Introduction
Cholangiolocellular carcinoma (CoCC) was first
reported as an adenocarcinoma originating from the smallest
cholangioles and ductules/Canals of Hering (1). CoCC is categorized as a subtype of
intrahepatic cholangiocarcinoma (ICC) based on the criteria of the
World Health Organization (WHO) (2).
However, it is currently classified as a subtype of combined
hepatocellular carcinoma (HCC) and cholangiocarcinoma
(cholangiocellular subtype with stem cell characteristics), based
on the criteria of the 2010 WHO classification (3). In Japan, CoCC was classified as an
independent primary liver cancer in 2008 (4). In a Japanese study, four cases of CoCC
were identified among 708 (0.56%) consecutively resected cases of
primary liver cancer (5).
Due to its low frequency, the clinicopathological
characterisitcs of CoCC have not yet been fully elucidated.
Moreover, only few cases have been reported in the English
literature. Surgery is the only curative treatment for CoCC.
Ariizumi et al reported that hepatectomy in patients with
CoCC produced favorable long-term survival rates due to the less
invasive histopathological characteristics of this tumor (6). However, several cases may be discovered
at an advanced stage; thus, chemotherapy is indispensable for the
treatment of advanced CoCC. S-1 is an orally administered drug that
combines three pharmacological agents: Tegafur (FT), a prodrug of
5-fluorouracil; 5-chloro-2,4-dihydroxypyridine (CDHP), which
inhibits dihydroxypyridine dehydrogenase (DPD) activity; and
potassium oxonate (Oxo), which reduces gastrointestinal toxicity
(7). In a phase II trial for biliary
tract cancer (BTC), including ICC, S-1 monotherapy showed promising
results, with a favorable response rate (35%) and median survival
time (9.4 months), with mild toxicity (8). Therefore, S-1 is considered to be a
promising agent for the treatment of advanced BTC, and has been
approved for advanced BTC by social insurance in Japan. S-1 has
also been demonstrated to have potent antitumor activity against
various solid tumors in clinical studies (9–12).
We herein present a case of CoCC diagnosed following
liver biopsy and effectively treated by S-1 monotherapy.
Case report
A 71-year-old man was admitted to the Kansai Medical
University Takii Hospital (Osaka, Japan) for investigation of
hepatic lesions in a background of alcoholic cirrhosis, Child-Pugh
class B (score 7). The hepatitis C virus antibody and hepatitis B
surface antigen tests were negative. The levels of tumor markers,
including carbohydrate antigen (CA) 19-9 and protein induced by
vitamin K absence/antagonist-II (PIVKA-II) were marginally elevated
(Table I). Ultrasound surveillance
followed by a triple-phase computed tomography (CT) scan revealed
various hypervascular lesions, measuring 54 mm in segment 5/8, 17
mm in segment 4 and 8 mm in segment 2. These tumors showed
enhancement in the early phase of dynamic enhanced CT and
persistent enhancement in the delayed phase. The margins of the
tumors ware not clear. The patient also underwent hepatic
angiography. On common hepatic angiography, the entire tumor showed
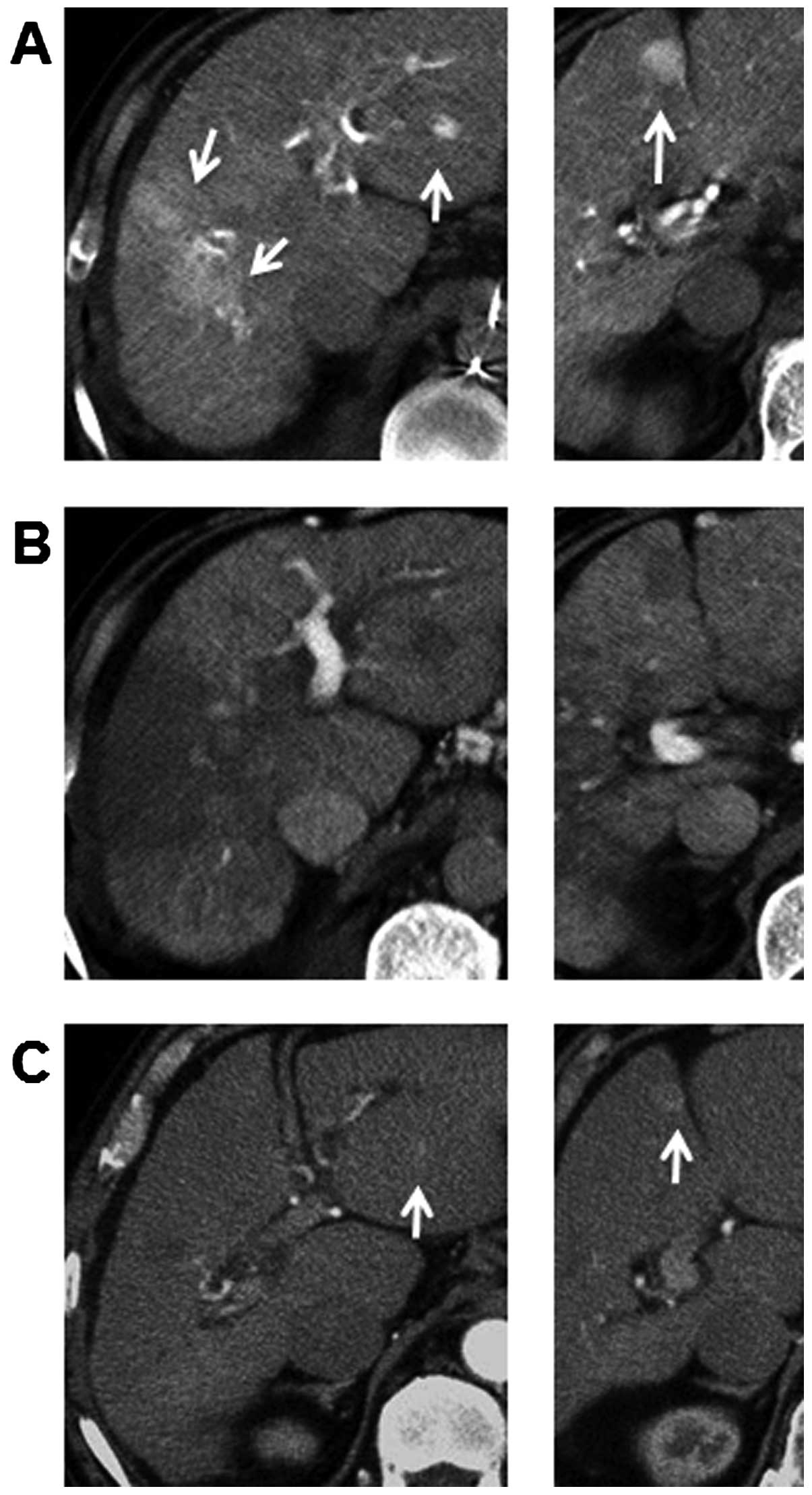
hypervascularity and pooling on the delayed images. CT during
hepatic arteriography (CTA) showed that the tumors were enhanced
(Fig. 1A), whereas the tumors were
depicted as a complete contrast defect using CT during arterial
portography (CTAP) (Fig. 1B).
Transarterial chemoembolization (TACE) was performed for these
hypervascular lesions via the right and left hepatic arteries. A
solution of lipiodol 5 ml, epirubicin 50 mg, and Gelfoam fine
particles was injected. However, 1 week after TACE, a follow-up CT
of the liver revealed the absence of almost any lipiodol
granules.
 | Table I.Laboratory findings. |
Table I.
Laboratory findings.
| Marker | Measurement | Range |
|---|
| Hematology |
|
|
| WBC | 3,200/µl |
|
| RBC |
375×104/µl | ↓ |
| Hb | 13.8 g/dl |
|
| Ht | 40.5% |
|
| Plt |
10.3×104/µl | ↓ |
| Coagulation |
|
|
| PT | 52% | ↓ |
| INR | 1.30 |
|
| Biochemistry |
|
|
| AST | 210 U/l | ↑ |
| ALT | 72 U/l | ↑ |
|
T-Bil | 3.1 mg/dl | ↑ |
|
D-Bil | 1.3 mg/dl | ↑ |
| ALP | 335 U/l |
|
|
γ-GTP | 257 U/l | ↑ |
| LDH | 398 U/l | ↑ |
| TP | 7.3 g/dl |
|
| Alb | 3.8 g/dl |
|
| BUN | 7 mg/dl | ↓ |
|
Creatine | 0.95 mg/dl |
|
|
ICG-R15 | 52.8% | ↑ |
| Tumor markers |
|
|
| AFP | 4.3 ng/ml |
|
|
AFP-L3 | 0.5% |
|
|
PIVKA-II | 69 AU/l | ↑ |
| CEA | 4.4 ng/ml |
|
|
CA19-9 | 46.3 U/ml | ↑ |
| Viral tests |
|
|
|
HCV-Ab | (−) |
|
|
HBs-Ag | (−) |
|
Given the characteristic appearance of well-enhanced
nodules with ill-defined margins, the differential diagnosis
included CoCC, ICC, combined HCC and cholangiocarcinoma, focal
nodular hyperplasia, or nodular transformation. An
ultrasound-guided biopsy of the lesion at segment 5/8 was
performed. The pathological findings revealed that the tumor cells
exhibited mild atypia and invasively proliferated in a ductular
morphology without mucinous fluid production. Immunohistochemical
analysis for hepatocyte paraffin 1 was negative, whereas staining
was positive for cytokeratin (CK) 7, CK19, epithelial cell adhesion
molecule and epithelial membrane antigen (EMA); mucicarmine
staining was negative (Fig. 2). The
staining pattern for EMA showed positivity in the membranous area
of the lumen; this membranous staining pattern of EMA is
characteristic of CoCC (13). Thus,
the tumor was histologically confirmed to be a CoCC.
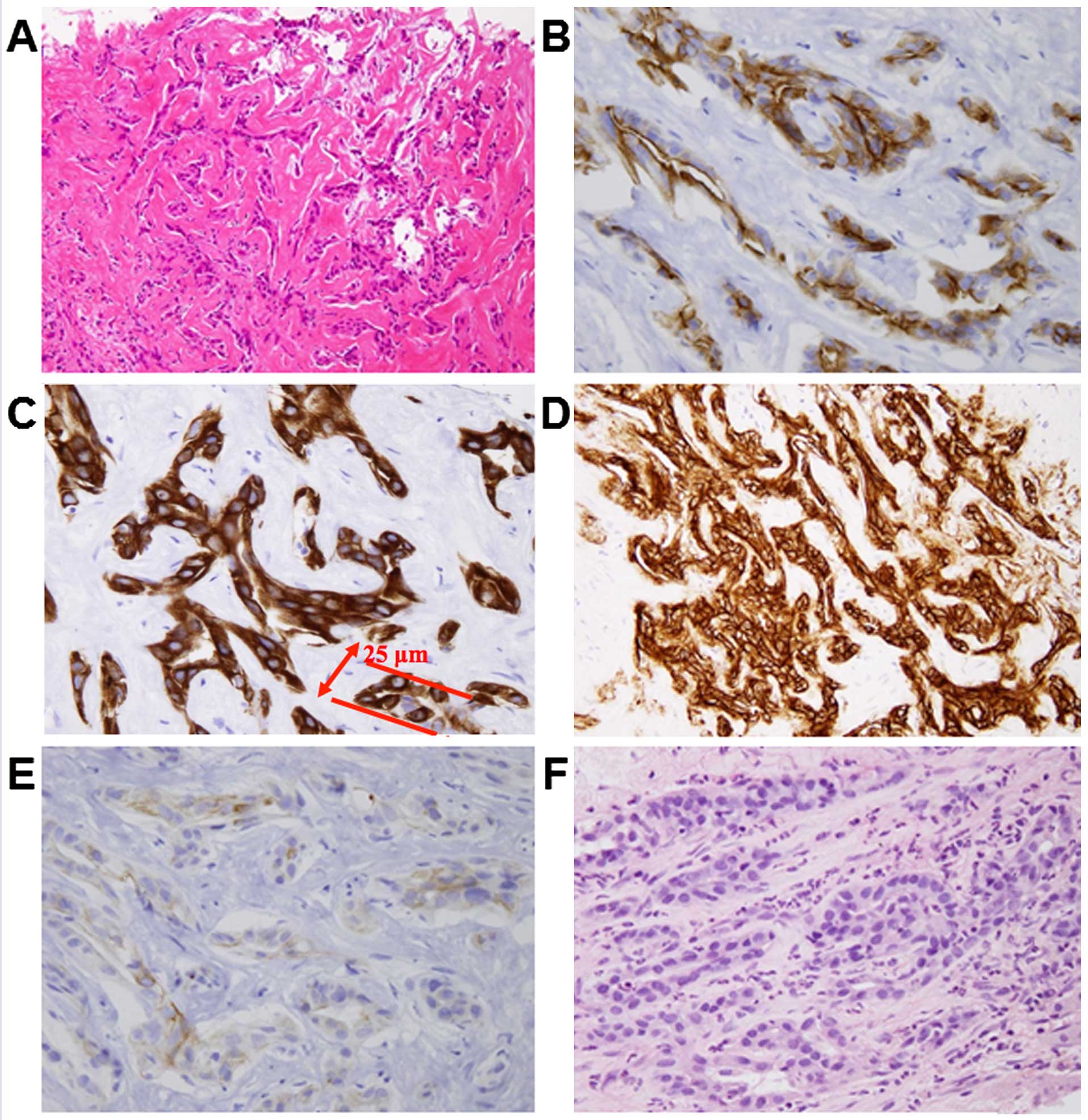 | Figure 2.(A) Microscopic examination revealed
that the tumor was composed of small cells with ovoid nuclei and
eosinophilic cytoplasm, with mild atypia and proliferating in an
anastomosing pattern of Hering's canal-like small glands with a
fibrous stroma. However, a representative cancer duct was 25 µm in
diameter and thicker than a true Hering's canal. There was no
production of mucinous fluid in the ducts (hematoxylin and eosin
staining; magnification, ×50). On immunohistochemistry, the tumor
was positive for (B) epithelial membrane antigen (magnification,
×200), (C) cytokeratin (CK) 7 (magnification, ×200), (D) epithelial
cell adhesion molecule (magnification, ×100) and (E) CK19
(magnification, ×200) and (F) negative for mucicarmine stain
(magnification, ×100). |
The tumor was already at an advanced stage and
surgical treatment was not possible due to insufficient function of
the residual liver. In inoperable cases, palliative treatments are
available, such as intra-arterial chemoembolization and systemic
chemotherapy (14). The patient
refused hospitalization and intra-arterial chemoembolization is
only applied if systemic chemotherapy fails. The most promising
approaches involve the use of single-agent gemcitabine or
combination regimens with gemcitabine and cisplatin (15). However, the patient preferred
systemic chemotherapy using only an oral administration agent.
Thus, S-1 monotherapy was recommended.
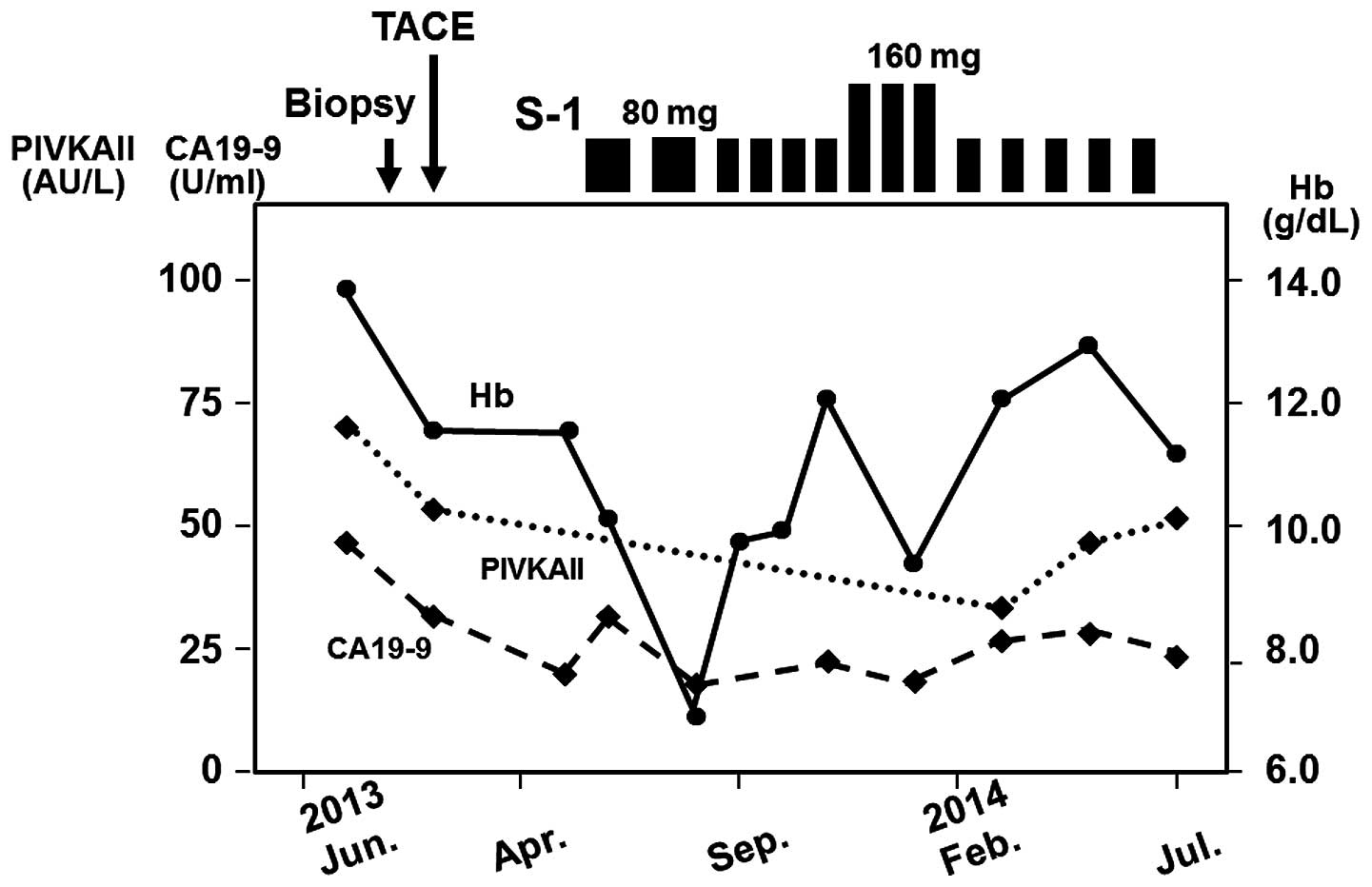
S-1 was initially administered orally at a dose of
80 mg/day in May, 2013. The schedule was 4 weeks of S-1
administration followed by a 2-week drug-free interval. The
clinical course following administration of S-1 is presented in
Fig. 3. Although macrocytic anemia
occurred after 2 cycles, the treatment was again implemented
following an interruption. After a regimen of a 2-week treatment
followed by 1-week rest was performed for 4 cycles, the dosage was
increased to 160 mg/day. However, macrocytic anemia occurred again
after 3 cycles; as a result, a regimen of 2 weeks of treatment at a
dose of 80 mg/day followed by 2 weeks of rest has been used
thereafter. Side effects other than hematological toxicity, such as
skin pigmentation and diarrhea, were not observed for 15 months.
Although tumor marker levels (PIVKAII and CA19-9) were marginally
elevated, their values did not change over the entire course.
Pleural effusion and ascites have not appeared during treatment. In
addition, the tumor has not spread to lymph nodes or other organs.
One year after S-1 monotherapy, a dynamic CT scan showed
disappearance of the intratumoral arterial enhancement in the
lesion at segment 5/8, whereas the lesions at segments 4 and 2 were
both smaller in diameter (Fig.
1C).
Two standard sets of criteria were used to evaluate
the tumor response to S-1 monotherapy: The Response Evaluation
Criteria in Solid Tumors (RECIST) (16) and the modified RECIST (mRECIST)
(17). According to RECIST, the sum
of the longest diameters of all target lesions decreased by 53%,
which was classified as partial response (PR) by the end of the
1-year treatment (Fig. 1C).
According to mRECIST, the sum of the longest diameters of the
enhanced tumor areas during the arterial phase decreased by 84%,
which was also classified as PR (Fig.
1C).
Discussion
This case report describes a successful outcome
using salvage chemotherapy with S-1 for unresectable CoCC in a
patient resistant to prior TACE. As CoCC is an extremely rare
primary malignant tumor in the liver, with a frequency as low as
0.56% in Japan (5), the optimal
treatment option for unresectable CoCCs has not yet been
determined.
The majority of CoCC cases exhibit hyperenhancement
in the early phase of contrast-enhanced CT and hypervascularity on
angiography (18,19). Persistent enhancement in the late
phase of contrast-enhanced CT has often been confirmed. These
characteristics may reflect slow diffusion of the contrast agent
into the fibrotic component of the tumor, which is similarly seen
in cases of ICC (20). The
characteristics of this case were comparable to previous
reports.
As CoCC is considered to be derived from the Canals
of Hering (1), it has been suggested
that CoCC is pluripotent and may develop into HCC and/or ICC
(21). This case contained a CoCC
component only according to histological analysis of the liver
biopsy specimen. As a biopsy specimen was used, the histological
findings may not reflect all the characteristics of the whole tumor
and the other unexamined tumors. However, the imaging findings were
uniform in each tumor and were similar among all tumors. In
addition, the reduction of tumor size was relatively uniform during
chemotherapy.
The origin of CoCC has been controversial since the
first report by Steiner et al (1). CoCC was considered to be derived from
the Canals of Hering. However, the interlobular ducts have also
been hypothesized to be the origin of CoCC (1). According to the recent morphometric and
immunohistochemical studies, CoCC closely resembles interlobular
duct structure (22,23). In addition, the CoCC cases in those
studies did not include HCC components. Although CoCCs were
considered to be pluripotent and may develop into HCC and/or ICC
(21), a considerable number of
CoCCs are pure adenocarcinomas, without evidence of a HCC
component. This fact may be the reason why the Liver Cancer Study
Group of Japan does not recognize CoCC as combined
HCC-cholangiocarcinoma (4). As the
present case only contained a CoCC component and the imaging
findings were uniform in each tumor and similar among all tumors,
this case may be a CoCC of pure adenocarcinoma type. Based on the
cancer duct size (25 µm), our case may originate from interlobular
ducts. However, CoCC is categorized as a subtype of combined
HCC-cholangiocarcinoma in the 2010 WHO classification (3). There are also CoCC cases with an HCC
component. Kondo et al described a small CoCC component
located in a large background area of HCC (23). It may also be hypothesized that only
the CoCC component was biopsied from a combined
HCC-cholangiocarcinoma. As a biopsy specimen was used, the
histological findings may not reflect all the characteristics of
the whole tumor and the other unexamined tumors.
To the best of our knowledge, there is only one
report of correct diagnosis of CoCC using a needle biopsy tissue
sample (24). The majority of
reported cases of CoCC have been diagnosed using pathological
findings from resected tumors (6,19,25).
Therefore, if pathological diagnosis is not available due to
advanced stage, CoCC may be clinically misdiagnosed as HCC due to
the HCC-like early enhancement observed on dynamic CT. Chemotherapy
for CoCC has not yet been reported. Further study is required
regarding the response of CoCC to chemotherapy. Therefore,
clinicians should consider the use of needle biopsy to ensure the
correct diagnosis of atypical hypervascular lesions.
The prognosis of CoCC has not yet been clearly
determined due to its low incidence. Motosugi et al reported
that several-year follow-up studies indicate a quite varied
doubling time of CoCC (26).
Ariizumi et al reported that patients with CoCC exhibited
less invasive histopathological characteristics with favorable
long-term survival (6). However, the
insufficient number of previously reported cases and the diversity
of stage at diagnosis or postoperative treatment make it difficult
to make any generalizations regarding clinical outcome. Further
studies are required to elucidate the prognosis of CoCC.
In conclusion, S-1 monotherapy was generally
well-tolerated and exhibited promising efficacy against advanced
CoCC. Randomized controlled trials are required to establish
effective treatment strategies for unresectable CoCC. However, it
is difficult to recruit patients for trials, as CoCC is very rare.
Multicenter trials are required to establish optimal treatments and
improve the prognosis of patients with this disease.
References
|
1
|
Steiner PE and Higginson J:
Cholangiolocellular carcinoma of the liver. Cancer. 12:753–759.
1959. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamilton SR and Aaltonen LA: Intrahepatic
cholangiocarcinoma. In: World Health Organization Classification of
TumorsPathology and Genetics of Tumours of the Digestive System.
IARC Press; Lyon: 2000
|
|
3
|
Theise ND, Nakashima O, Park YN and
Nakanuma Y: Combined hepatocellular-cholangiocarcinomaWorld Health
Organization Classification of Tumors. WHO classification of tumors
of the digestive system. Bosman FT, Carneiro F, Hruban RH and
Theise ND: IARC Press; Lyon: pp. 225–227. 2010
|
|
4
|
Liver Cancer Study Group of Japan, .
General rules for the clinical and pathological study of primary
liver cancer. 5th. Revised version. Tokyo, Kanehara: pp. 462009
|
|
5
|
Shiota K, Taguchi J, Nakashima O,
Nakashima M and Kojiro M: Clinicopathologic study on
cholangiolocellular carcinoma. Oncol Rep. 8:263–268.
2001.PubMed/NCBI
|
|
6
|
Ariizumi S, Kotera Y, Katagiri S, Nakano
M, Nakanuma Y, Saito A and Yamamoto M: Long-term survival of
patients with cholangiolocellular carcinoma after curative
hepatectomy. Ann Surg Oncol. 21:(Suppl 3). S451–S458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miura K, Shirasaka T, Yamaue H and Sasaki
I: S-1 as a core anticancer fluoropyrimidine agent. Expert Opin
Drug Deliv. 9:273–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furuse J, Okusaka T, Boku N, Ohkawa S,
Sawaki A, Masumoto T and Funakoshi A: S-1 monotherapy as first-line
treatment in patients with advanced biliary tract cancer: A
multicenter phase II study. Cancer Chemother Pharmacol. 62:849–855.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi
K, Mitachi Y and Taguchi T: Late phase II study of novel oral
fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1
M otastat potassium) in advanced gastric cancer patients. Eur J
Cancer. 34:1715–1720. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohtsu A, Baba H, Sakata Y, Mitachi Y,
Horikoshi N, Sugimachi K and Taguchi T: Phase II study of S-1, a
novel oral fluorophyrimidine derivative, in patients with
metastatic colorectal carcinoma. S-1 Cooperative Colorectal
Carcinoma Study Group. Br J Cancer. 83:141–145. 2000.PubMed/NCBI
|
|
11
|
Kawahara M, Furuse K, Segawa Y, Yoshimori
K, Matsui K, Kudoh S, Hasegawa K and Niitani H: S-1 Cooperative
Study Group (Lung Cancer Working Group): Phase II study of S-1, a
novel oral fluorouracil, in advanced non-small-cell lung cancer. Br
J Cancer. 85:939–943. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saeki T, Takashima S, Sano M, Horikoshi N,
Miura S, Shimizu S, Morimoto K, Kimura M and Taguchi T: A late
phase II clinical study of S-1 in patients with progressed,
refractory breast cancer. Cancer & chemotherapy. 31:539–547.
2004.(In Japanese).
|
|
13
|
Nakano M: Histopathological characteristic
of cholangiolocellular carcinoma. J Biliary Tract Pancreas.
25:343–349. 2004.
|
|
14
|
Vogl TJ, Schwarz W, Eichler K, Hochmuth K,
Hammerstingl R, Jacob U, Scheller A, Zangos S and Heller M: Hepatic
intraarterial chemotherapy with gemcitabine in patients with
unresectable cholangiocarcinomas and liver metastases of pancreatic
cancer: A clinical study on maximum tolerable dose and treatment
efficacy. J Cancer Res Clin Oncol. 132:745–755. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park I, Lee JL, Ryu MH, Kim TW, Lee S
Sook, Park D Hyun, Soo Lee S, Wan Seo D, Koo Lee S and Kim MH:
Prognostic factors and predictive model in patients with advanced
biliary tract adenocarcinoma receiving first-line palliative
chemotherapy. Cancer. 115:4148–4155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuda M, Hara M, Suzuki T, Kono H and
Fujii H: Synchronously resected double primary hepatic cancers -
hepatocellular carcinoma and cholangiolocellular carcinoma. J
Hepatobiliary Pancreat Surg. 13:571–576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanamoto M, Yoshizumi T, Ikegami T, Imura
S, Morine Y, Ikemoto T, Sano N and Shimada M: Cholangiolocellular
carcinoma containing hepatocellular carcinoma and cholangiocellular
carcinoma, extremely rare tumor of the liver: A case report. J Med
Invest. 55:161–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lacomis JM, Baron RL, Oliver JH III,
Nalesnik MA and Federle MP: Cholangiocarcinoma: Delayed CT contrast
enhancement patterns. Radiology. 203:98–104. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Komuta M, Spee B, Borght S Vander, De Vos
R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H, et
al: Clinicopathological study on cholangiolocellular carcinoma
suggesting hepatic progenitor cell origin. Hepatology.
47:1544–1556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maeno S, Kondo F, Sano K, Takada T and
Asano T: Morphometric and immunohistochemical study of
cholangiolocellular carcinoma: Comparison with non-neoplastic
cholangiole, interlobular duct and septal duct. J Hepatobiliary
Pancreat Sci. 19:289–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kondo F and Fukusato T: Pathogenesis of
cholangiolocellular carcinoma: Possibility of an interlobular duct
origin. Intern Med. 54:1685–1694. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Joshita S, Ichijo T, Suzuki F, Yokoyama T,
Sugiyama Y, Fukushima M, Kamijo A, Komatsu M, Umemura T, Yoshizawa
K, et al: A case of well-differentiated cholangiolocellular
carcinoma visualized with contrast-enhanced ultrasonography using
Sonazoid. Hepatol Res. 39:207–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maeda T, Hashimoto K, Ishida T, Yamashita
Y, Saeki H, Kawanaka H, Uchiyama H, Ikeda T, Tsujitani S and
Maehara Y: Repeat hepatectomy for intrahepatic recurrence of
cholangiolocellular carcinoma. Fukuoka Acta Medica. 104:564–568.
2013.
|
|
26
|
Motosugi U, Ichikawa T, Nakajima H, Araki
T, Matsuda M, Suzuki T, Fujii H, Nakazawa T and Yamaguchi H:
Cholangiolocellular carcinoma of the liver: Imaging findings. J
Comput Assist Tomogr. 33:682–688. 2009. View Article : Google Scholar : PubMed/NCBI
|