Introduction
Lung cancer is the most common type of cancer
worldwide, and is the leading cause of cancer-related mortality in
men as well as in women. Approximately 85% of lung cancers are
non-small-cell lung cancer (NSCLC), and >70% of NSCLC patients
present with inoperable and locally advanced (stage IIIB) or
metastatic (stage IV) disease (1).
Among patients with advanced NSCLC, the use of cytotoxic
chemotherapy is associated with a response rate of 20–40% and a
median survival time of 7–12 months (2–4).
However, the prognosis of NSCLC patients has recently improved with
the use of targeted therapies and new anticancer drugs.
Bevacizumab (BEV) is a monoclonal antibody targeted
against vascular endothelial growth factor, and has been found to
benefit patients with a variety of cancers. A phase III trial
(E4599 study) established that the addition of BEV to first-line
carboplatin + paclitaxel is an effective treatment for patients
with advanced non-squamous (non-Sq) NSCLC (5). However, in the AVAiL study [BEV or
placebo + cisplatin (CDDP) and gemcitabine], the progression-free
survival (PFS) benefit did not translate into a significant overall
survival (OS) benefit (6). Thus, the
benefit of adding BEV to the CDDP regimen has not been
demonstrated.
The randomized phase III AVAPERL study evaluated
whether combination treatment with PEM and BEV in the maintenance
setting could further improve the efficacy (compared with BEV
monotherapy) in patients with advanced non-Sq NSCLC whose disease
had not progressed after first-line induction treatment with CDDP,
PEM and BEV (7,8). The median PFS and OS with the induction
treatment in the PEM+BEV arm were 10.2 and 19.8 months,
respectively, and these results were considered favorable for the
treatment of advanced NSCLC. However, the AVAPERL study had several
limitations, such as the survival data being based on selected
patients who were eligible for BEV and maintenance therapy, and the
lack of an arm for PEM alone as maintenance therapy. Therefore, the
results of the AVAPERL study should be interpreted with extreme
caution.
The present study retrospectively reviewed
consecutive patients who received first-line chemotherapy with
CDDP+PEM in order to assess the efficacy of this regimen in
selected patients who were eligible for BEV and maintenance therapy
(similar to the AVAPERL study).
Materials and methods
Patients
Consecutive patients with stage IIIB/IV or recurrent
non-Sq NSCLC who were initiated on first-line chemotherapy with
CDDP (75 mg/m2) and PEM (500 mg/m2) between
July, 2009 and January, 2013 at the Shizuoka Cancer Center
(Shizuoka, Japan) were reviewed. The eligibility criteria for BEV
according to the AVAPERL study were the absence of a history of
hemoptysis, absence of evidence of a tumor invading the major
vessels, absence of current or recent use of antithrombotic agents
and absence of clinically significant cardiovascular disease. The
eligibility criteria for maintenance therapy were the absence of
disease progression and the absence of unacceptable toxicity at
completion of four cycles of the induction therapy. The PFS and OS
were then compared among patients who were eligible or ineligible
for BEV therapy. Finally, the PFS and OS were compared among the
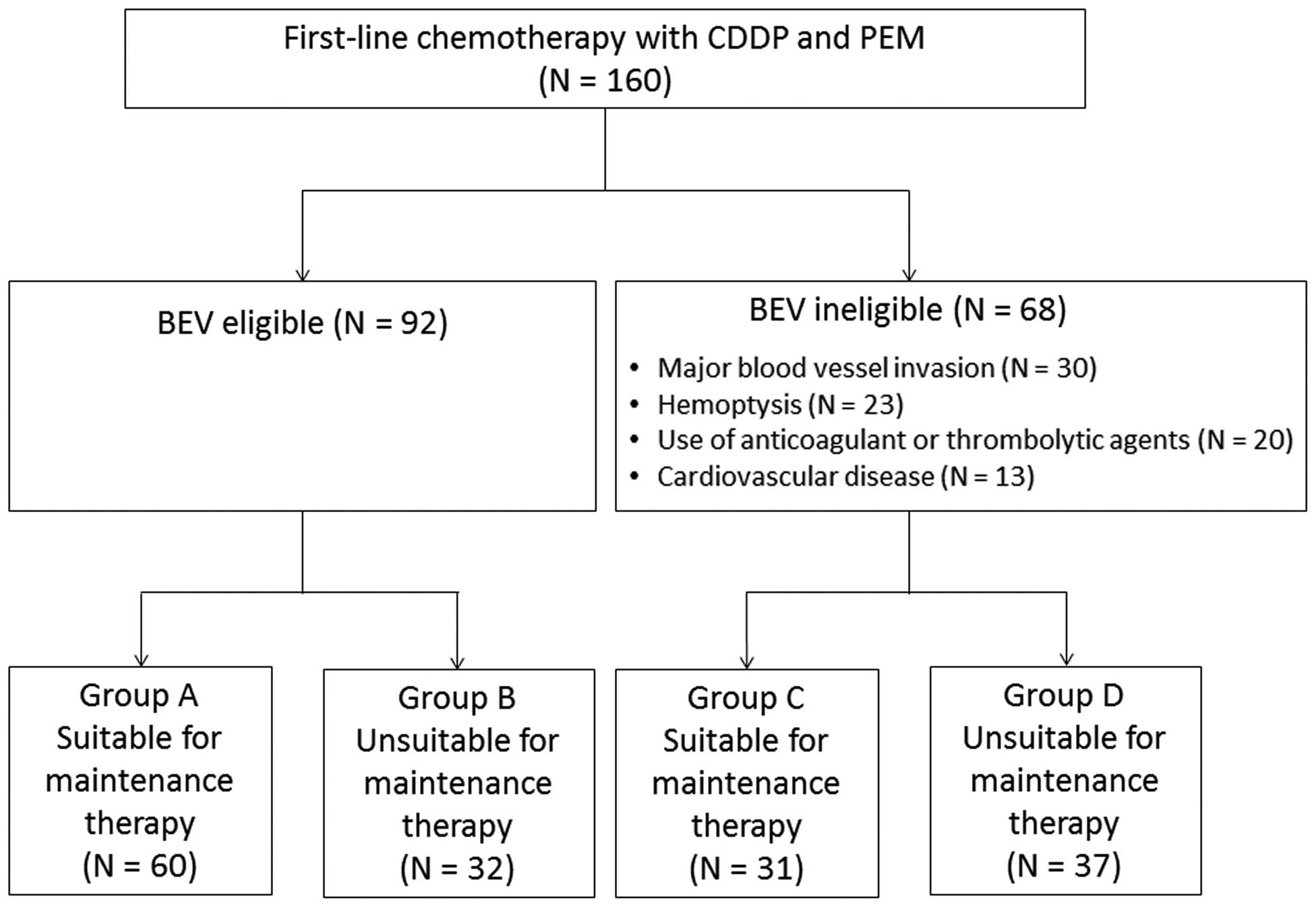
maintenance therapy eligibility subgroups. The patient grouping
process is summarized in Fig. 1.
The protocol of the present study was approved by
the Institutional Review Board of the Shizuoka Cancer Center.
Statistical analysis
Differences in characteristics between the
BEV-eligible and -ineligible groups were evaluated using Fisher's
exact test. The PFS and OS were estimated using the Kaplan-Meier
method, and the log-rank test was used for inter-group comparisons.
The PFS and OS data were analyzed using JMP software, version 10.0
(SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 160 patients with stage IIIB/IV or
recurrent non-Sq NSCLC who received first-line chemotherapy with
CDDP and PEM were reviewed in the present study. A total of 92
patients were eligible for BEV therapy, whereas 68 patients were
ineligible due to major blood vessel invasion (n=30), hemoptysis
(n=23), use of anticoagulant or thrombolytic agents (n=20) or
cardiovascular disease (n=13). The baseline characteristics,
including gender, histology and stage, were similar between the
BEV-eligible and -ineligible groups (Table I). However, compared with the
BEV-ineligible group, the BEV-eligible group contained
significantly more patients aged <65 years, with an Eastern
Cooperative Oncology Group performance status of 0, epidermal
growth factor receptor (EGFR) mutation-positive and never
smokers.
 | Table I.Baseline patient characteristics. |
Table I.
Baseline patient characteristics.
| Characteristics | BEV-eligible
(n=92) | BEV-ineligible
(n=68) | P-value |
|---|
| Age (years) |
|
|
| Median
(range) | 63 (39–76) | 67 (37–75) | 0.0036 |
| <65, n
(%) | 61 (66) | 29 (43) |
|
| Gender, n (%) |
|
| 0.085 |
| Male | 58 (63) | 52 (76) |
|
|
Female | 34 (37) | 16 (24) |
|
| Histology, n (%) |
|
| 0.65 |
|
Adenocarcinoma | 90 (98) | 65 (96) |
|
|
Other | 2 (2) | 3 (4) |
|
| Stage, n (%) |
|
| 1 |
| IIIB | 5 (5) | 3 (4) |
|
| IV | 87 (95) | 65 (96) |
|
| PS, n (%) |
|
| 0.004 |
| 0 | 48 (52) | 19 (28) |
|
| 1 | 43 (47) | 47 (69) |
|
| 2 | 1 (1) | 2 (3) |
|
| EGFR
mutation-positive, n (%) | 22 (24) | 7 (10) | 0.037 |
| Smoking status, n
(%) |
|
| 0.006 |
|
Current/former smoker | 60 (65) | 58 (85) |
|
|
Never | 32 (35) | 10 (15) |
|
Response to first-line CDDP+PEM in
BEV-eligible vs. -ineligible non-Sq NSCLC patients
The overall response rate was 33% in the
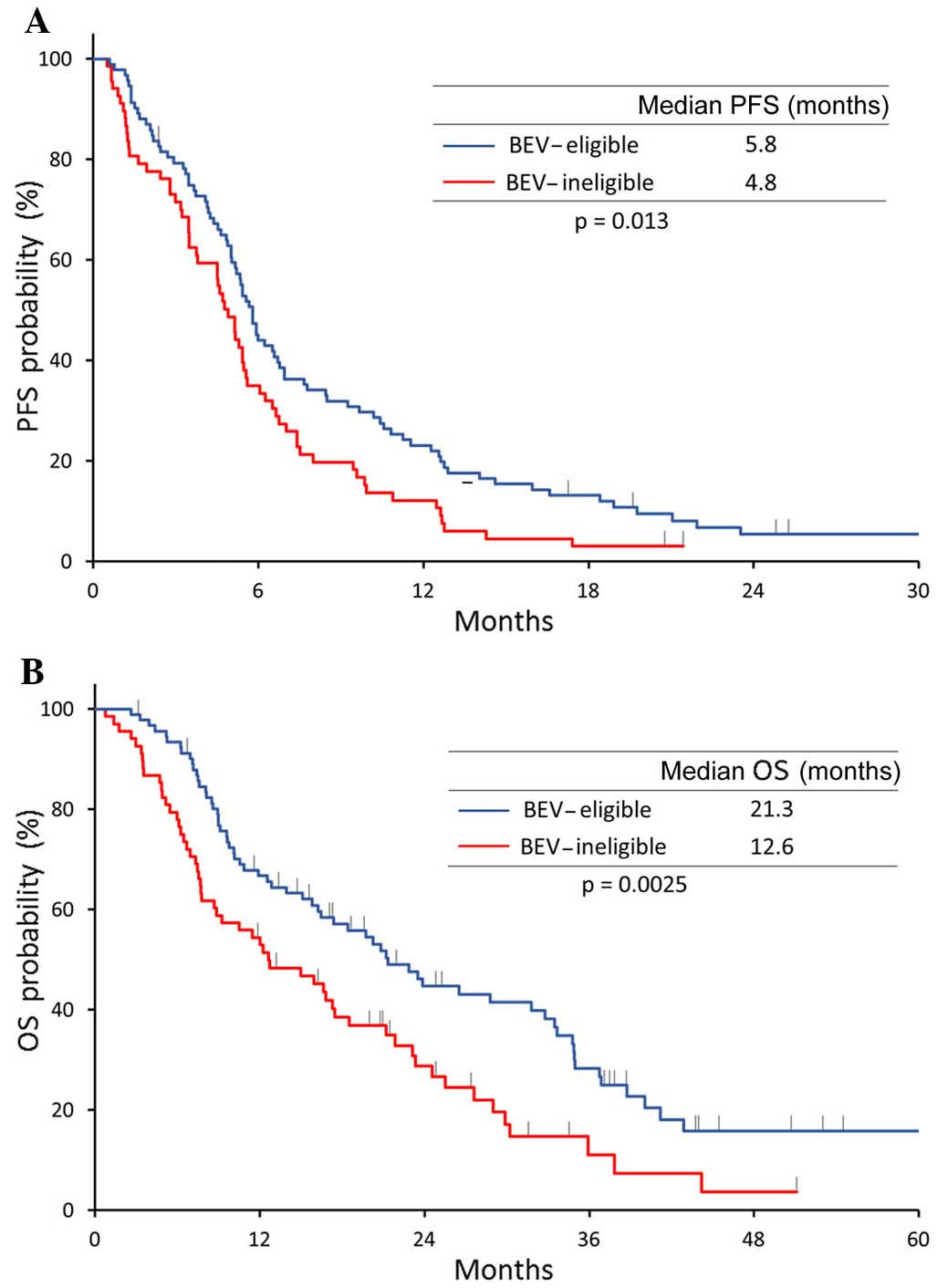
BEV-eligible and 35% in the BEV-ineligible group (P=0.74). However,
treatment efficacy in the BEV-eligible group was significantly
superior compared with that in the BEV-ineligible group in terms of
survival (median PFS, 5.8 vs. 4.8 months, respectively, P=0.013;
and median OS, 21.3 vs. 12.6 months, respectively, P=0.0025)
(Fig. 2).
Maintenance therapy with PEMs
following first-line CDDP+PEM
The patients were stratified into groups A-D
according to their eligibility for BEV and maintenance therapy
(Fig. 1). In the BEV-eligible group,
60 patients were suitable for maintenance therapy (group A) whereas
32 were unsuitable (group B) due to adverse events or disease
progression. In the BEV-ineligible group, 31 patients were suitable
for maintenance therapy (group C) and 37 patients were unsuitable
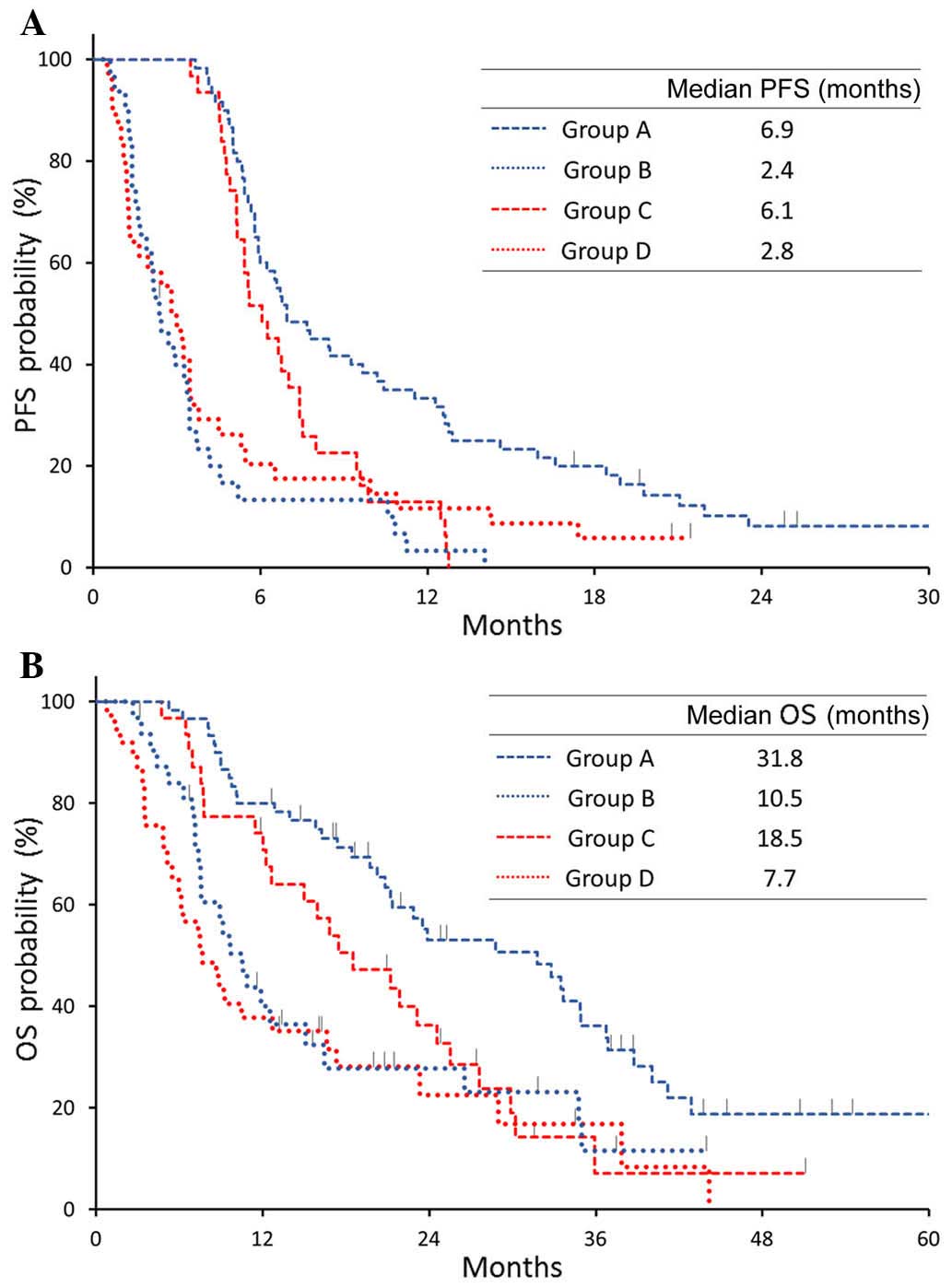
(group D). In groups A and C, 22 (37%) and 16 patients (52%),
respectively, received PEM maintenance therapy. The median PFS and
OS were longer in groups A and C (maintenance
therapy-eligible)compared with those in groups B and D
(BEV-ineligible). In group A, the PFS and OS were 6.9 and 31.8
months, respectively, compared with 2.4 and 10.5 months in group B,
6.1 and 18.5 months in group C, and 2.8 and 7.7 months in group D
(Fig. 3). The PFS and OS in group A
(eligible for both BEV and maintenance therapy) were significantly
better compared with those in the other groups.
Discussion
In the present study, combination chemotherapy with
CDDP and PEM achieved favorable PFS and OS in patients who were
eligible for both BEV and maintenance therapy. This result supports
the concept that eligibility for BEV represents a favorable
prognostic factor, in accordance with the findings of a
retrospective study by Takagi et al (9). In addition, patients who were eligible
for maintenance therapy were found to have a better prognosis,
which was expected, as patients with induction therapy failure were
excluded. The favorable results of the AVAPERL study may have been
affected by a similar selection bias. However, the AVAPERL study
included a higher proportion of patients who were eligible for
maintenance therapy (67%) compared with the present study (57%).
Thus, the regimen used in the AVAPERL study (triplet induction
chemotherapy with CDDP, PEM and BEV, followed by maintenance
therapy with PEM and BEV) may be efficacious, although a similar OS
was observed between the AVAPERL study and the BEV-ineligible group
in the present study. In this context, although the addition of BEV
improves tumor response, it may also lead to characteristic adverse
events, such as hypertension, proteinuria, bleeding, hemoptysis and
pulmonary embolism (5,7,10).
Furthermore, the AVAiL study failed to demonstrate an OS benefit
with BEV therapy (6). The regimen
used in the PARAMOUNT study (doublet induction therapy with CDDP
and PEM, followed by continuation maintenance therapy with PEM), is
a standard treatment for patients with advanced non-Sq NSCLC, based
on the results of phase III trials (11,12).
However, whether the AVAPERL study regimen is superior to the
PARAMOUNT study regimen remains unclear.
The present study has several limitations. First,
the number of patients who were included in this retrospective
study was relatively small. Furthermore, the subjects were Japanese
patients, and their OS may be affected by subsequent therapy with
EGFR tyrosine kinase inhibitors.
In conclusion, doublet induction chemotherapy with
CDDP and PEM was associated with a favorable outcome in a patient
population similar to that of the AVAPERL trial. Therefore, the
PARAMOUNT study regimen is a reasonable treatment option for
patients with advanced non-Sq NSCLC, regardless of their BEV
eligibility status.
Acknowledgements
The authors would like to thank Editage (www.editage.jp) for the English language editing.
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small-cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Azzoli CG, Baker S Jr, Temin S, Pao W,
Aliff T, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, et
al: American Society of Clinical Oncology Clinical Practice
Guideline update on chemotherapy for stage IV non-small-cell lung
cancer. J Clin Oncol. 27:6251–6266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wakelee H and Belani CP: Optimizing
first-line treatment options for patients with advanced NSCLC.
Oncologist. 10:(Suppl 3). S1–S10. 2005. View Article : Google Scholar
|
|
4
|
Sandler A: Bevacizumab in non small cell
lung cancer. Clin Cancer Res. 13:(Suppl). S4613–S4616. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, et
al: Overall survival with cisplatin-gemcitabine and bevacizumab or
placebo as first-line therapy for nonsquamous non-small-cell lung
cancer: Results from a randomised phase III trial (AVAiL). Ann
Oncol. 21:1804–1809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barlesi F, Scherpereel A, Rittmeyer A,
Pazzola A, Ferrer Tur N, Kim JH, Ahn MJ, Aerts JG, Gorbunova V,
Vikström A, et al: Randomized phase III trial of maintenance
bevacizumab with or without pemetrexed after first-line induction
with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous
non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol.
31:3004–3011. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baresi F, Scherpereel A, Gorbounova V,
Gervais R, Vikström A, Chouaid C, Chella A, Kim JH, Ahn MJ, Reck M,
et al: Maintenance bevacizumab-pemetrexed after first-line
cisplatin-pemetrexed-bevacizumab for advanced nonsquamous
nonsmall-cell lung cancer: Updated survival analysis of the AVAPERL
(MO22089) randomized phase III trial. Ann Oncol. 25:1044–1052.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takagi Y, Totiihara A, Nakahara Y, Yomota
M, Okuma Y, Hosomi Y, Shibuya M and Okamura T: Eligibility for
bevacizumab as an independent prognostic factor for patients with
advanced non-squamous non-small cell lung cancer: A retrospective
cohort study. PLoS One. 8:e597002013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N and
Manegold C: Phase III trial of cisplatin plus gemcitabine with
either placebo or bevacizumab as first-line therapy for nonsquamous
non-small-cell lung cancer: AVAiL. J Clin Oncol. 27:1227–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paz-Ares L, de Marinis F, Dediu M, Thomas
M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, et
al: Maintenance therapy with pemetrexed plus best supportive care
versus placebo plus best supportive care after induction therapy
with pemetrexed plus cisplatin for advanced non-squamous
non-small-cell lung cancer (PARAMOUNT): A double-blind, phase 3,
randomised controlled trial. Lancet Oncol. 13:247–255. 2012.
View Article : Google Scholar : PubMed/NCBI
|