Introduction
Anti-N-methyl-D-aspartate-receptor (NMDAR)
encephalitis is an autoimmune disorder with a wide spectrum of
neuropsychiatric symptoms, which was first described in 2005 by
Vitalini et al (1). It has
recently been demonstrated that this potentially fatal disease is
significantly underdiagnosed and may contribute to the majority of
cases of non-infectious encephalitis of previously unknown
aetiology (2). Vitalini et al
(1) retrospectively analysed >500
cases of intensive care unit (ICU) admission and identified 7
patients with encephalitic signs (psychiatric and cognitive
disturbances, as well as focal or generalized seizures progressing
into status epilepticus), cerebrospinal fluid (CSF) inflammation
and exclusion of bacterial or viral infection, and in 6 cases the
presence of anti-NMDAR antibodies was confirmed. A positive serum
or CSF sample screening for antibodies to the NMDAR subunit remains
the gold-standard in diagnosing the disease and must be performed
in all patients presenting with an acute onset of psychiatric
symptoms with atypical features or unusual movements. However,
emerging data exists to suggest that electroencephalograms (EEG)
can also be very useful in aiding clinicians to diagnose anti-NMDAR
encephalitis (3).
Over the past years, increasing awareness of
autoimmune mediated forms of encephalitis with antibodies against
neuronal surface antigens have led to an improvement in prognosis,
predominantly due to wider application of immunomodulatory
therapies. Notably, despite major progress in understanding the
pathophysiology of anti-NMDAR encephalitis, there remains a clear
requirement for a good quality data regarding the optimal treatment
of the disease, predominantly since the type of immunotherapy that
is most effective in controlling the symptoms of the disease
remains a matter of debate. Numerous patients, particularly with a
prolonged or severe form of the disease, do not respond to
first-line immunotherapy [steroids or intravenous immunoglobulins
(IVIg)] and may require therapeutic plasma exchange (TPE), which is
commonly used to treat a number of neurological conditions,
including Guillain-Barré syndrome, myasthenia gravis, chronic
inflammatory demyelinating polyneuropathy, Lambert-Eaton syndrome,
multiple sclerosis, neuromyelitis optica, paraproteinemic
polyneuropathy, Sydenham's chorea and natalizumab-associated
progressive multifocal leukoencephalopathy (4). Despite the increasing number of
potential indications for TPE in the treatment of neurological
disorders, the proven efficacy, side effects and costs must be
taken into consideration prior to a decision being made to
implement this therapy.
Case report
A 23-year-old female was admitted to a psychiatric
ward, presenting with acute confusion, agitation and behavioural
changes. The initial diagnosis was the first episode of
schizophrenia or schizoaffective disorder. Her past medical history
was non-significant and no prior psychiatric or psychological
problems were reported. An episode of an upper respiratory tract
infection, which preceded the psychotic symptoms by few days was
notable. Upon admission, agitation and restlessness were observed,
followed by progressive mutism and somnolence, which were the
predominant symptoms of the disease within 5 days. The patient was
administered standard antipsychotic treatment during her entire
stay in the psychiatric ward, which included haloperidol,
olanzapine and aposulpiryd, without any improvement in her
psychiatric condition. On day 5, the patient's neurological
condition significantly deteriorated; decreased level of
consciousness and loss of muscle tone were observed. Involuntary
movements of upper limbs, jaw and eyes were also noticed, as well
as clonic seizures, which were treated with intravenous diazepam.
The initial diagnosis was changed to infectious encephalitis and
the patient was transferred to a neurological ward at the state
hospital, where CSF and blood samples were obtained and imaging
studies of the brain, as well as EEG were performed (Fig. 1).
Results of the CSF analysis revealed a lymphocytic
pleocytosis (60 white cells/µl), a normal protein level (28.3
mg/dl; normal value range: 15–45 mg/dl) and a normal glucose level
(81 mg/dl). EEG recordings revealed generalized rhythmic delta
activity with superimposed rhythmic beta frequency activity
(‘extreme delta brush’) (5).
Computed tomography (CT) of the head revealed no pathological
changes. The magnetic resonance imaging (MRI), which was performed
upon admission to the neurological ward, revealed only two
hyperintense lesions in subcortical white matter in the frontal
lobes on T2/FLAIR. Additionally, initial focal seizures evolved
into generalized tonic-clonic repetitive seizures, which did not
respond to various anticonvulsants, including clonazepam, sodium
valproate, phenytoin and carbamazepine. Subsequently, treatment
with acyclovir was initiated for presumptive viral encephalitis.
Immunotherapy was also implemented and the patient received a daily
dose of 20 g of IVIg for 3 consecutive days. The CSF antibody tests
were negative for Epstein-Barr virus, cytomegalovirus, human
immunodeficiency virus, herpes simplex virus and varicella-zoster
virus. The patient's condition deteriorated further; she developed
a refractory status epilepticus with concomitant respiratory
failure and autonomic instability and was referred to a tertiary
ICU on the 27th day of hospitalization.
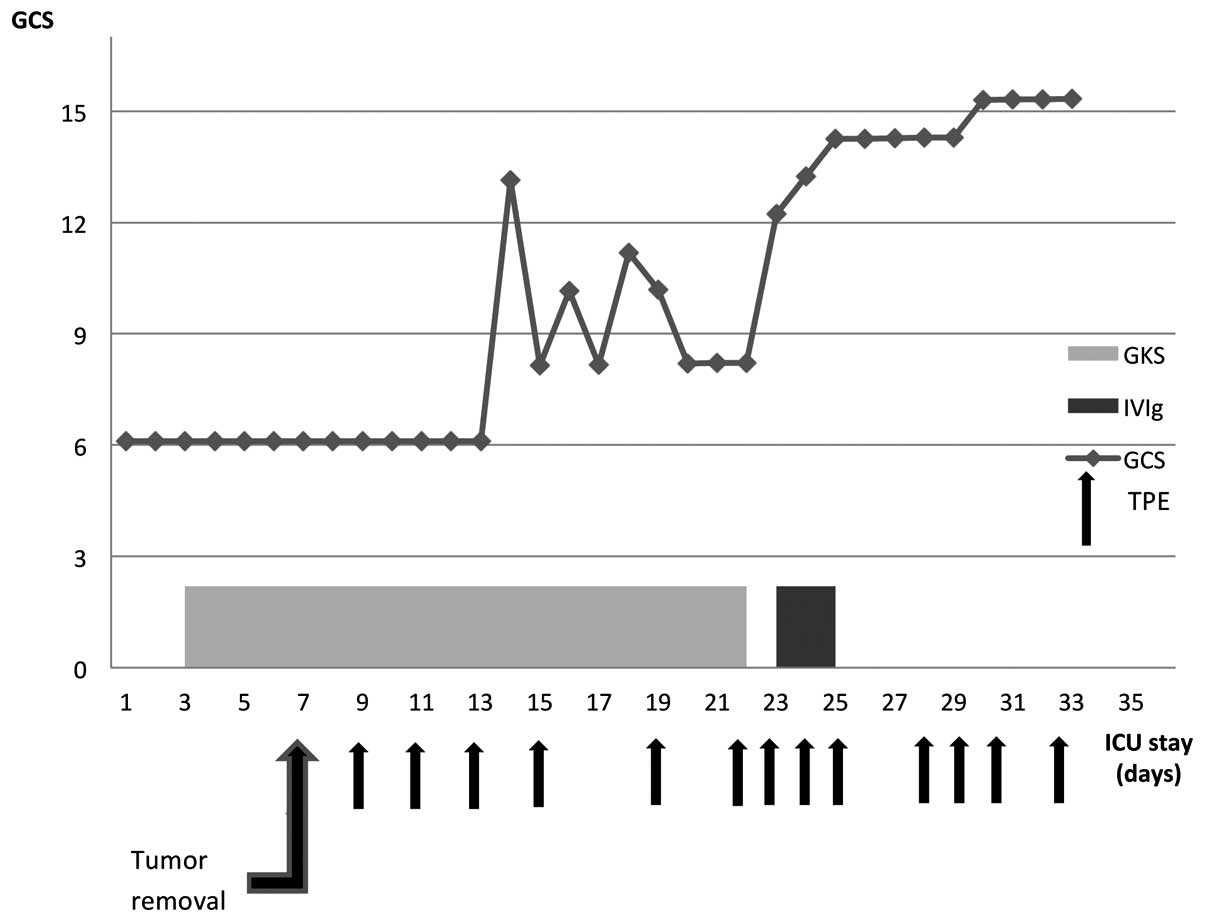
The clinical course and the treatment protocol are
summarized in Fig. 2. Upon admission
to the ICU, the patient was comatose with a Glasgow Coma Scale
(GCS) score of 6. Neurological examination revealed that the
patient exhibited spontaneous and provoked involuntary movements of
the eyes, jaw, and upper and lower limbs, despite receiving a
constant infusion of clonazepam and thiopental. Excessive
salivation was also noted. The patient's trachea was intubated and
mechanical ventilation was initiated. Sedatives (propofol,
midazolam) and opioids (remifentanil) were given in continuous
infusion to facilitate mechanical ventilation, yet orofacial and
limb dyskinesia were still present and difficult to control despite
increasing infusion rates of propofol and midazolam. The
subsequently obtained test results of CSF and blood were irrelevant
with only slightly elevated white bloodcell count. Results of both
blood and CSF cultures remained negative, and repeated CT scan of
the brain was also normal. Since the infectious background of the
disease was unable to be confirmed, autoimmunological encephalitis
was suspected and high dose corticosteroids (methyloprednisolone 1
g daily for 6 days, followed by prednisone 40 mg twice daily) were
administered, without any improvement in the patient's neurological
status. On the 7th day of her stay in the ICU, a CT scan of the
abdomen and the pelvis revealed an ovarian cyst. Additionally, a
transvaginal ultrasound confirmed a left ovarian mass suggestive of
a teratoma. Laparoscopic oophorectomy was immediately performed.
The blood and CSF samples were collected to search for neuronal
surface antibodies and the anti-NMDAR antibodies were identified in
the CSF (titers, 1:3.2), but not in the plasma. The diagnosis of
anti-NMDAR encephalitis was made based on the above results. Two
days later, on the 36th day of hospitalization, TPE was initiated
and after the third course, the patient's neurological condition
markedly improved (an increase of GCS score from 6 to 11). She
gradually started to open her eyes, obey simple commands and answer
uncomplicated questions. Additionally, involuntary movements
disappeared, yet salivation, autonomic instability and muscle
weakness were still observed. The courses of TPE were continued to
a total of 13 courses during her stay in the ICU. Meanwhile,
another course of supplementary immunotherapy with IVIg (20 g every
second day) was implemented due to its proven position as a first
line treatment in the armamentarium against autoimmunological
encephalitis. The relatively high frequency of TPE resulted in
haemodynamic instability and coagulopathy, which required plasma
transfusion; therefore, the intervals between TPE were increased
from 1 to 3 days and the anticoagulant was changed from
unfractionated heparin to citrate. The autonomic dysfunction
persisted despite obvious improvement in cognitive status and
involved severe bradycardia and hypersalivation, which made all the
tracheal extubation efforts futile. Therefore, on the 25th day of
the ICU stay, a tracheostomy was performed. The patient was
subsequently weaned from mechanical ventilation and transferred to
a neurological ward to continue intensive rehabilitation. She was
discharged in a good clinical condition nearly 3 months after the
onset of the disease. The follow-up assessment was performed 3
months following discharge. The patient's neurological state was
tested with modified Rankin Scale (mRS) and she gained 1 point. She
reported no significant disability and was able to perform all
usual activities. The only neurological sequel included parasomnia
and complete loss of memory during the entire hospitalization
period.
Discussion
The present case study described a clinical course
of anti-NMDAR encephalitis, which is one of the central nervous
system (CNS) diseases, where the presence of the neuronal surface
antibodies can be demonstrated (6).
This relatively uncommon disorder has a typical sequential
presentation: sudden onset with prodromal, fever-like symptoms,
followed by a psychiatric disorders, decreased level of conscious
accompanied by intractable focal and clonic seizures, dyskinesias
and autonomic instability (7). In
the present case, all of the above-mentioned symptoms were
observed, so the clinical course of the anti-NMDAR encephalitis was
typical. In addition, the patient was a young female, which is also
a common feature of this type of autoimmunological encephalitis,
since this patient population accounts for ~80% of reported cases
(8). Furthermore, the coexistence of
an ovarian tumour, which was confirmed to be a teratoma in this
case, is also a typical for anti-NMDAR encephalitis. This type of
encephalitis is suggested to be strongly associated with
malignancies, including ovarian, mediastinal and testicular
teratomas, Hodgkin's lymphoma, small cell lung carcinoma, and
neuroblastomas. However, more recent studies have challenged these
data showing that up to 80% of patients may present with a
non-paraneoplastic type of the disease, making the diagnosis even
more difficult (9). When anti-NMDAR
is suspected the diagnostic tests typically include detection of
NMDAR autoantibodies in CSF and/or in the serum as the core of
diagnosis, predominantly because other laboratory tests and imaging
studies of the brain appear to be irrelevant (10,11). On
the other hand, MRI scans of the patient's CNS revealed multifocal
subcortical white matter lesions in T2/FLAIR, which were also
described by other authors and may be concomitant with anti-NMDAR
encephalitis (9).
There is ongoing debate as to whether serum or CSF
must be tested for in the presence of anti-NMDAR antibodies. In the
present case, high levels of anti-NMDAR antibodies were detected in
the CSF, but not in the plasma. The association between the
prodromal flu-like symptoms and the antibodies against NMDAR is
also a matter of debate. Certain authors emphasise the connection
between a viral infection and damage of the blood-brain-barrier,
which facilitates transfer of NMDAR autoantibodies to the CNS,
whilst others claim that flu-like symptoms are only a part of an
early immunological activation (12). Nevertheless, when anti-NMDAR
encephalitis is suspected it appears absolutely crucial to search
for the antibodies in both serum and CSF, yet other more readily
available diagnostic measures must also be implemented in order to
obtain the final diagnosis. In the present case, a retrospective
analysis of the first EEG recordings revealed an unique
electrographic pattern, which is referred to as “extreme delta
brush”, due to of its resemblance to waveforms observed in
premature infants (13). Although
the specificity of this finding remains unclear, its presence is
usually associated with a more prolonged and severe course of
illness. An EEG maybe extremely valuable in distinguishing between
encephalitis and a primary psychiatric disorder, since a vast
majority of patients with anti-NMDAR encephalitis exhibit a
non-specific slowing at a certain stage during the illness. In
addition, characteristic pattern of generalized rhythmic delta
activity with superimposed rhythmic beta frequency activity may
appear, which was described in a large series of patients unique to
this disorder (8).
Treatment of anti-NMDAR encephalitis remains
challenging, since no comprehensive guidelines have been published
to date. The majority of authors agree that treatment must target
both the cause and the clinical consequences of the encephalitis.
It is generally accepted that the anti-NMDAR encephalitis treatment
is much more effective in patients who have an underlying tumour
removed and there are numerous cases described in which ovarian
teratomas were discovered years after initial onset of symptoms,
particularly in patients who experienced a slow recovery (14). Certain authors advocate early
oophorectomy even in cases when the presence of an ovarian tumour
cannot be confirmed in imaging studies. In a case described by
Peery et al (14),
postoperative biopsy revealed an occult teratoma, and the surgical
removal of the tumour resulted in an improvement of clinical
symptoms. In the majority of cases, immunotherapy is the first-line
treatment and typically includes corticosteroids, IVIg and TPE,
administered alone or in combination (15). The second-line treatment includes
rituximab and cyclophosphamide, and is used predominantly in
patients who either exhibit a delayed diagnosis or did not have an
underlying malignancy (16). It is
worth mentioning that the role of specific treatment options
remains a matter of debate and numerous authors provide conflicting
data on the subject, with special regards to the role of TPE, which
in the opinion of Dalmau et al (17) should not be used routinely. On the
other hand, numerous recently published case studies, where
implementation of TPE was associated with an improvement of
clinical status, exist (18,19). In a recent paper published by DeSena
et al (20), the efficacy of
intravenous methylprednisolone administered alone or following TPE
was compared in the treatment of anti-NMDAR receptor encephalitis.
The obtained results were clearly in favour of administering
steroids only after TPE was performed in the studied patient
population.
In the present case, the patient was initially
treated in the neurological ward with the use of corticosteroids
(methylprednisolone) and IVIg due to suspected infectious
encephalitis. This first-line immunosuppressive treatment failed to
produce any improvement of the patient's clinical condition.
Similarly, the second course of high-dose corticosteroids
administered in the ICU was not effective. The patient's clinical
condition only improved following the third course of TPE, which is
in line with the case study published by Nunez-Enamorado et
al (21). By contrast, clinical
improvement in cases of anti-NMDAR encephalitis is not always
achieved with TPE (22,23). According to the guidelines published
by the American Society for Apheresis, the use of TPE must be
considered as a third-line treatment in paraneoplastic neurological
syndromes (grade 2C) (24).
In conclusion, NMDAR encephalitis is a potentially
reversible disorder with a good clinical outcome if diagnosed and
treated promptly. A multimodal immunosuppresive therapy may result
in an improvement in neurological symptoms in >60% of patients
(25). In the present case, a
long-term follow-up examination revealed a good clinical outcome,
without any major neurological or psychiatric complications
following the anti-NMDAR encephalitis. A multidisciplinary team,
including psychiatrists, neurologists and intensivists, must be
involved in the process of recognition and management of the
disease. The gold standard for diagnosing anti-NMDAR encephalitis
is still detecting the antibodies in either the CSF or plasma;
however, other diagnostic measures, including EEG or MRI may also
aid clinicians in obtaining the final diagnosis. Tumour removal and
pharmacotherapy remain the first-line treatment in the majority of
cases, yet TPE must also be considered in the clinician's
armamentarium, particularly in cases where initial treatment has
failed. Available data remains ambiguous and therefore there
remains an urgency for good quality clinical trials.
References
|
1
|
Vitaliani R, Mason W, Ances B, Zwerdling
T, Jiang Z and Dalmau J: Paraneoplastic encephalitis, psychiatric
symptoms, and hypoventilation in ovarian teratoma. Ann Neurol.
58:594–604. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prüss H, Dalmau J, Harms L, Höltje M,
Ahnert-Hilger G, Borowski K, Stoecker W and Wandinger KP:
Retrospective analysis of NMDA receptor antibodies in encephalitis
of unknown origin. Neurology. 75:1735–1739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barry H, Byrne S, Barrett E, Murphy KC and
Cotter DR: Anti-N-methyl-d-aspartate receptor encephalitis: Review
of clinical presentation, diagnosis and treatment. BJPsych Bull.
39:19–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gwathmey K, Balogun RA and Burns T:
Neurologic indications for therapeutic plasma exchange: 2013
update. J Clin Apher. 29:211–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmitt SE, Pargeon K, Frechette ES,
Hirsch LJ, Dalmau J and Friedman D: Extreme delta brush: A unique
EEG pattern in adults with anti-NMDA receptor encephalitis.
Neurology. 79:1094–1100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zuliani L, Graus F, Giometto B, Bien C and
Vincent A: Central nervous system neuronal surface antibody
associated syndromes: Review and guidelines for recognition. J
Neurol Neurosurg Psychiatry. 83:638–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Granerod J, Ambrose HE, Davies NW, Clewley
JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ,
Solomon T, et al: UK Health Protection Agency (HPA) Aetiology of
Encephalitis Study Group: Causes of encephalitis and differences in
their clinical presentations in England: A multicentre,
population-based prospective study. Lancet Infect Dis. 10:835–844.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Titulaer MJ, McCracken L, Gabilondo I,
Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I,
Martinez-Hernandez E, et al: Treatment and prognostic factors for
long-term outcome in patients with anti-NMDA receptor encephalitis:
An observational cohort study. Lancet Neurol. 12:157–165. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Irani SR, Bera K, Waters P, Zuliani L,
Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, et
al: N-methyl-D-aspartate antibody encephalitis: Temporal
progression of clinical and paraclinical observations in a
predominantly non-paraneoplastic disorder of both sexes. Brain.
133:1655–1667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dalmau J, Gleichman AJ, Hughes EG, Rossi
JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R and
Lynch DR: Anti-NMDA-receptor encephalitis: Case series and analysis
of the effects of antibodies. Lancet Neurol. 7:1091–1098. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motta E, Gołba A, Kazibutowska Z, Huć M
and Stęposz A: Anti-NMDA receptor encephalitis-case report. Neurol
Neurochir Pol. 46:288–293. 2012.(In Polish). PubMed/NCBI
|
|
12
|
Hammer C, Stepniak B, Schneider A, Papiol
S, Tantra M, Begemann M, Sirén AL, Pardo LA, Sperling S, Jofrry S
Mohd, et al: Neuropsychiatric disease relevance of circulating
anti-NMDA receptor autoantibodies depends on blood-brain barrier
integrity. Mol Psychiatry. 19:1143–1149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iizuka T, Sakai F, Ide T, Monzen T, Yoshii
S, Iigaya M, Suzuki K, Lynch DR, Suzuki N, Hata T, et al: Anti-NMDA
receptor encephalitis in Japan: Long-term outcome without tumor
removal. Neurology. 70:504–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peery HE, Day GS, Doja A, Xia C, Fritzler
MJ and Foster WG: Anti-NMDA receptor encephalitis in children: The
disorder, its diagnosis, and treatment. Handb Clin Neurol.
112:1229–1233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mann AP, Grebenciucova E and Lukas RV:
Anti-N-methyl-D-aspartate-receptor encephalitis: Diagnosis, optimal
management, and challenges. Ther Clin Risk Manag. 10:517–525. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Florance NR, Davis RL, Lam C, Szperka C,
Zhou L, Ahmad S, Campen CJ, Moss H, Peter N, Gleichman AJ, et al:
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children
and adolescents. Ann Neurol. 66:11–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dalmau J, Lancaster E, Martinez-Hernandez
E, Rosenfeld MR and Balice-Gordon R: Clinical experience and
laboratory investigations in patients with anti-NMDAR encephalitis.
Lancet Neurol. 10:63–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Gao D, Ye H and Su Y: [Safety of
plasma exchange therapy in patients with anti-NMDA receptor
encephalitis. Zhonghua Yi Xue Za Zhi. 95:1505–1508. 2015.(In
Chinese). PubMed/NCBI
|
|
19
|
Shahani L: Steroid unresponsive anti-NMDA
receptor encephalitis during pregnancy successfully treated with
plasmapheresis. BMJ Case Rep 2015 (apr29 1). 292015.
|
|
20
|
DeSena AD, Noland DK, Matevosyan K, King
K, Phillips L, Qureshi SS, Greenberg BM and Graves D: Intravenous
methylprednisolone versus therapeutic plasma exchange for treatment
of anti-N-methyl-D-aspartate receptor antibody encephalitis: A
retrospective review. J Clin Apher. 30:212–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nunez-Enamorado N, Camacho-Salas A,
Belda-Hofheinz S, Cordero-Castro C, Simon-De Las Heras R, Saiz-Diaz
R, Martinez-Sarries FJ, Martinez-Menendez B and Graus F: Fast and
spectacular clinical response to plasmapheresis in a paediatric
case of anti-NMDA encephalitis. Rev Neurol. 54:420–424. 2012.(In
Spanish). PubMed/NCBI
|
|
22
|
Mirza MK, Pogoriler J, Paral K,
Ananthanarayanan V, Mandal S, Mazin A, Baron B and Richa E: Adjunct
therapeutic plasma exchange for anti-N-methyl-D-aspartate receptor
antibody encephalitis: A case report and review of literature. J
Clin Apher. 26:362–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ikeguchi R, Shibuya K, Akiyama S, Hino S,
Kubo H, Takeda T, Shibata N and Yamamoto K: Rituximab used
successfully in the treatment of anti-NMDA receptor encephalitis.
Intern Med. 51:1585–1589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwartz J, Winters JL, Padmanabhan A,
Balogun RA, Delaney M, Linenberger ML, Szczepiorkowski ZM, Williams
ME, Wu Y and Shaz BH: Guidelines on the use of therapeutic
apheresis in clinical practice-evidence-based approach from the
Writing Committee of the American Society for Apheresis: The sixth
special issue. J Clin Apher. 28:145–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ehrlich S, Fassbender CM, Blaes C, Finke
C, Günther A, Harms L, Hoffmann F, Jahner K, Klingel R, Kraft A, et
al: Therapeutic apheresis for autoimmune encephalitis: A nationwide
data collection. Nervenarzt. 84:498–507. 2013.(In German).
View Article : Google Scholar : PubMed/NCBI
|