Introduction
Multiple myeloma (MM) is the main type of
hematological malignancy originating from plasma cells. MM is
currently an incurable disease. With conventional chemotherapeutic
regimens, such as melphalan and prednisone (MP), vincristine plus
adriamycin and dexamethasone (VAD) and high-dose dexamethasone, the
overall response rate (RR) of MM patients is 60%, with only <5%
achieving a complete response (CR). The median survival is 2–3
years (1). High-dose chemotherapy
followed by autologous stem cell transplantation (ASCT) may achieve
a higher RR and longer progression-free survival (PFS) and overall
survival (OS) (2–4). Over the last decade, the introduction of
the proteasome inhibitor bortezomib and the immunomodulatory agents
thalidomide and lenalidomide, has revolutionised MM treatment
(5,6).
The high RR and CR rate achieved by these novel agents have also
raised the question whether ASCT should still be considered as
first-line therapy in MM. Several phase 3 trials comparing
chemotherapy with first-line ASCT have reported an improved PFS,
but no difference in OS (7,8), while others support ASCT as part of the
treatment strategy (9,10). It is clear that ASCT enhances the
response, even after the most active first-line regimens, including
bortezomib plus thalidomide and dexamethasone (VTD) and
lenalidomide plus bortezomib, pegylated liposomal doxorubicin and
dexametasone (RVDD) (11,12). ASCT is currently considered the
standard of care in younger MM patients according to the current
National Comprehensive Cancer Network guidelines (13).
However, in China, the majority of the patients may
be less likely to undergo transplantation due to multiple reasons,
and data on which group of patients may benefit more from ASCT are
currently limited. In this study, we performed a retrospective
analysis of 114 MM patients, aged <65 years, who were treated
with or without ASCT following novel agent-containing induction
therapy in our hospital.
Patients and methods
Patients
From January, 2008 to December, 2012, 136 patients
with de novo MM, aged <65 years, received bortezomib- or
thalidomide-containing induction therapy at the Department of
Hematology of Ruijin Hospital (Shanghai, China). Following
induction therapy, 114 patients who achieved at least a PR and had
no severe comorbidities, were eligible for ASCT. Among these, 42
patients received ASCT within 1 year of diagnosis (ASCT group). The
remaining 72 patients declined ASCT for personal reasons (non-ASCT
group). This study was approved by the Ethics Committee of Ruijin
Hospital and conformed to the principles of the Declaration of
Helsinki. Written informed consent was obtained from all the
patients.
Evaluation
MM was diagnosed according to the uniform response
criteria of the International Myeloma Working Group (IMWG)
(14). All 114 patients had
symptomatic MM, with measurable disease. Non-secretory MM cases
were excluded. The response was classified as complete response
(CR), very good partial response (VGPR), partial response (PR) and
progressive disease (PD), according to the IMWG criteria.
Treatment regimens
All the patients were induced with chemotherapeutic
regimens, including bortezomib plus adriamycin and dexamethasone
(PAD), or bortezomib plus cyclophosphamide and dexamethasone (PCD),
or VAD and thalidomide (VADT). Bortezomib was administered at a
dose of 1.3 mg/m2 i.v. twice/week for 2 weeks in a 21-
or 28-day cycle. Thalidomide was administered at a dose of 100 mg
daily. In the ASCT group, all the patients received peripheral
blood progenitor cell mobilization with cyclophosphamide (4
g/m2) and conditioning therapy with high-dose melphalan
(100–200 mg/m2). The main maintenance therapy (MT)
regimen was thalidomide (50–150 mg/day), which, if tolerated,
continued until disease progression.
Statistical analysis
PFS was calculated from the date of the initiation
of induction therapy to the date of disease progression, relapse,
or death. OS was measured from the date of diagnosis to the date of
death; data on survivors were censored at the last follow-up. All
analyses were performed with SPSS 19.0 statistical software (IBM
SPSS, Armonk, NY, USA). Survival outcomes were analyzed with the
Kaplan-Meier method and compared with the log-rank test. The Cox
proportional hazards model was used for multivariate analysis, and
all the variables achieving P<0.25 in the univariate ananlysis
were considered.
Results
Patient characteristics
The study population comprised 114 patients, of whom
73 (64%) were male. The median age of the patients was 56 years
(range, 26–64 years) and 85 (75%) patients had Durie-Salmon stage
III disease at diagnosis. A total of 80 patients received
bortezomib-containing induction therapy, whereas the remaining
patients received thalidomide-containing therapy. Following
induction therapy, all the patients underwent response evaluation:
A total of 32 patients had achieved CR, 36 patients had VGPR and 46
patients had PR. Of the 114 patients, 42 received high-dose
chemotherapy followed by ASCT (ASCT group), whereas the remaining
72 patients did not receive ASCT (non-ASCT group). The baseline
characteristics of the ASCT and non-ASCT groups are listed in
Table I. No significant difference in
clinical characteristics was observed between the two groups.
 | Table I.Baseline characteristics of the 114
multiple myeloma patients. |
Table I.
Baseline characteristics of the 114
multiple myeloma patients.
| Characteristics | Non-ASCT (n=72) | ASCT (n=42) |
|---|
| Age, years |
|
|
| Median
(range) | 57 (29–65) | 53 (41–65) |
| Gender,
male/female | 43/29 | 30/12 |
| M component, n
(%) |
|
|
| IgG | 41 (57.0) | 25 (59.5) |
| IgA | 19 (26.4) | 6 (14.3) |
| IgD | 2 (2.7) | 5 (11.9) |
| Light
chain | 10 (13.9) | 6 (14.3) |
| DS stage, n (%) |
|
|
| I | 0 | 0 |
| II | 15 (20.8) | 14 (33.3) |
| III | 57 (79.2) | 28 (66.7) |
| ISS stage, n (%) |
|
|
| I | 1 (1.4) | 1 (2.4) |
| II | 61 (84.7) | 32 (76.2) |
| III | 10 (13.9) | 9 (21.4) |
| Number of induction
cycles, median (range) | 5 (2–10) | 4 (3–8) |
| Best response after
induction therapy, n (%) |
|
|
| CR | 19 (26.4) | 13 (31.0) |
| VGPR | 22 (30.6) | 14 (33.3) |
| PR | 31 (43.0) | 15 (35.7) |
| Induction regimen, n
(%) |
|
|
|
Bortezomib | 55 (76.4) | 25 (59.5) |
|
Thalidomide | 17 (23.6) | 17 (40.5) |
| Maintenance therapy,
n (%) |
|
|
| Yes | 40 (55.6) | 25 (59.5) |
| No | 32 (44.4) | 17 (40.5) |
Disease outcome
The median follow-up time of the 114 patients was 39
months (range, 5–74 months). At the time of the last follow-up
(March 31st, 2014), 52 of the 72 (72.2%) patients in the non-ASCT
group had progressed or relapsed, whereas 24 patients (33.3%) had
succumbed to the disease. In the ASCT group, 20 of the 42 (47.6%)
patients had progressed or relapsed and 6 patients (14.3%) had
succumbed to the disease. The median PFS in the non-ASCT and ASCT
groups was 23 and 42 months, respectively (P=0.001, Fig. 1A). The median OS was not reached in
neither of the groups. The 5-year OS rate was 58.9 and 81.2% in the
non-ASCT and ASCT groups, respectively (P=0.03, Fig. 1B).
Prognostic indicators
The univariate analysis in the non-ASCT group
(Table II) revealed that the
prognostic factors associated with prolonged PFS and OS included CR
(P=0.01 and 0.007, respectively) and MT (P=0.015 and 0.004,
respectively). Lower International Staging System (ISS) stage
(I+II) was associated with superior PFS (P=0.004), but not OS
(P=0.171).
 | Table II.Univariate analysis of the prognostic
factors for survival in the non-ASCT group. |
Table II.
Univariate analysis of the prognostic
factors for survival in the non-ASCT group.
| Factors | n | PFS (months) | P-value | OS (months) | P-value |
|---|
| DS stage |
|
| 0.600 |
| 0.357 |
| II | 15 | 24±3.618 |
| NR |
|
| III | 57 | 23±2.589 |
| 65±15.156 |
|
| ISS stage |
|
| 0.004 |
| 0.171 |
| I+II | 62 | 26±2.828 |
| 65±8.145 |
|
| III | 10 | 15±2.324 |
| 42±19.322 |
|
| Best response after
induction therapy |
|
| 0.010 |
| 0.007 |
| CR | 19 | 38±9.413 |
| NR |
|
|
VGPR | 22 | 23±4.462 |
| 65±4.605 |
|
| PR | 31 | 18±2.226 |
| 42±10.882 |
|
| Induction
regimen |
|
| 0.982 |
| 0.765 |
|
Bortezomib | 54 | 23±2.902 |
| NR |
|
|
Thalidomide | 18 | 25±3.135 |
| 55±9.832 |
|
| Maintenance
therapy |
|
| 0.015 |
| 0.004 |
|
Yes | 40 | 27±2.726 |
| NR |
|
| No | 32 | 17±2.62 |
| 36±6.924 |
|
The univariate analysis in the ASCT group (Table III) revealed that the prognostic
factors of a favorable outcome included CR post-ASCT, which
prolonged PFS (P=0.02), but not OS (P=0.067). MT significantly
prolonged OS (P=0.038).
 | Table III.Univariate analysis of the prognostic
factors for survival in the ASCT group. |
Table III.
Univariate analysis of the prognostic
factors for survival in the ASCT group.
| Factors | n | PFS
(months)a | P-value | 3-year OS (%) | P-value |
|---|
| DS stage |
|
| 0.903 |
| 0.407 |
| II | 14 | 42±7.574 |
| 81.3 |
|
|
III | 28 | 39±7.907 |
| 88.5 |
|
| ISS stage |
|
| 0.781 |
| 0.625 |
|
I+II | 33 | 42±6.674 |
| 86.7 |
|
|
III | 9 |
38±10.474 |
| 83.3 |
|
| Response after
ASCT |
|
| 0.020 |
| 0.067 |
| CR | 29 | 45±2.553 |
| 94.7 |
|
|
VGPR | 10 | 30±4.714 |
| 62.5 |
|
| PR | 3 | 17±5.715 |
| 66.7 |
|
| Maintenance
therapy |
|
| 0.408 |
| 0.038 |
|
Yes | 25 | 42±4.812 |
| 92.9 |
|
| No | 17 | 34±5.837 |
| 77.0 |
|
The multivariate analysis revealed that, for all the
patients, CR post-induction therapy, ASCT and MT were prognostic
factors of improved PFS and OS, whereas ISS stage affected PFS, but
not OS (Table IV).
 | Table IV.Multivariate analysis of prognostic
factors for survival in all multiple myeloma patients. |
Table IV.
Multivariate analysis of prognostic
factors for survival in all multiple myeloma patients.
| Variables | P-value | HR | 95% CI |
|---|
| PFS |
|
|
|
| ISS
stage III vs. I–II | 0.009 | 2.202 | 1.223–3.965 |
| No CR
vs. CR | 0.004 | 2.284 | 1.301–4.011 |
| No ASCT
vs. ASCT | 0.000 | 2.871 | 1.659–4.966 |
| No MT
vs. MT | 0.002 | 2.170 | 1.336–3.525 |
| OS |
|
|
|
| ISS
stage III vs. I–II | 0.720 | 1.184 | 0.471–2.978 |
| No CR
vs. CR | 0.041 | 2.743 | 1.040–7.233 |
| No ASCT
vs. ASCT | 0.023 | 2.912 | 1.161–7.305 |
| No MT
vs. MT | 0.001 | 3.917 | 1.795–8.549 |
Effect of ASCT and MT on
subgroups
Furthermore, we performed a subgroup analysis base
on the response evaluation. Following induction therapy, the
patients were divided into three subgroups, namely the CR, VGPR and
PR subgroups. For each subgroup, the effect of ASCT and MT on PFS
and OS was analyzed. According to the two factors (ASCT and MT),
four arms were formed as follows: ASCT/MT, ASCT/non-MT,
non-ASCT/MT, and non-ASCT/non-MT. In the CR subgroup (Fig. 2), no significant differences in PFS
and OS were observed among the four arms (P=0.099 and P=0.511,
respectively); patients without ASCT but with MT still reached the
median PFS of 58±15.88 months, with a 3-year OS rate of 80%. In the
VGPR subgroup (Fig. 3), PFS
prolongation was achieved by the patients undergoing ASCT following
MT (P=0.012), but there was no statistical difference in OS
(P=0.582) among the four arms. In the PR subgroup (Fig. 4), the greatest benefit from ASCT and
MT was observed. There were statistically significant differences
in PFS (P=0.008) and OS (P=0.005) among the four arms.
 | Figure 2.ASCT and MT did not significantly
affect the outcome of the patients achieving a complete response
prior to ASCT. (A) The median PFS ± standard deviation of the four
arms (ASCT/MT, ASCT/non-MT, non-ASCT/MT, non-ASCT/non-MT) was
38±11.688, 39±6.736, 58±15.88 and 26±2.947, respectively. (B) The
3-year OS rate of the four arms (ASCT/MT, ASCT/non-MT, non-ASCT/MT,
non-ASCT/non-MT) was 100, 80, 80 and 53.6%, respectively. PFS,
progression-free survival; OS, overall survival; ASCT, autologous
hematopoietic stem cell transplantation; MT, maintenance
therapy. |
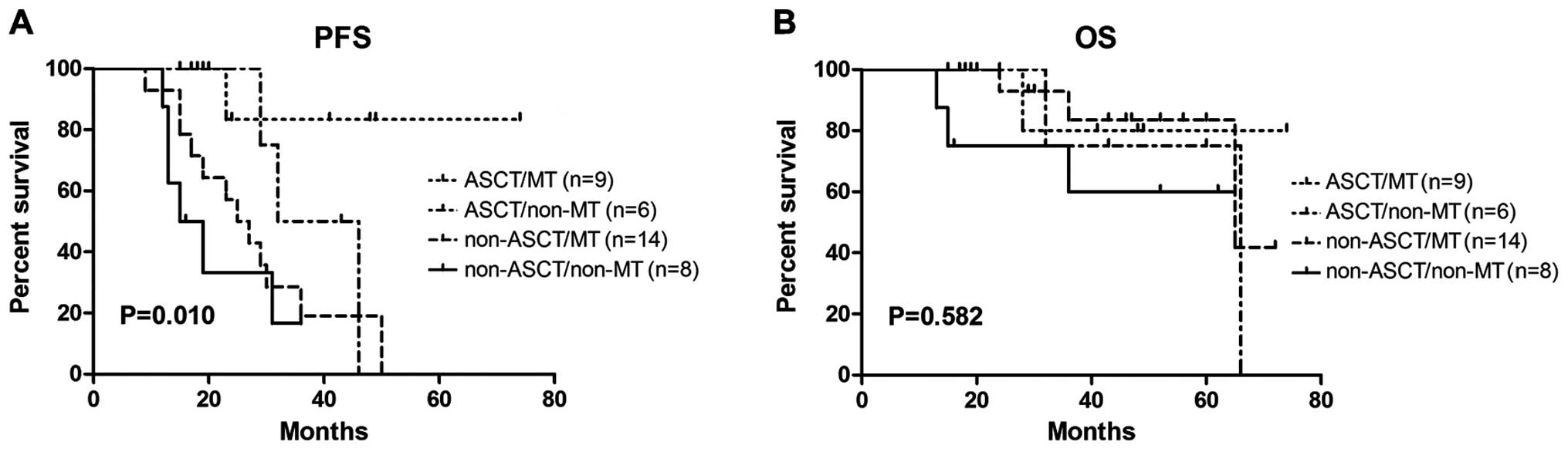 | Figure 3.ASCT and MT affected PFS but not OS in
the patients achieving a very good partial response prior to ASCT.
(A) The median PFS ± standard deviation of the four arms (ASCT/MT,
ASCT/non-MT, non-ASCT/MT, non-ASCT/non-MT) was NR (not reached),
32±5.667, 25±3.742, 15±3.637, respectively. (B) The 3-year OS rate
of the four arms was 80, 75, 83 and 60%, respectively. PFS,
progression-free survival; OS, overall survival; ASCT, autologous
hematopoietic stem cell transplantation; MT, maintenance
therapy. |
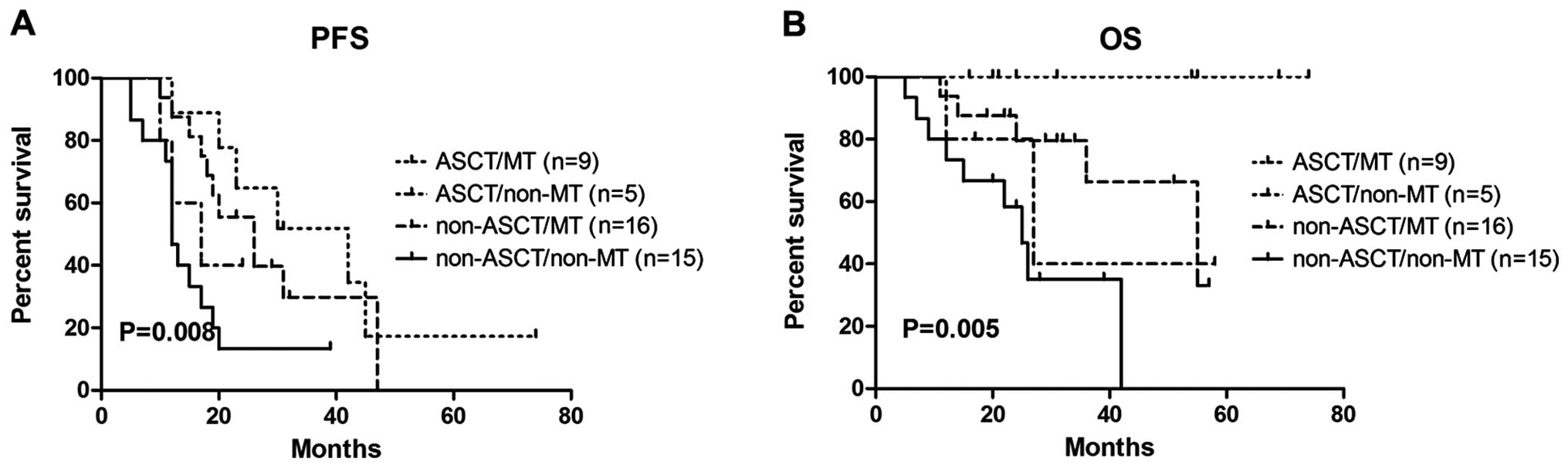 | Figure 4.ASCT and MT affected both PFS and OS
in the patients achieving a partial response prior to ASCT. (A) The
median PFS ± standard deviation of the four arms (ASCT/MT,
ASCT/non-MT, non-ASCT/MT, non-ASCT/non-MT) was 42±11.52, 17±5.477,
26±4.942 and 12±7.73, respectively. (B) The 3-year OS rate of the
four arms (ASCT/MT, ASCT/non-MT, non-ASCT/MT, non-ASCT/non-MT)
(ASCT/MT, ASCT/non-MT, non-ASCT/MT, non-ASCT/non-MT) was 100, 40,
66 and 35%, respectively. PFS, progression-free survival; OS,
overall survival; ASCT, autologous hematopoietic stem cell
transplantation; MT, maintenance therapy. |
Discussion
Multiple myeloma is currently considered incurable.
The aim of the treatment of this disease is to prolong PFS, and
eventually OS. The majority of the available studies indicate that
achievement of CR was associated with prolonged PFS and OS
(6,15). With conventional chemotherapy, the CR
rate is currently 5–8%. High-dose therapy followed by ASCT
increases the response rate and improves response to treatment,
achieving a CR rate of 22–44% (16,17). More
recently, novel agent-containing induction therapy has achieved a
high response rate and CR rate. In the phase 3 VISTA study of
bortezomib plus MP (VMP) vs. MP alone, the CR rate was 28% in the
VMP group (18). In other
bortezomib-containing induction regimens, the CR rate reached
22–47%, with a 1-year PFS of 83–100% (5). The improved outcome of the novel agents
may challenge the role of ASCT in the treatment of MM.
In this retrospective study, CR rate reached 28%
(32/114)with the novel-agent induction therapy. Further benefits
were obtained in the ASCT group, with the CR rate increasing from
31% (pre-ASCT) to 69% (post-ASCT). The survival analysis also
revealed that PFS and OS were significantly prolonged in the ASCT
group, supporting the beneficial role of ASCT in younger MM
patients in the era of novel agents (19,20).
However, different results were reported by other studies.
Boccadoro et al (7) compared
MP/lenalidomide (MPR) vs. ASCT plus lenalidomide maintenance or no
maintenance and indicated that ASCT improved PFS, while the effect
on OS was insignificant, suggesting that lenalidomide maintenance
may balance the OS in the two groups. The improved OS in our study
may be due to a higher number of cases achieving CR in the ASCT
group (n=29 case, 69%).
Thalidomide, commonly used as MT and sequential
therapy, was shown to improve PFS and OS (21,22). In
our study, the multivariate analysis revealed that MT was an
independent factor of improved PFS and OS in the non-ASCT as well
as the ASCT group. However, a study conducted by Barlogie et
al (23) reported a beneficial
effect on PFS, but not OS and suggested that the similar OS between
the two groups is partially due to the shorter survival following
relapse in the thalidomide maintenance group. In addition, Attal
et al (22,24) reported an OS advantage from
thalidomide maintenance with a follow-up of 39 months, while the OS
advantage was lost when the follow-up was prolonged to 5.7 years.
In our study, the median OS time has not yet been reached, and
elucidating the survival advantages of MT requires a longer
follow-up and further investigation.
CR achievement was another independent factor in our
study, which was consistent with previous studies (4,6). Attaining
a CR has been the major objective in the management of younger
patients. However, the data on whether patients who achieved CR
following induction therapy may further benefit from ASCT are
currently limited. We performed a subgroup analysis according to
the response status following induction therapy. Considering the
important role of MT on survival, we analyzed ASCT as well as MT.
Interestingly, in the subgroup of CR, with or without ASCT/MT, no
statistically significant difference in PFS or OS was observed. It
was previously demonstrated that stringent complete response (sCR)
was an attainable goal following ASCT, which significantly improved
survival outcome compared with conventional CR (25). As shown in our study, a better OS
(5-year OS rate of 100%) may be achieved in the ASCT/MT arm, which
may be due to more sCRs obtained, although the difference was not
statistically significant. Our findings support the role of ASCT
and MT in deepening the CR.
In the VGPR subgroup, ASCT and MT prolonged the PFS
(P=0.010), but without an OS benefit (P=0.582). However, through
constructing Kaplan-Meier curves, modest differences were observed
among arms. Since the median OS time has not been reached, we
hypothesized that different findings may emerged after long-term
follow-up. In the PR subset, the patients undoubtedly benefited the
most from ASCT and MT in terms of survival (P<0.01). The median
PFS of the ASCT/MT arm was 42 months, with a 3-year OS rate of
100%, whereas in the non-ASCT/non-MT arm, the median PFS was only
12 months, with a 3-year OS rate of 35%. A poor PFS (17 months) and
a 3-year OS rate of 40% was noted in the ASCT/no-MT arm. In this
arm, the sample was too small (n=5) and 3 patients remained in PR
following ASCT. Similar to other reports, any response less than
VGPR following ASCT was always associated with a poor outcome
(26,27).
In summary, novel-agent induction therapy resulted
in a higher response rate and response quality. ASCT further
increased the CR rate and improved PFS and OS, particularly in
patients achieving a PR following induction therapy. In the
patients who achieved CR following induction therapy, the role of
ASCT requires further investigation. As an independent prognostic
factor of better PFS and OS, MT has become an important part of the
treatment strategy in MM.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81302038 and 81201863).
References
|
1
|
Collaborative Group: Combination
chemotherapy versus melphalan plus prednisone as treatment for
multiple myeloma: An overview of 6,633 patients from 27 randomized
trials. Myeloma Trialists' Collaborative Group. J Clin Oncol.
16:3832–3842. 1998.PubMed/NCBI
|
|
2
|
Harousseau JL and Attal M: The role of
autologous hematopoietic stem cell transplantation in multiple
myeloma. Semin Hematol. 34(1 Suppl 1): 61–66. 1997.PubMed/NCBI
|
|
3
|
Blade J, Rosinol L, Cibera MT, Rovira M
and Carreras E: Hematopoietic stem cell transplantation for
multiple myeloma beyond 2010. Blood. 115:3655–3663. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van de Velde HJ, Liu X, Chen G, Cakana A,
Deraedt W and Bayssas M: Complete response correlates with
long-term survival and progression-free survival in high-dose
therapy in multiple myeloma. Haematologica. 92:1399–1406. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar S, Flinn I, Richardson PG, Hari P,
Callander N, Noga SJ, Stewart AK, Turturro F, Rifkin R, Wolf J, et
al: Randomized, multicenter, phase 2 study (EVOLUTION) of
combinations of bortezomib, dexamethasone, cyclophosphamide and
lenalidomide in previously untreated multiple myeloma. Blood.
119:4375–4382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chanan-Kahn AA and Giralt S: Importance of
achieving complete response in multiple myeloma and the impact of
novel agents. J Clin Oncol. 28:2612–2624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boccadoro M, Cavallo F, Nagler A, Ben
Yehuda D, Omedè P, Cavalli M, Levi A, Crippa C, Siniscalchi A,
Brasca P, et al: Melphalan/prednisone/lenalidomide (MPR) versus
high-dose melphalan and autologous transplantation (MEL200) in
newly diagnosed multiple myeloma (MM) patients: a phase III trial.
J Clin Oncol. 29(Supp 1): 8020–8027. 2011.
|
|
8
|
Gay F, Hajek R, Diramondo F, et al:
Cyclophosphamide lenalidomide-dexamethasone vs. autologous
transplant in newly diagnosed myeloma: a phase 3 trial. Clin
Lymphoma Myeloma Leuk. 13(Suppl 1): S402013.
|
|
9
|
Fermand JP, Katsahian S, Divine M, et al:
High-dose therapy and autologous blood stem-cell transplantation
compared with conventional treatment in myeloma patients aged 55 to
65 years: long-term results of a randomized control trial from the
Group Myelome-Autogreffe. J Clin Oncol. 23:9227–9233. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hahn T, Wingard JR, Anderson KC, et al:
The role of cytotoxic therapy with hematopoietic stem cell
transplantation in the therapy of multiple myeloma: an
evidence-based review. Biol Blood Marrow Transplant. 9:4–37. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moreau P, Avet-Loiseau H, Facon T, Attal
M, Tiab M, Hulin C, Doyen C, Garderet L, Randriamalala E, Araujo C,
et al: Bortezomib plus dexamethasone versus reduced-dose
bortezomib, thalidomide plus dexamethasone as induction treatment
before autologous stem cell transplantation in newly diagnosed
multiple myeloma. Blood. 118:5752–5758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jakubowiak AJ, Griffith KA, Reece DE,
Hofmeister CC, Lonial S, Zimmerman TM, Campagnaro EL, Schlossman
RL, Laubach JP, Raje NS, et al: Lenalidomide, bortezomib, pegylated
liposomal doxorubicin and dexamethasone in newly diagnosed multiple
myeloma: a phase 1/2 Multiple Myeloma Research Consortium trial.
Blood. 118:535–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kenneth C Anderson, Melissa Alsina,
William Bensinger, et al: Multiple Myeloma. Version 1.2013 J Natl
Compr Canc Netw. 11:11–17. 2013.
|
|
14
|
Durie BG, Harousseau JL, Miguel JS, et al:
International uniform response criteria for multiple myeloma.
Leukemia. 20:1467–1473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haroussear JL, Attal M and Avet-Loiseau H:
The role of complete response in multiple myeloma. Blood.
114:3139–3146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Attal M, Harousseau JL, Stoppa AM, Sotto
JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah
N, et al: A prospective, randomized trial of autologous bone marrow
transplantation and chemotherapy in multiple myeloma. Intergroupe
Français du Myélome. N Engl J Med. 335:91–97. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Child JA, Morgan GJ, Davies FE, Owen RG,
Bell SE, Hawkins K, Brown J, Drayson MT and Selby PJ: Medical
Research Council Adult Leukaemia Working Party: High-dose
chemotherapy with hematopoietic stem-cell rescue for multiple
myeloma. N Engl J Med. 348:1875–1883. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harousseau JL, Palumbo A, Richardson PG,
Schlag R, Dimopoulos MA, Shpilberg O, Kropff M, Kentos A, Cavo M,
Golenkov A, et al: Superior outcomes associated with complete
response in newly diagnosed multiple myeloma patients treated with
nonintensive therapy: analysis of the phase 3 VISTA study of
bortezomib plus melphalan-prednisone versus melphalan-prednisone.
Blood. 116:3743–3750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reeder CB, Reece DE, Kukreti V, Chen C,
Trudel S, Hentz J, Noble B, Pirooz NA, Spong JE, Piza JG, et al:
Cyclophosphamide, bortezomib and dexamethasone induction for newly
diagnosed multiple myeloma: high response rates in a phase II
clinical trial. Leukemia. 23:1337–1341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar L, Iqbal N, Mookerjee A, Verma RK,
Sharma OD, Batra A, Pramanik R and Gupta R: Complete response after
autologous stem cell transplant in multiple myeloma. Cancer Med.
3:939–946. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spencer A, Prince HM, Roberts AW, Prosser
IW, Bradstock KF, Coyle L, Gill DS, Horvath N, Reynolds J and
Kennedy N: Consolidation therapy with low-dose thalidomide and
prednisolone prolongs the survival of multiple myeloma patients
undergoing a single autologous stem-cell transplantation procedure.
J Clin Oncol. 27:1788–1793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Attal M, Harousseau JL, Leyvraz S, Doyen
C, Hulin C, Benboubker L, Agha Yakoub I, Bourhis JH, Garderet L,
Pegourie B, et al: Inter-Groupe Francophone du Myélome (IFM).:
Maintenance therapy with thalidomide improves survival in patients
with multiple myeloma. Blood. 108:3289–3294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barlogie B, Tricot G, Anaissie E,
Shaughnessy J, Rasmussen E, van Rhee F, Fassas A, Zangari M,
Hollmig K, Pineda-Roman M, et al: Thalidomide and hematopoietic
cell transplantation for multiple myeloma. N Engl J Med.
354:1021–1030. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Attal M and Roussel M: Maintenance therapy
for myeloma. How much, how long and at what cost? Am Soc Clin Oncol
Educ Book. 32:515–522. 2012.
|
|
25
|
Kapoor P, Kumar SK, Dispenzieri A, et al:
Importance of achieving stringent complete response after
autologous stem-cell transplantation in multiple myeloma. J Clin
Oncol. 31:4529–4535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bergantim R, Trigo F and Guimaraes JE:
Impact of tandem autologous stem cell transplantation and response
to transplant in the outcome of multiple myeloma. Exp Hematol
Oncol. 1:35–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martinez-Lopez J, Blade J, Mateos MV, et
al: Long-term prognostic significance of response in multiple
myeloma after stem cell transplantation. Blood. 118:529–534. 2011.
View Article : Google Scholar : PubMed/NCBI
|


















