Introduction
As carcinoma of the vulva is rare among young women,
reports of pregnancy following treatment for vulvar carcinoma are
extremely uncommon. The incidence of vulvar carcinoma, however, has
recently increased among younger women; thus, there may have been
an increase in pregnancy rates following vulvar carcinoma treatment
(1). Pregnant women who undergo
surgical treatment for vulvar carcinoma, including radical
vulvectomy, may have an increased incidence of caesarean delivery
(2). In the literature, vulvar
scarring following radical vulvectomy was the major reason for
pregnant women undergoing caesarean section (2–7). To
date, no cases of pregnancy following vulvar carcinoma have been
reported in patients who had undergone surgery and
radiotherapy.
We herein describe a case in which caesarean section
was performed due to the presence of extensive vulvar scarring
following multimodal therapy for vulvar carcinoma, including
chemotherapy, surgery and radiotherapy.
Case report
In April, 2003, a 17-year-old, nulligravida Japanese
woman, who was a smoker, presented with a 4-month history of a mass
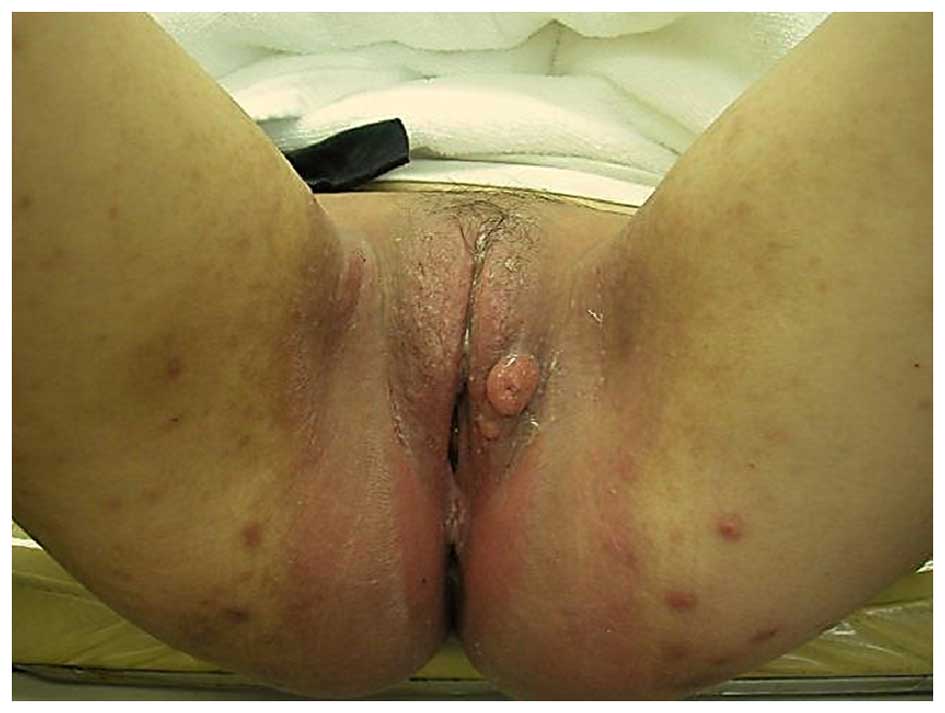
(sized >2 cm) with accompanying pruritus in the left labium
majus (Fig. 1). Examination of
biopsy specimens revealed invasive squamous cell carcinoma with
keratin pearl formation and stromal invasion >1 mm in depth
(Fig. 2). Screening for sexually
transmitted infections, including HIV serology, syphilis serology,
Chlamydia trachomatis polymerase chain reaction and
Neisseria gonorrhoeae polymerase chain reaction were
performed, and were all negative. The serum squamous cell carcinoma
antigen level was 2.6 ng/ml (normal value, <1.5 ng/ml). Magnetic
resonance imaging (MRI) revealed enlarged inguinal lymph nodes
bilaterally. The patient first underwent neoadjuvant chemotherapy
(pepleomycin, 5 mg/body intramuscular injection twice a week for
2.5 weeks, 5 cycles of administration in total). The tumor of the
vulva was reduced in size following chemotherapy. Subsequently,
wide local excision with bilateral inguinal lymph node dissection
was performed (Figs. 3 and 4). All surgical margins (≥1 cm) were free
of carcinoma cells. The lymph nodes were negative for malignancy
and there was no evidence of lymphovascular invasion. The tumor was
classified as ypT1bN0M0, and a diagnosis of International
Federation of Gynecology and Obstetrics (2008) stage IB
keratinizing squamous cell carcinoma of the vulva was confirmed.
The specimen was negative for p16 immunohistostaining. The skin
surrounding the cancer lesion displayed non-specific chronic
eczema, without any vulvar intraepithelial neoplasia. One month
after the vulvar surgery, the patient received adjuvant
radiotherapy of the vulvar and inguinal regions bilaterally
(external-beam irradiation, 1.8 Gy/day in 25 fractions over 5
weeks; total radiation dose, 45.0 Gy).
The patient was prescribed an ointment of steroids
and zinc oxide for radiation dermatitis of the vulva and perineum
by the dermatologist. There was no dysuria or dyschezia following
radiotherapy. No stenosis of the vaginal orifice occurred, but the
patient occasionally experienced mild dyspareunia, for which she
used lubricants during coitus. The menstrual cycles following
multimodal therapy were regular.
The patient became pregnant spontaneously in
January, 2012, at the age of 26 years, 9 years after the diagnosis
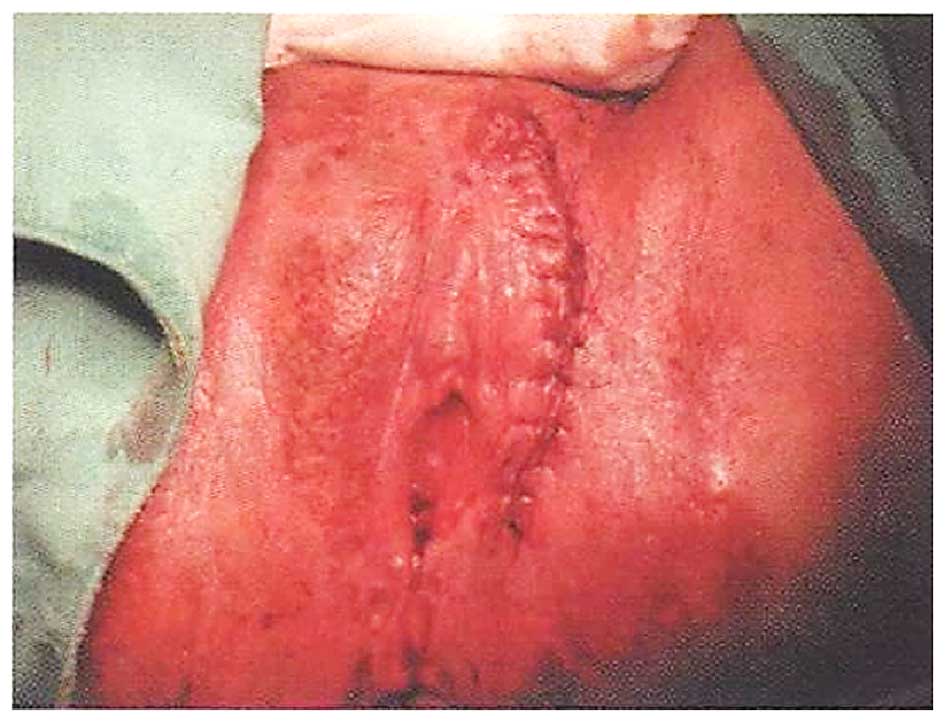
and treatment of her malignancy. Delivery by caesarean section was
planned, due to vulvar scarring extending to the perineum and anal
granulation following multimodal therapy, particularly radiotherapy
(Fig. 5). There was no edema on the
vulva or the lower limbs during pregnancy. The antenatal course was
good, except for mild acute pyelonephritis at 25 weeks of
gestation, which was considered to be unrelated to her previous
treatment. The patient improved after 3 days of intravenous
administration of cefmetazole, and there was no relapse of
pyelonephritis until delivery. In August, 2012, a healthy female
infant was delivered by elective caesarean section under combined
spinal epidural anesthesia, at a gestational age of 38 weeks and 2
days. The amount of intraoperative hemorrhage was 1,926 ml,
containing amniotic fluid. The newborn weighed 2,625 g and was 45.0
cm tall, with an Apgar score of 8 both at 1 and 5 min. The
umbilical cord arterial pH was 7.330 and base excess was −3.1.
Placental pathology revealed no abnormal findings.
In April, 2015 (12 years after the initial diagnosis
of vulvar carcinoma), the patient was alive and well, without signs
of recurrence or metastasis. At the last follow-up, the child was
aged 2 years and exhibited good growth and development, except for
a case of lymphadenitis purulenta of the neck with febrile
convulsions at the age of 1 year.
Discussion
Reports of pregnancy following treatment for vulvar
carcinoma are extremely uncommon. Our case involved a Japanese
woman who became pregnant after having been treated for vulvar
carcinoma. We reviewed 15 cases reported in the literature
(Table I) and all the patients
remained alive (2–7). Only 1 patient had stage IV disease
(metastasis to the pelvic lymph nodes), whereas the others had
stage I disease. Vulvar surgery involved 1 case undergoing wide
local excision and 14 cases of radical vulvectomy. Radiation
therapy was not administered in any of the cases. Recurrence of
vulvar lesions was confirmed in 3 of the 14 cases with a long
follow-up reported in the literature. All 3 recurrent cases were
treated by local excision. Of the 10 cases who were nulliparous at
treatment for vulvar cancer, 8 underwent caesarean section; the
remaining 2 cases had a normal vaginal delivery (premature labor in
1 case and labor induction for intrauterine fetal death in the
other case). Of the 5 parous cases at treatment for malignancy, 4
had a normal vaginal delivery and the remaining patient underwent a
caesarean section. Thus, the mode of delivery following treatment
for vulvar carcinoma may be affected by parity at treatment.
 | Table I.Previous studies reporting pregnancy
and delivery outcomes following treatment for vulvar carcinoma. |
Table I.
Previous studies reporting pregnancy
and delivery outcomes following treatment for vulvar carcinoma.
| First author, year
(Refs.) | Age, years | Parity | Cancer stage | Vulvar surgery | Radiation
therapy | Time from treatment
to pregnancy confirmation | Delivery | Gestation at
delivery | Complication | Birth weight, g | Recurrence | Outcome from
treatment |
|---|
| Rubin, 1953 (6) | 37 | 1 | I | RV | – | 9 months | NVD– | 36 weeks | Premature labor | 2,190 | – | 27 months |
| Dahle, 1959 (3) | 27 | 0 | I | RV | – | 3 years | CS (obstetric) | 38 weeks | Twins, PIH | 3,110/2,380 | – | 5 years |
| Collins, 1960
(4) | 35 | 0 | IV | RV | – | 1 year | CS (2nd-look
laparotomy) | Term | – | 3,500 | – | 11 years |
| Gemmel, 1960
(5) | 19 | 0 | I | RV | – | 24 months | CS (vulvar
scarring) | Preterm | – | 1,930 | – | 108 months |
|
| 28 | 0 | I | RV | – | 19 months | CS (vulvar
scarring) | Term | – | 3,175 | Early SCC at
perineum | 171 months |
|
| 30 | 0 | I | RV | – | 33 months | CS (obstetric) | Term | PIH | 2,495 | – | 70 months |
|
| 30 | 3 | I | RV | – | 19 months | NVD– | Term | PIH | 3,400 | – | 7 years |
|
| 31 | 3 | I | RV | – | 6 months | NVD+ | Term | Proteinuria | 3,685 | – | 66 months |
|
| 32 | 0 | I | RV | – | 13 months | NVD– | 36 weeks | Premature labor,
retained placenta | 2,015 | – | 114 months |
|
| 32 | 1 | I | RV | – | 31 months | NVD+ | Term | Episiotomy
detachmen | 3,345 | – | 73 months |
|
| 33 | 0 | I | RV | – | 2 years | CS (vulvar
scarring) | Term | – | 3,345 | – | 79 months |
|
| 39 | 0 | I | RV | – | 33 months | CS (obstetric) | Preterm | PIH, NRFS,
stillbirth | 1,815 | – | 10 years |
| Palmer, 2009
(2) | 29 | 1 | I | RV | – | 2 years | CS (vulvar
scarring) | 38 weeks | – | NA | AIN | 20 years |
|
| 36 | 0 | I | WLE | – | 20 months | NVD? | 29 weeks | IUFD, labor
induction | NA | VIN | 9 years |
| Arjona, 2011
(7) | 26 | 0 | I | RV | – | 89 months | CS (vulvar
scarring) | 39 weeks | – | NA | NA | NA |
| Present case | 17 | 0 | I | WLE | + | 9 years | CS (vulvar
scarring) | 38 weeks | PN | 2,625 | – | 12 years |
The major reason for caesarean section being
performed in the reported cases was vulvar scarring following
treatment for vulvar carcinoma; however, the obstetrical reasons
were minor. In our review, 5 of 9 cases who had undergone caesarean
delivery had vulvar scarring following radical vulvectomy for
vulvar carcinoma. In contrast to the reported cases, however, the
vulva of our patient was scarred following multimodal therapy for
vulvar cancer, including chemotherapy, surgery and radiotherapy.
For vulvar carcinoma, surgical therapy, including wide local
excision, simple vulvectomy and radical vulvectomy, with or without
bilateral inguinal lymph node dissection, are currently the
standard treatment. In certain cases, however, radiotherapy with or
without chemotherapy may also be performed in addition to surgical
therapy (8,9). Our patient first presented with a
vulvar tumor sized >2 cm and enlarged inguinal lymph nodes on
MRI at the age of 17 years. Following tumor shrinkage with
neoadjuvant chemotherapy, wide local excision was performed.
Subsequently, although the inguinal lymph nodes were negative for
malignancy, adjuvant radiotherapy was administered for potential
future local recurrence and inguinal lymph node metastasis.
Although our case did not undergo major surgery on the vulva,
radiotherapy caused extensive skin scarring, extending from the
vulva to the perineum. Moreover, granulation tissue developed
around the anus. Thus, an elective caesarean delivery was planned
to avoid complicated lacerations during delivery.
In conclusion, pregnancy following treatment for
vulvar carcinoma may be accompanied by post-treatment vulvar
scarring. In addition to radical vulvectomy, multimodal therapy,
particularly radiotherapy, may also cause extensive skin scarring.
When considering radiotherapy for vulvar carcinoma in young
patients, the risk of vulvar scarring and the probability of future
delivery by caesarean section should be explained to the
patients.
References
|
1
|
Hampl M, Deckers-Figiel S, Hampl JA, Rein
D and Bender HG: New aspects of vulvar cancer: Changes in
localization and age of onset. Gynecol Oncol. 109:340–345. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palmer JE and Tidy JA: Pregnancy following
vulvar squamous cell carcinoma: A report of two cases. J Gynecol
Oncol. 20:254–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dahle T: Carcinoma of the vulva and
subsequent successful pregnancy. Acta Obstet Gynecol Scand.
38:448–452. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collins JH, Birch HW, Pailet M and Avent
JK: Pregnancy and delivery following extensive vulvectomy. Am J
Obstet Gynecol. 80:167–171. 1960.PubMed/NCBI
|
|
5
|
Gemmell AA and Haines M: Pregnancy
following radical vulvectomy for carcinoma of the vulva. J Obstet
Gynaecol Br Emp. 67:199–207. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubin A and Lewis GC Jr: Pregnancy and
vaginal delivery following radical surgery for cancer of the vulva;
review of the literature and case report. Am J Obstet Gynecol.
65:1347–1349. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arjona JE, Velasco E, Cervelo P, Espejo E,
Pizarro I, Carrasco S and Castelo-Branco C: Pregnancy following
radical vulvectomy for carcinoma of the vulva: A case report and
literature review. Eur J Obstet Gynecol Reprod Biol. 158:113–114.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mahner S, Jueckstock J, Hilpert F, Neuser
P, Harter P, de Gregorio N, Hasenburg A, Sehouli J, Habermann A,
Hillemanns P, et al: AGO-CaRE 1 investigators: Adjuvant therapy in
lymph node-positive vulvar cancer: The AGO-CaRE-1 Study. J Natl
Cancer Ins. 107:dju4262015. View Article : Google Scholar
|
|
9
|
Matsuo K, Whitman SA, Blake EA, Conturie
CL, Ciccone MA, Jung CE, Takiuchi T and Nishimura M: Feto-maternal
outcome of pregnancy complicated by vulvar cancer: A systematic
review of literature. Eur J Obstet Gyn Reprod Biol. 179:216–223.
2014. View Article : Google Scholar
|