrhea has been less frequently reported
in most bevacizumab studies and is not a typical symptom of bowel
perforation (10). A literature
search identified no reported cases of small bowel ileitis
associated with bevacizumab and irinotecan administration. The
patient in case 1 did not have another episode of ileitis during
subsequent cycles of irinotecan and bevacizumab treatment following
discontinuation of capecitabine.
In summary, we presented two cases of adult Chinese
patients who developed ileitis associated with capecitabine
treatment. Ileitis may occur despite previous good tolerance to
capecitabine. A high index of suspicion is crucial for prompt
diagnosis. Ileitis may be reversible with early recognition, close
monitoring and proper supportive treatment.
References
|
1
|
Saif MW, Katirtzoglou NA and Syrigos KN:
Capecitabine: An overview of the side effects and their management.
Anticancer Drugs. 19:447–464. 2008.PubMed/NCBI
|
|
2
|
Radwan R, Namelo WC, Robinson M, Brewster
AE and Williams GL: Ileitis secondary to oral capecitabine
treatment? Case Rep Med. 154981(2012)2012.
|
|
3
|
Bouma G and Imholz AL: Ileitis following
capecitabine use. Ned Tijdschr Geneeskd. 155:A30642011.([In
Dutch]). PubMed/NCBI
|
|
4
|
Al-Gahmi AM, Kerr IG, Zekri JM and Zagnoon
AA: Capecitabine-induced terminal ileitis. Ann Saudi Med.
32:661–662. 2012.PubMed/NCBI
|
|
5
|
Shoemaker LK, Arora U and Rocha Lima CM:
5-fluorouracil-induced coronary vasospasm. Cancer Contr. 11:46–49.
2004.
|
|
6
|
Jensen SA and Sørensen JB:
5-fluorouracil-based therapy induces endovascular injury having
potential significance to development of clinically overt
cardiotoxicity. Cancer Chemother Pharmacol. 69:57–64. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuzel T, Esparaz B, Green D and Kies M:
Thrombogenicity of intravenous 5-fluorouracil alone or in
combination with cisplatin. Cancer. 65:885–889. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bucaloiu ID, Dubagunta S, Pachipala KK,
Kamal N and Fata F: Small-cell cancers, and an unusual reaction to
chemotherapy: Case 4. Fluorouracil-related small bowel vasculitis.
J Clin Oncol. 21:2442–2443. 2003.PubMed/NCBI
|
|
9
|
Soares PM, Mota JM, Souza EP, Justino PF,
Franco AX, Cunha FQ, Ribeiro RA and Souza MH: Inflammatory
intestinal damage induced by 5-fluorouracil requires IL-4.
Cytokine. 61:46–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Badgwell BD, Camp ER, Feig B, Wolff RA,
Eng C, Ellis LM and Cormier JN: Management of
bevacizumab-associated bowel perforation: A case series and review
of the literature. Ann Oncol. 19:577–582. 2008. View Article : Google Scholar : PubMed/NCBI
|