Introduction
Syncope is a common event, accounting for ~1% of all
emergency visits to the hospital (1). There are three major categories of
syncope, namely cardiac, orthostatic and neutrally mediated.
Structural heart disease and orthostatic hypotension in elderly
patients are associated with an increased mortality risk due to
comorbidities (2). Syncopal attacks
should draw clinicians' attention, as they may be associated with
life-threatening events in such patients. Syncope is commonly
attributed to cardiogenic or cerebral factors when considering
differential diagnosis. It has been reported that episodic syncope
is rarely caused by small-cell lung cancer (SCLC) (3), and clinicians should bear this
possibility in mind, as it may be associated with life-threatening
events. However, the mechanism underlying the occurrence of this
type of syncope has not been fully elucidated. Therefore, we herein
report a case treated at the Changhai Hospital of the Second
Military Medical University (Shanghai, China) and review 8 cases
(3–10) of SCLC accompanied with episodic
syncope, with the aim of analysing potential mechanisms associated
with lung tumour anatomy, the neurobiology of SCLC and the inducing
factors of syncope, in order to provide optimal management
recommendations for clinicians to identify and treat episodic
syncope patients.
Case report
A 61-year-old man presented to the outpatient
department with two incidents of recurrent loss of consciousness.
The patient was admitted to our hospital for further evaluation of
the syncopal attacks. Five months prior to admission, the patient
had suffered a syncope after physical exertion; he reported
dizziness and dyspnea, which lasted for 1 h, before he recovered
consciousness spontaneously. One week prior to admission, the
patient suffered a second syncope attack, with similar
symptoms.
On physical examination, the patient appeared
healthy; his pulse rate was 62 beats per minute, his blood pressure
(BP) was 110/70 mmHg, his respiratory rate was 18 breaths per
minute and his temperature was 36.2̊C. His body mass index was 21.8
kg/m2, and the physical examination revealed no
significant abnormalities. The sinus rhythm was found by 24-h
dynamic electrocardiogram (ECG) to be 72 beats per minute, with 16
atrial premature beats, short paroxysmal atrial tachycardia, and 8
multifocal ventricular premature beats. An ultrasonic cardiogram
(UCG) revealed mild mitral and tricuspid regurgitation with normal
heart chamber size and wall movement. Coronary angiography was
performed, as for syncope patients with myocardial ischaemia, with
no obvious abnormalities detected.
The laboratory tests revealed elevated serum tumour
marker levels [progastrinreleasing peptide, 398.47 pg/ml (normal
range, 0–70 pg/ml); carbohydrate antigen 199, 3.46 U/ml (normal
range, 0–37 U/ml); carcinoembryonic antigen, 1.39 ng/ml (normal
range, 0–5 ng/ml); cytokeratin-19 fragment, 4.18 ng/ml (normal
range, 0–2.08 ng/ml); and neuron-specific enolase (NSE), 64.2 µg/l
(normal range, 0–12.5 µg/l)]. Based on these tumour markers, a
contrast-enhanced chest CT scan was scheduled and revealed a mass
measuring 6.8×6.0 cm in the left hilum, accompanied by multiple
enlarged mediastinal lymph nodes and multiple pulmonary bullae of
the upper lobes bilaterally (Fig.
1A). Bronchoscopy revealed an upper left lobe neoplasm with
surrounding infiltrative changes. Pathological diagnosis combined
with immunohistochemistry for SCLC (Fig.
2) showed no evidence of metastasis. Therefore, the diagnosis
was limited-disease SCLC (T4N2M0, IIIB).
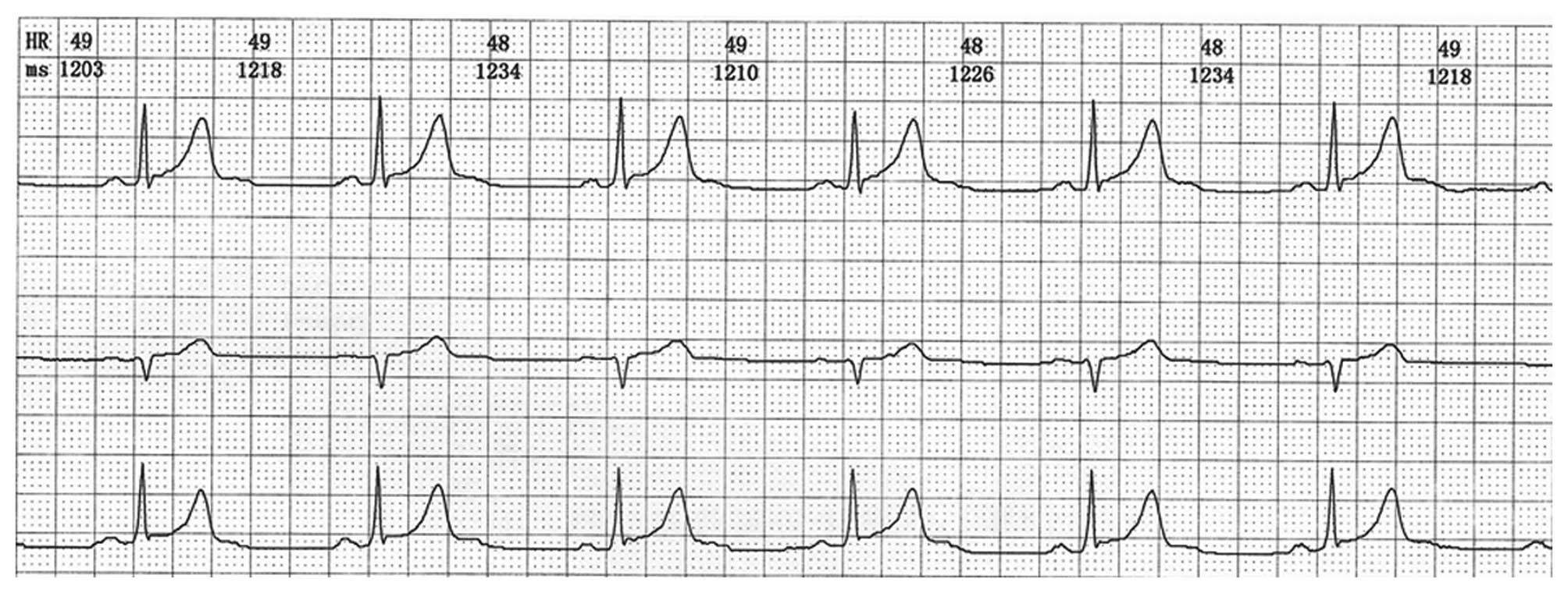
On day 5 of admission, the patient stood up and,
after taking a few steps, he experienced a third episode of
syncope, with sweating and numbness of the extremities. The
duration of the episode was ~1 min, and the BP dropped to 69/39
mmHg. An ECG showed sinus bradycardia at a rate of 48 beats per
minute (Fig. 3). The BP and heart
rate immediately increased following administration of a 0.5-mg
intravenous bolus of atropine and continuous intravenous infusion
of Ringer's lactate. A quick assessment of arterial blood gas,
renal function and serum electrolytes did not reveal significant
abnormalities. On the following day, a magnetic resonance
angiography of the head and neck and a coronary angiography were
performed, but no significant abnormalities were detected.
According to the abovementioned tests, the possibility of a brain
or cardiac origin of the syncope was excluded. Neurogenic syncope
was suspected, associated with the SCLC. The patient was then
administered chemotherapy (carboplatin injection 400 mg on day 1
and etoposide injection 100 mg on days 1–5). The patient exhibited
a partial response (PR) according to the Response Evaluation
Criteria in Solid Tumours (RECIST) guidelines, version 1.1
(11), with the mass measuring
2.0×2.0 cm (Fig. 1B) after 4 cycles
of chemotherapy (carboplatin injection 400 mg on day 1 and
etoposide injection 100 mg on days 1–5). The patient refused
further radiotherapy. After the first course of chemotherapy, no
further syncopal attacks were observed. The patient received 6
courses of chemotherapy in our hospital and he was then discharged.
Follow-up has been performed every 3 months by telephonical
communication, and the disease is currently stable, with occasional
cough and expectoration, for which he receives symptomatic
treatment at a local hospital.
Written informed consent was obtained from the
patient regarding the publication of the case details and
accompanying images.
Discussion
Episodic syncope associated with SCLC is rare, and
the association of episodic syncope with SCLC is not well
understood. The definition of syncope is brief loss of
consciousness with rapid spontaneous recovery, often triggered by
transient reduction in cerebral perfusion (12). Based on the mechanisms involved,
syncope may be divided into three major categories, namely cardiac,
orthostatic and neutrally mediated syncope. Based on the various
triggers, neutrally mediated syncope may be categorized into
carotid sinus, vasovagal and situational syncope. In the present
case, there was no evidence associating the episodic syncope with
cardiac or cerebral mechanisms based on the MRA, UCG and CTA
findings. Moreover, syncope was controlled after the first course
of chemotherapy as the tumour size decreased. Therefore, we
consider that this may have been a type of vasovagal syncope (VVS)
associated with lung cancer.
Using PubMed, a literature search was performed, and
only 8 previously reported cases of SCLC with recurrent syncope
were identified (Table I) (3–10). A
total of 9 cases are included in the review, including the present
case, comprising a total of 8 men and 1 woman, with a mean age of
62.33±2.12 years.
 | Table I.Characteristics of 9 cases of
small-cell lung cancer with episodic syncope. |
Table I.
Characteristics of 9 cases of
small-cell lung cancer with episodic syncope.
| Cases | Age,
years/gender | Complaints | Stage | Location | Size, cm | Inducement | Chemotherapy | Radiation | Relapse therapy | Response | Refs. |
|---|
| Present case | 61/M | Syncope twice | Limited T4N2M0 | LH | 6.8×6.0 | Upright position | EC | − | − | PR | − |
| Case 1 | 64/M | Chest discomfort,
syncope twice | Limited T4N2M0 | LH |
4.2×3.7 | Walking,
coughing | EC | − | + | CR | (3) |
| Case 2 | 57/M | Lightheadedness
syncope once | Limited T2aN2M0 | LUL | 4.6×3.8 | Bending forward | EP | + | − | PR | (4) |
| Case 3 | 57/M | Dyspnea, sweating
syncope twice | Limited T2N2M0 | LM | 3.0×4.0 | Upright position | EP | + | + | PR | (5) |
| Case 4 | 69/M | Recurrent syncopal
episodes | Limited T4M0N0 | LUL | 5.6×4.3 | Pain | CE | − | − | PR | (6) |
| Case 5 | 67/M | Chest pain, syncopal
attack | Limited T1N2M0 | LH | 4.0×3.0 | Upright position | CE | − | − | CR | (7) |
| Case 6 | 66/F | Syncopal attack | Limited T2bN2M0 | LH | 6.0×4.0 | Upright position | CE | + | − | CR | (8) |
| Case 7 | 56/M | Chest pain, syncopal
attack | Limited T4N2M0 | LH | 7.2×2.4 | Standing and lying
down | CE | + | − | PR | (9) |
| Case 8 | 64/M | Chest pain, syncopal
attack | Limited T4N2M0 | LH | 8.0×6.0 | After spirometry | CE | + | − | PR | (10) |
According to Table I,
almost all cases had a tumour located in the left hilum. This
conclusion is similar to the findings reported by Shimizu et
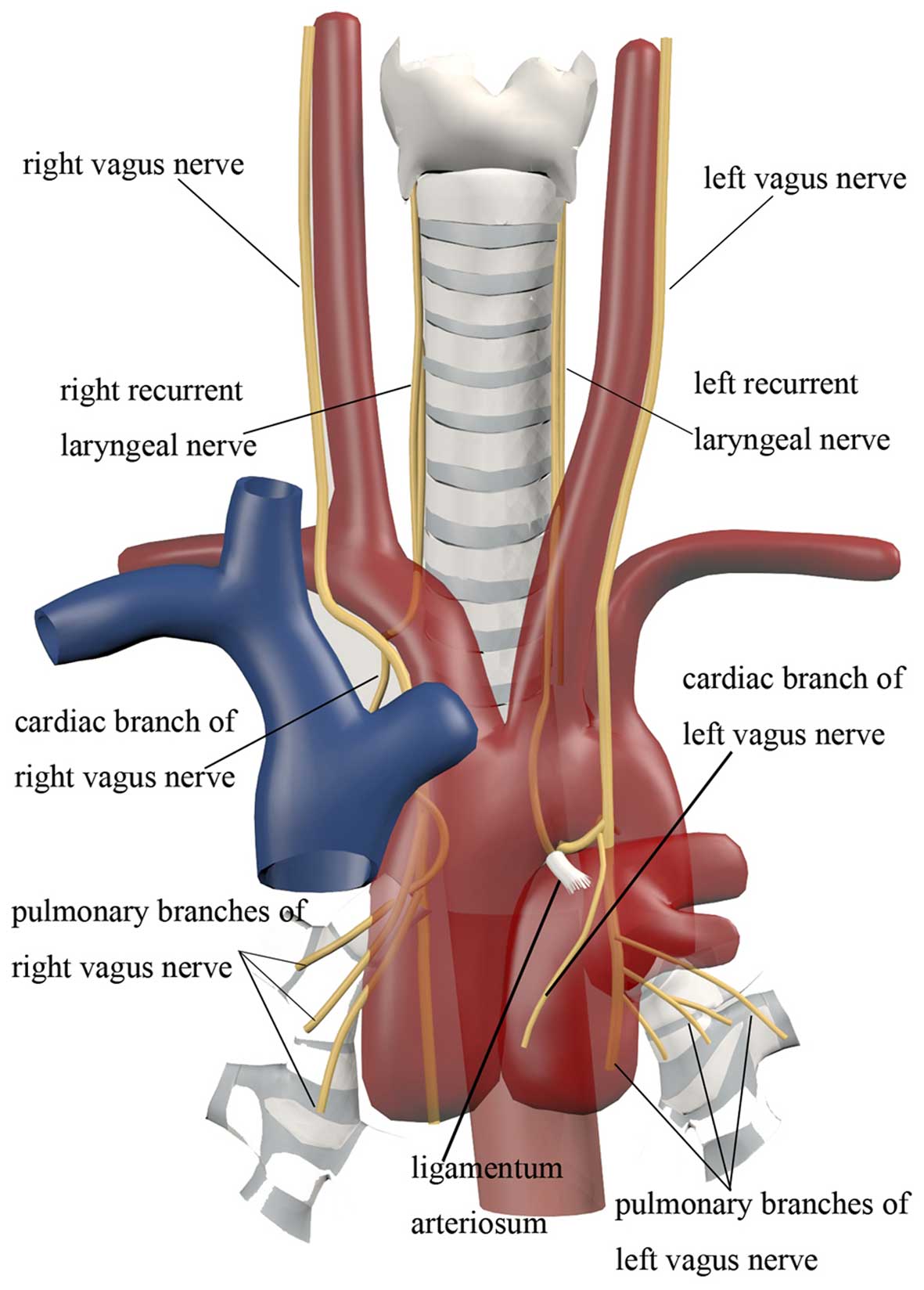
al (10). Yu et al and
Wang et al (13,14) found that the cardiac branches arising
from the left vagus nerve are lower and more closely located to the
hilum compared with those from the right (Fig. 4). The cardiac branch of the left
vagus nerve originates from the middle and lower part of the vagus
nerve, whereas on the right side the cardiac branch mainly
originates from the right recurrent laryngeal nerve, with the point
of origin located higher. This is in agreement with the 88.9%
incidence of the lesion in the left lung mentioned above, and
indicates that the left cardiac branches are more easily
infiltrated by lung cancer masses, which may account for the sinus
bradycardia (48 beats/min) in our patient after the syncopal
attack. Therefore, it is considered that the tumour location
contributes to syncope. However, direct compression usually causes
sustainable sinus bradycardia rather than episodic bradycardia.
Except for anatomical factors, SCLC, particularly of
the oat cell type with neuroendocrine granules, may secrete and
release hormones or peptides and other substances (such as
catecholamines and 5-HT) (15),
which play an important role in the pathogenesis of VVS. NSE was
abnormally elevated in this case, which is associated with a
variant of neurogenic paraneoplastic syndrome. Alboni et al
(16) and Benditt et al
(17) observed that the level of
noradrenaline was higher in patients prior to syncope compared with
that in the control group, and the difference was statistically
significant. In the clinical trials of Theodorakis et al
(18), 83% (105/126) of patients had
a positive response to clomipramine head-up tilt testing
(clom-HUT), which is significantly higher compared with the 41%
positive response rate to conventional HUT, indicating that the
activity of 5-HT was enhanced in VVS patients, resulting in low
blood pressure and sinus bradycardia. Thus, the neuroendocrine
mechanism of SCLC clearly promoted the syncope to a certain extent.
However, in the majority of the cases mentioned above, indicators
of neurogenic paraneoplastic syndrome were not detected. When
dealing with such cases, attention should be paid to changes in
these indicators, which may be associated with the frequency of
syncope.
The syncope symptoms of SCLC may be triggered by the
upright position or severe coughing. Of the 8 patients reviewed in
Table I, in 4 syncope was induced by
the upright position, in 1 by coughing, and in 1 by bending
forward. In the case reported in this study, all the syncope
attacks occurred in the upright position.
The ventricular theory notes that it is easier for
VVS to occur while standing upright when there is ~650 ml of blood
pooled in the peripheral veins, accompanied by decreasing BP
(19). According to the classic
Bezold-Jarisch reflex theory, ventricular hypovolemia results in an
increase in the sympathetic tone, which stimulates the ‘empty
chamber effect’ and, in turn, activates the ventricular
mechanoreceptors (C fibres). Then, with the impulse transmitted to
the brainstem nucleus, the vagal tone is enhanced, leading to
bradycardia and low cerebral perfusion (20). Emotional stress and intense coughing
may also account for VVS (21).
Therefore, the presence of these predisposing factors explains the
characteristics of the onset of syncope to a certain extent, rather
than its persistence.
In conclusion, tumour location, the neuroendocrine
characteristics of SCLC, a change in body position and other
factors, are all associated with syncope in SCLC. The precise role
of those factors in triggering VVS remains unknown. Furthermore,
based on our current understanding of the mechanisms underlying
syncope in SCLC, a recommended diagnostic and treatment approach
for unexplained syncope has been outlined.
Syncopal attack is a common complaint on admission,
particularly for elderly patients. Other causes of syncope are
usually neglected when cardiac and cerebral syncope are initially
suspected. This may delay diagnosis and effective intervention and
may result in patient death.
For any patients with a history of syncope, MRI,
ECG, UCG and CTA are required to identify cerebral and cardiac
diseases. The exclusion of glycopenia, epilepsy, carotid sinus
syndrome, arrhythmia and steno-occlusive vascular disease and
electrolyte disturbances may lead practitioners to consider VVS,
particularly if lesions are found in the left hilum. HUT is
recommended, if possible.
Preventing syncope episodes is crucial for treatment
(22). Instructing the patients to
avoid inducing factors may be beneficial. The sitting and semiprone
position may be helpful during presyncope. If a syncope attack
occurs, the Trendelenburg position may help to maintain the blood
supply to the brain. In 9 reports reviewed herein, all the patients
were evaluated as CR or PR according to the RECIST guidelines.
Additionally, 7 patients experienced no relapse. In those cases, we
consider that standard chemotherapy was crucial for preventing
syncopal attacks in patients with SCLC. It appears to be more
effective to use concurrent chemoradiotherapy for limitedstage
disease. For extensive-stage SCLC, chemotherapy is the first choice
of treatment. Etoposide plus cisplatin or carboplatin is the
standard chemotherapeutic regimen for SCLC. A recent randomized
phase III trial (23) suggested that
irinotecan plus carboplatin in extensive-stage SCLC prolonged the
overall survival compared with oral etoposide plus carboplatin,
without compromising the quality of life (enrolled by NCCN in
2016).
References
|
1
|
Veltmann C, Borggrefe M, Wolpert C and
Schimpf R: Evaluation and management of syncope. Minerva
Cardioangiol. 58:701–715. 2010.PubMed/NCBI
|
|
2
|
Brignole M and Hamdan MH: New concepts in
the assessment of syncope. J Am Coll Cardiol. 59:1583–1591. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang D, Wang L and Yang Z: Recurrent
Syncope Associated with Lung Cancer. Case Rep Med.
2015:3097842015.PubMed/NCBI
|
|
4
|
Koga T, Kaseda S, Miyazaki N, Kawazoe N,
Abe I, Sadoshima S, Onoyama K and Koga T: Neurally mediated syncope
induced by lung cancer-a case report. Angiology. 51:263–267. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li YB, Yao ZH, Cao YJ and Wang R: Lung
cancer: A rare cause of recurrent syncope after pacemaker
implantation. Chin Med J. 126:1992–1993. 2013.PubMed/NCBI
|
|
6
|
Campagna D, Amaradio MD, Battaglia E,
Demma S, Russo C and Polosa R: An uncommon cause of syncope. Intern
Emerg Med. 11:425–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Angelini P and Holoye PY: Neurocardiogenic
syncope and Prinzmetal's angina associated with bronchogenic
carcinoma. Chest. 111:819–822. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demura Y, Mizuno S, Wakabayashi M, Totani
Y, Okamura S, Shiozaki K, Ameshima S, Sasaki F, Ishizaki T and
Miyamori I: Neurally mediated syncope in association with small
cell lung carcinoma. Nihon Kokyuki Gakkai Zasshi. 38:229–232.
2000.(In Japanese). PubMed/NCBI
|
|
9
|
Martin MG, Ardati AK, Dunlay SM, Abernethy
AP and Blazing MA: Small cell lung cancer presenting as a
paraneoplastic syndrome characterized by recurrent episodic
hypotension and bradycardia: Case report. Chest. 131:290–293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimizu K, Yoshii Y, Watanabe S, Hosoda C,
Takagi M, Tominaga T, Kawaishi M and Kuwano K: Neurally mediated
syncope associated with small cell lung cancer: A case report and
review. Intern Med. 50:2367–2369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishino M, Jagannathan JP, Ramaiya NH and
Van den Abbeele AD: Revised RECIST guideline version 1.1: What
oncologists want to know and what radiologists need to know. AJR Am
J Roentgenol. 195:281–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ntusi NA, Coccia CB, Cupido BJ and Chin A:
An approach to the clinical assessment and management of syncope in
adults. S Afr Med J. 105:690–693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu C: Clinical Applied Anatomy of Highly
Selective Vagotomy of Hilum Pulmonis in Human. [D]. Minimally
Invasive Thoracic Surgery of ZhongShan University; Guangzhou: pp.
1–27. 2007
|
|
14
|
Wang J, Li J, Liu G and Deslauriers J:
Nerves of the mediastinum. Thorac Surg Clin. 21:239–249. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Graus F and Dalmau J: Paraneoplastic
neurological syndromes. Curr Opin Neurol. 25:795–801. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alboni P, Bondanelli M, Dinelli M,
Gruppillo P, Franceschetti P, Marchi P and Uberti EC degli: Role of
the serotonergic system in the genesis of vasovagal syncope.
Europace. 2:172–180. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benditt DG, Ermis C, Padanilam B, Samniah
N and Sakaguchi S: Catecholamine response during haemodynamically
stable upright posture in individuals with and without tilt-table
induced vasovagal syncope. Europace. 5:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Theodorakis GN, Livanis EG, Leftheriotis
D, Flevari P, Markianos M and Kremastinos DT: Head-up tilt test
with clomipramine challenge in vasovagal syndrome-a new tilt
testing protocol. Eur Heart J. 24:658–663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fenton AM, Hammill SC, Rea RF, Low PA and
Shen WK: Vasovagal syncope. Ann Intern Med. 133:714–725. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosqueda-Garcia R, Furlan R, Tank J and
Fernandez-Violante R: The elusive pathophysiology of neurally
mediated syncope. Circulation. 102:2898–2906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lurie KG, Benditt D and Mosqueda-Garcia R:
Syncope and the autonomic nervous system. J Cardiovasc
Electrophysiol. 7:760–776. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morillo CA: Evidence-based common sense:
The role of clinical history for the diagnosis of vasovagal
syncope. Eur Heart J. 27:253–254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hermes A, Bergman B, Bremnes R, Ek L,
Fluge S, Sederholm C, Sundstrøm S, Thaning L, Vilsvik J, Aasebø U,
et al: Irinotecan plus carboplatin versus oral etoposide plus
carboplatin in extensive small-cell lung cancer: A randomized phase
III trial. J Clin Oncol. 26:4261–4267. 2008. View Article : Google Scholar : PubMed/NCBI
|