Introduction
Traditional Chinese medicine (TCM) has been used for
over 2,000 years in China, and has recently become an accepted
alternative therapy in Western countries. TCM is based on syndrome
patterns that are diagnosed through complex symptoms, and with the
goal of reaching a ‘yin-yang’ balance in a person's body prior to
the onset of disease. TCM includes Chinese herbal medicine,
remedial massage (termed ‘An-Mo-Tui-Na’), diet, lifestyle advice,
as well as acupuncture, exercise and breathing therapy (including
qigong). In 1950, in the People's Republic of China, TCM was
standardized, and nowadays is frequently administered through drug
formulas and other means for the treatment of diverse diseases. The
drug formulas predominantly are composed of extracts from medicinal
plants, animal and human tissue, as well as from medicinal minerals
(1). Certain of these components
represent the principal agent in TCM, and others serve as an
adjuvant to improve the effects of, or facilitate the delivery of,
the active component.
TCM theory and practice are not based upon
scientific knowledge, and its effectiveness is not well understood;
neither has it been well researched (2). There are concerns over a number of
potentially toxic plants, animal parts and mineral Chinese
medicinal compounds (1,3). Furthermore, due to the unknown
interactions among the various ingredients of drug formulations and
complex interactive biological systems, interpretation of the
effects due to TCM becomes highly complicated. Thus, improved
methods of evaluation in combination with randomized clinical
trials are warranted to assure the effectiveness of TCM for various
diseases.
Chinese medicinal philosophy believes that toxic
heat is the root cause of cancer progression. Thus, numerous herbs
used in TCM were developed to offset this toxic effect, and have
been used for treatment of cancer. Previous studies have also
suggested that the principal TCM effect may stem from its ability
to alter a patient's immune system, leading to cancer inhibition
(4,5). The present study aimed to investigate
the effectiveness of 150 drug formulations used in TCM for leukemia
induction and progression. A drug formulation, designated C54, was
identified that markedly inhibited leukemia cell proliferation
through cell cycle arrest and apoptosis. This TCM drug was
originally administered for the treatment of sore throats, although
it has never previously been tested for cancer therapy. The present
study also revealed that C54 inhibits the function of the oncogene,
Fli-1, in leukemic cells. Notably, when C54 was administered in a
mouse model of leukemia, the drug only moderately inhibited the
induction and progression of leukemia. It was also revealed that
this occurred, at least in part, through the induction of
inflammation and increased infiltration of tumor inhibitory
monocytes, processes that are known to accelerate cancer
progression. Taken together, the present study has revealed the
complexity of TCM, and the necessity to study the systemic effects
of individual components of drug formulation when applying the
identical methods to other diseases.
Materials and methods
Cell lines
The murine Friend virus-induced erythroleukemic cell
line, CB7, human erythroleukemic cell lines, K562 and HEL, the
human breast cancer cell line, MDA-MB-231, and the human melanoma
(WM9) and HEK293T cell lines were maintained in Dulbecco's modified
Eagle's medium supplemented with 5% fetal bovine serum at 37°C
(HyClone™ Cell Culture; GE Healthcare, Sydney, NSW, Australia).
Tumor induction and in vivo drug
studies
Viral supernatants from NIH-3T3 cells transduced
with Friend murine leukemia virus (F-MuLV) clone 57 plasmid
(6) were harvested and frozen at
−80°C. Newborn BABL/c mice were inoculated with F-MuLV injections
administered intraperitoneally (IP), as previously described
(6). At 5 weeks post-infection,
leukemic mice were injected IP every other day, for a total of six
injections with either the C54 drug (50 mg/kg) or dimethylsulfoxide
(DMSO) as a control, and monitored for any signs of disease. At the
end point of the treatment, when the mice were exhibiting signs of
morbidity from severe leukemia, the animals were sacrificed by
cervical dislocation, and were used to determine the survival rate,
spleen weight and hematocrit value. Hematocrit values were measured
by tail blood collection in 200 ml heparinized capillary tubes
(Drummond Scientific, Broomall, PA, USA); the blood was centrifuged
at 1,000 × g for 15 min, and subsequently evaluated using a
hematocrit gauge. Two groups of MDA-MB-231 cells (1×106)
were injected into the mammary fat pad of anesthetized severe
combined immunodeficiency (SCID) mice, as previously described
(7). After tumors had reached 0.5 cm
in diameter, mice were given a dose of 150 mg/kg drug via gavage,
every day for 2 weeks. All animal studies were conducted in
accordance with the ethical standards of China's animal care and
use of laboratory animals. The study protocol was approved by the
ethics committee on animal experiments of Guizhou Medical
University.
Determination of the IC50
values (i.e., the concentration of drug required to give
half-maximal inhibition), cell cycle and apoptosis analysis
Triplicate cultures of the CB7, HEL, K562, WM9,
MDA-MB-237 and PC3 cell lines were incubated with different
concentrations of C54 or DMSO as a control for three days. Cell
were subsequently subjected to an MTT assay by adding
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to the
culture for 4 h. Following removal of the supernatant, 200 ml DMSO
was added to dissolve the formazan crystals. The absorbance was
read using a Synergy 2 microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA) at 490 nm. For apoptosis and cell cycle
analysis, CB7, HEL and K562 cell lines were incubated at 37°C with
C54 or DMSO as a control for 24 h; subsequently, the cells were
washed in cold phosphate-buffered saline (PBS). For the apoptosis
experiments, cells were stained using an annexin V and propidium
iodide (PI) Apoptosis Detection kit (BD Biosciences, Franklin
Lakes, NJ, USA), prior to flow cytometric analysis. For cell cycle
analysis, cells were fixed in cold 75% ethanol overnight at −20°C.
Following a further wash with cold PBS, cells were stained in PI
for 40 min at 37°C, and then subjected to flow cytometric analysis.
IC50 values were calculated using appropriate
software.
Flow cytometric analysis
Immunofluorescence staining was performed to
determine the expression of Gr-1, Mac-1 (CD11b) and major
histocompatibility complex class II (MHCII) molecules on the tumors
and splenocytes of control (DMSO) and C54-treated mice, as
described previously (8). In brief,
1×106 cells were incubated with anti-CD16/CD32 blocking
antibody (cat. no. 14-0161-82; eBioscience, San Diego, CA, USA) for
10 min at 4°C. Cells were stained with primary antibodies for 30
min on ice. The primary antibodies were as follows:
Phycoerythrin-conjugated anti-mouse Gr1 (cat. no. 17-5931-81), Mac1
(cat. no. 17-0112-82) and MHCII (cat. no. 11-5322-82) antibodies
(all from eBioscience). Cells were subsequently washed and
resuspended in PI (0.1 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) to
exclude dead cells. A total of 104 events were collected
using a FACSCalibur™ flow cytometer and analyzed using
CellQuest™ Pro software (both from BD Biosciences).
Western blot analysis
Western blot analysis was performed as previously
described (6). Polyclonal rabbit
anti-rat Fli-1 (cat no. ab133485) were obtained from Abcam
(Cambridge, UK), extracellular signal-regulated kinase (ERK; cat.
no. 9102) and phospho-ERK (cat. no. 9101) antibodies were obtained
from Cell Signaling Technology, Inc. (CST; Danvers, MA, USA),
whereas the antibody against GAPDH (cat. no. G9545) was purchased
from Sigma-Alrich. All antibodies were used at a dilution of
1:1,000.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was prepared by using TRIzol (cat. no.
15596018l; Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA)
and cDNA was prepared by using a PrimeScript™ RT reagent kit (cat.
no. RR047A; Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's protocol. The β-actin gene was used to normalize the
expression level; SYBR® Select Master mix reagent (cat.
no. 4472908; Invitrogen; Thermo Fisher Scientific) was used for
detection using the 2−ΔΔCq method (9). The primers for GATA1 amplification
were: Sense, 5′-TGGTGGCTTTATGGTGGTG-3′, anti-sense,
5′-CCTTGGTAGAGATGGGCAGT'-3; and the primers for β-actin
amplification were: Sense, 5′-CATGTACGTTGCTATCCAGGC-3′, anti-sense,
5′-CTCCTTAATGTCACGCACGAT-3′.
Luciferase reporter assay
HEK293T cells, plated in triplicate, were
transfected with the indicated amounts of DNA (1 µg of pGL3Fli-1-BS
using Lipofectamine™ 2000 (Life Technologies; Thermo Fisher
Scientific, Inc., Beijing, China) following the manufacturer's
protocol. After 48 h of transfection, luciferase assays were
performed in triplicate, as previously described (7).
Survival and statistical analysis
Mice survival rates were computed and plotted
according to the nonparametric Kaplan-Meier analysis. Statistical
analysis was performed using the two-tailed Student's t-test
with analysis of variance, using Origin 3.5 software (Microcal
Software, Northampton, MA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Anti-cancer activity of 150 TCM drug
formulations on various cancer cell lines
TCM drug formulations are widely used in China for
the treatment of various diseases with or without doctors'
prescriptions. The present study aimed to examine the effect of 150
TCM drugs used without drug prescription for anti-cancer
activities. It was hypothesized that a novel anti-cancer TCM might
be able to be administered in the clinic with fewer regulatory
requirements. These drugs were dissolved in DMSO, and added to
proliferating cultures of several cancer cell lines.
A drug, designated C54, was found to inhibit
proliferation of the cell lines CB7, HEL and K562
(erythroleukemias), PC3 (prostate cancer), WM9 (melanoma) and
MDA-MB-231 (breast cancer), with IC50 values in the
range of 0.07–4.2 µM (Table I). The
IC50 values were determined to be higher in the PC3,
MDA-MB-231 and WM9 cell lines, which are known to have
drug-resistant properties (10–12). C54
is a TCM formulation prescribed as pills (‘Hou-Tong-Jie-Du-Wan’)
for the treatment of sore throats. It contains Realgar (α-As4S4, an
arsenic sulfide mineral), Bos taurus domestius Gmelin (dried bovine
gallstones), Borneol (a terpene) and cinobufagin venom toad organic
material (from toad gland extract). Notably, Realgar and Borneol
have been reported to have anti-cancer activity in various cancer
cell lines (13,14). Additionally, Borneol acts as a drug
absorber (15), and Bos taurus
domesticus Gmelin has been used to remove toxins from the body (see
http://old.tcmwiki.com/wiki/calculus-bovis).
 | Table I.Determination of the IC50
values for various cancer cell lines with C54. |
Table I.
Determination of the IC50
values for various cancer cell lines with C54.
| Cell line | IC50
(µM) |
|---|
| HEL | 0.081±0.008 |
| K562 | 0.073±0.001 |
| CB7 | 0.573±0.014 |
| MDA-MB-231 | 4.20±0.05 |
| WM9 |
1.17±0.024 |
| PC3 |
1.79±0.045 |
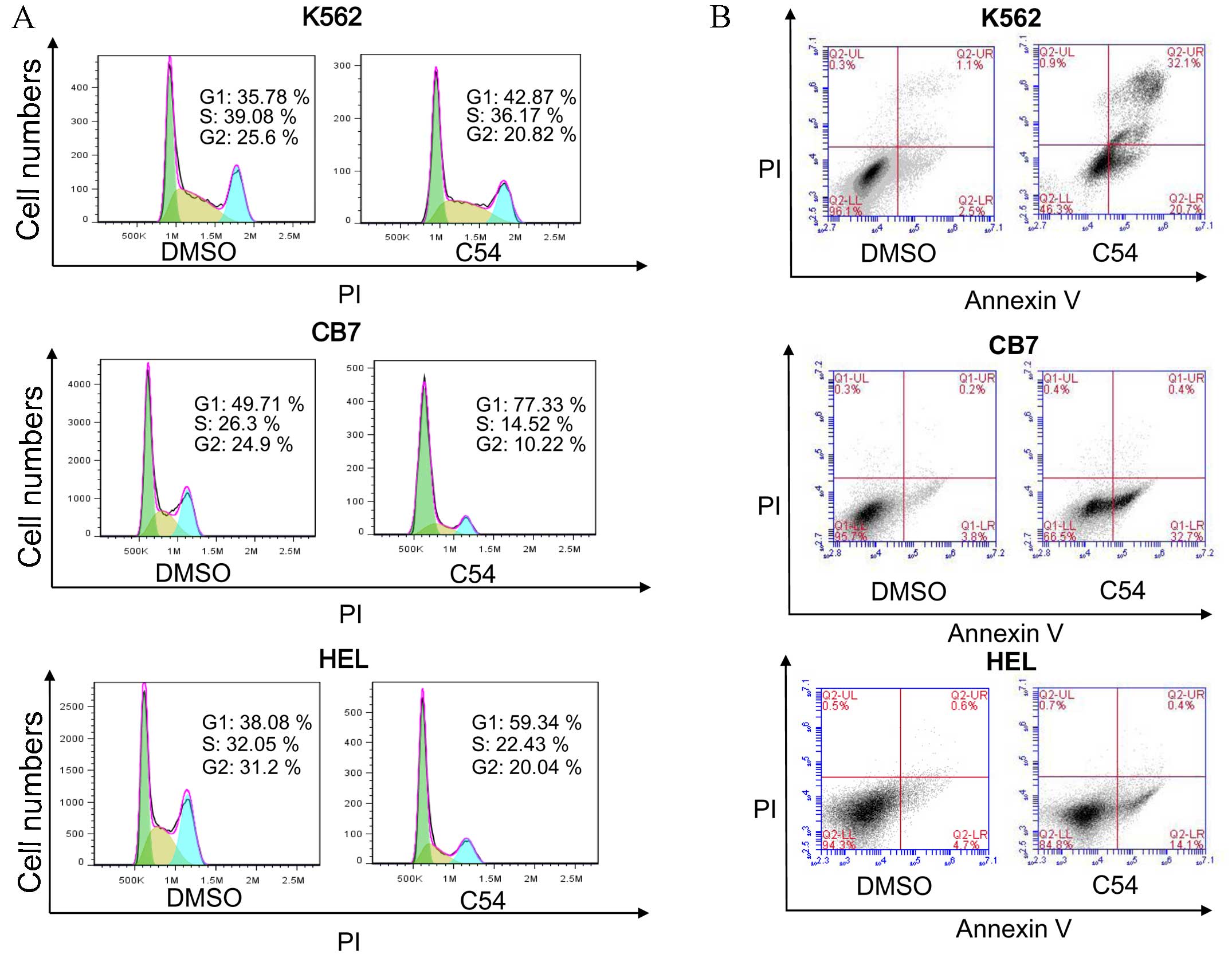
Subsequently, the mechanism of growth inhibition in
the cell lines HEL, K562 and CB7, which exhibited the highest
growth suppression activity with the C54 drug, was examined. Growth
arrest in these cell lines in culture was revealed to be
predominantly due to G1 cell cycle arrest and the
induction of apoptosis 24 h post-treatment (Fig. 1). HEL and CB7 cells expressed high
levels of the Fli-1 oncogene (Fig. 2A
and B). Fli-1 is a critical player in the induction and
progression of F-MuLV-induced erythroleukemia (6,16–18).
Notably, C54 markedly downregulated the expression of Fli-1 in HEL
and CB7 cells, as determined by western blot analysis (Fig. 2A and B). In addition, in HEK293T
cells transiently transfected with the Fli-1 expression vector, C54
inhibited the promoter activity of a PGL3-luciferase gene, driven
by a minimum promoter and two Fli-1 binding sites, as previously
described (Fig. 2C) (6). As anticipated, C54 upregulated the
transcription of GATA1, a downstream target of Fli-1 whose
expression is negatively regulated by this transcription factor
(Fig. 2D) (19). C54 also downregulated the
phosphorylation of mitogen-activated protein kinase (MAPK)/ERK in
the HEL and CB7 cell lines (Fig. 2E and
F). This growth signaling pathway is known to be activated by
Fli-1 overexpression in leukemic cells (20,21).
These data, therefore, demonstrate that C54 acts as a potent
inhibitor of Fli-1 expression and activity in leukemic cells.
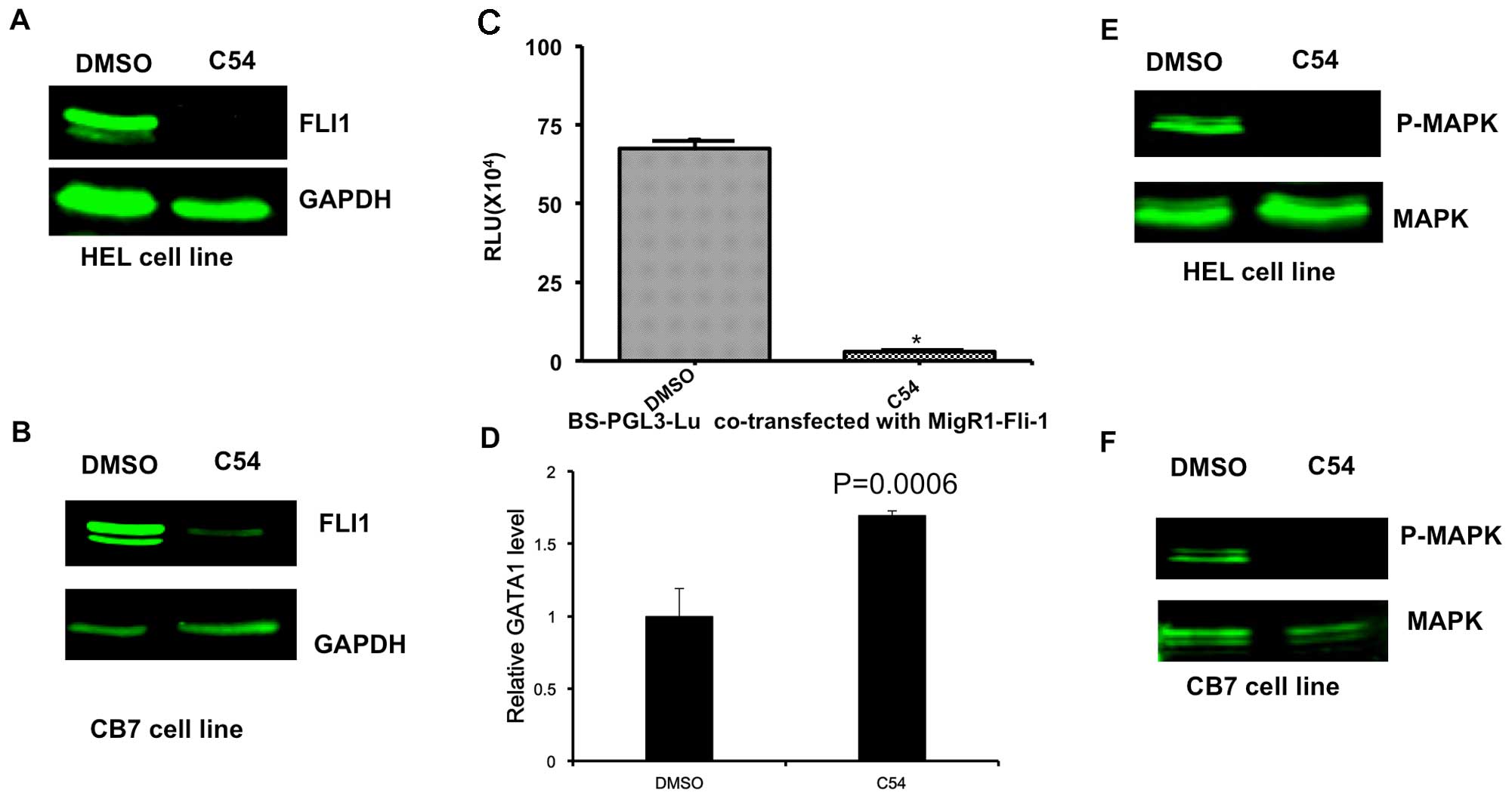 | Figure 2.C54 inhibits expression and function
of the Fli-1 oncogene in erythroleukemia cell lines. HEL (A) and
CB7 (B) cells were cultured in the presence of 5 µg/ml C54 for 12
h, and then subjected to western blot analysis, as previously
described (7). (C) 293T cells were
transfected with a Fli-1 expression reporter (MigR1-Fli-1) and
Fli-1-binding site promoter plasmid (BS-PGL3-Lu). As a control, the
empty vector, MigR1, was transfected with BS-PGL3-Lu into 293T
cells. After 48 h, cells were lysed and subjected to luciferase
assays, as described previously (7).
(D) HEL cells were incubated with C54 for 24 h, and then RNA was
isolated and subjected to RT-qPCR analysis using Fli-1 and control
β-actin primers. (E) HEL cells were incubated with C54 for 24 h,
proteins were isolated and subjected to western blot analysis using
a Fli-1 antibody. β-actin was used as the loading control. (F) CB7
cells were treated with C54 for 24 h and subjected to western blot
analysis using MAPK and P-MAPK antibodies. MAPK, mitogen-activated
protein kinase; P-MAPK, phosphorylated MAPK; DMSO,
dimethylsulfoxide; RLU, relative luciferase units. |
Anti-cancer activity of the TCM drug,
C54, on leukemia and breast cancer progression
To examine the anti-cancer activity of C54 in
vivo, the mouse model of F-MuLV-induced erythroleukemia was
used, in which Fli-1 activation through retroviral insertional
mutagenesis induces erythroleukemias (17,22).
Treatment of mice with F-MuLV-induced erythroleukemias with 50
mg/kg C54 resulted in no marked increase in leukemia-free survival
(Fig. 3A). C54 resulted in a small,
but significant reduction in the size of the spleen, the major site
of tumor infiltration, when compared with vehicle-treated mice
(Fig. 3B). Virus-induced leukemic
mice develop anemia, which becomes severe as a result of tumor
growth (6,17). Although the control group of
DMSO-treated leukemic mice developed massive anemia, C54-treated
mice exhibited significantly increased hematocrit values,
indicating therapeutic improvements and a slowing down of disease
progression (Fig. 3C). These data
suggested that, although C54 markedly inhibited Fli-1 in leukemic
cells in vitro, it only exerted moderate anti-cancer effects
in vivo.
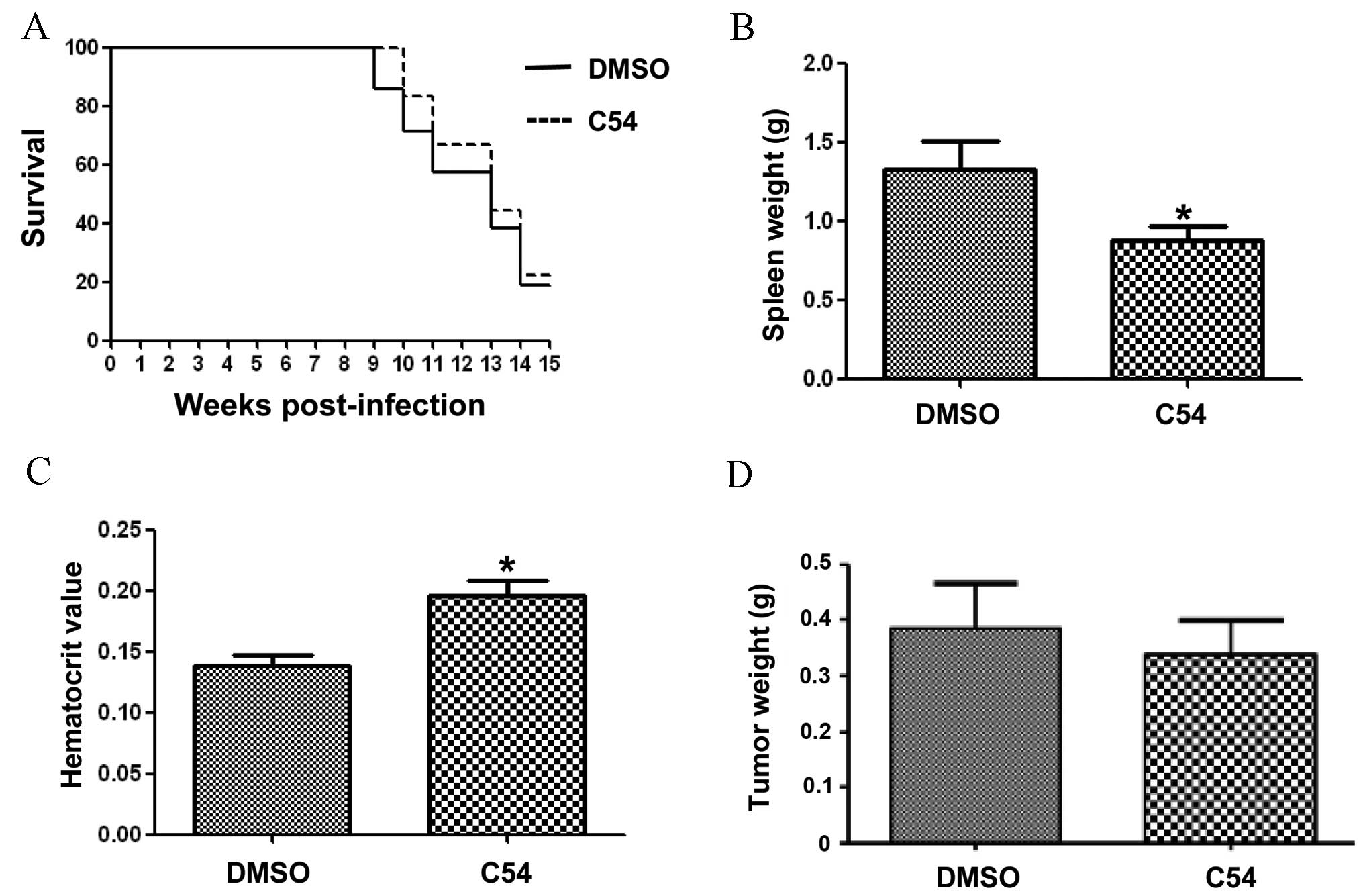 | Figure 3.C54 does not significantly inhibit
the progression of leukemia and breast cancer in mice. A group of
6-week-old mice (n=6), infected at birth with F-MuLV, were treated
every other day with C54 (50 mg/kg of body weight) via IP injection
for a period of 2 weeks. At the end points, when mice showed signs
of morbidity from severe leukemia, animals were sacrificed by
cervical dislocation and used to determine the survival rate (A),
spleen weight (B) and hematocrit value (C). Note that treatment
with C54 did not significantly delay leukemic development,
manifested in an increased hematocrit value and reduced spleen size
compared with the DMSO-injected control group. The statistical
significance (P-value) was calculated using two-tail Student's
t-tests. (D) A group of SCID mice (n=6) were anesthetized
with 10% chloral hydrate and injected orthotopically with
MDA-MB-231 cells (2×106). After the tumors had reached
0.5 cm in diameter, mice were treated with C54 (100 mg/kg) every
other day for 2 weeks via gavage. At 2 weeks following the drug
treatment, mice were sacrificed and tumor weights were measured. No
significant delays in tumor growth were observed. *P<0.05
compared with the DMSO-treatment group. DMSO, dimethylsulfoxid; IP,
intraperitoneal; F-MuLV, Friend murine leukemia virus; SCID, severe
combined immunodeficiency. |
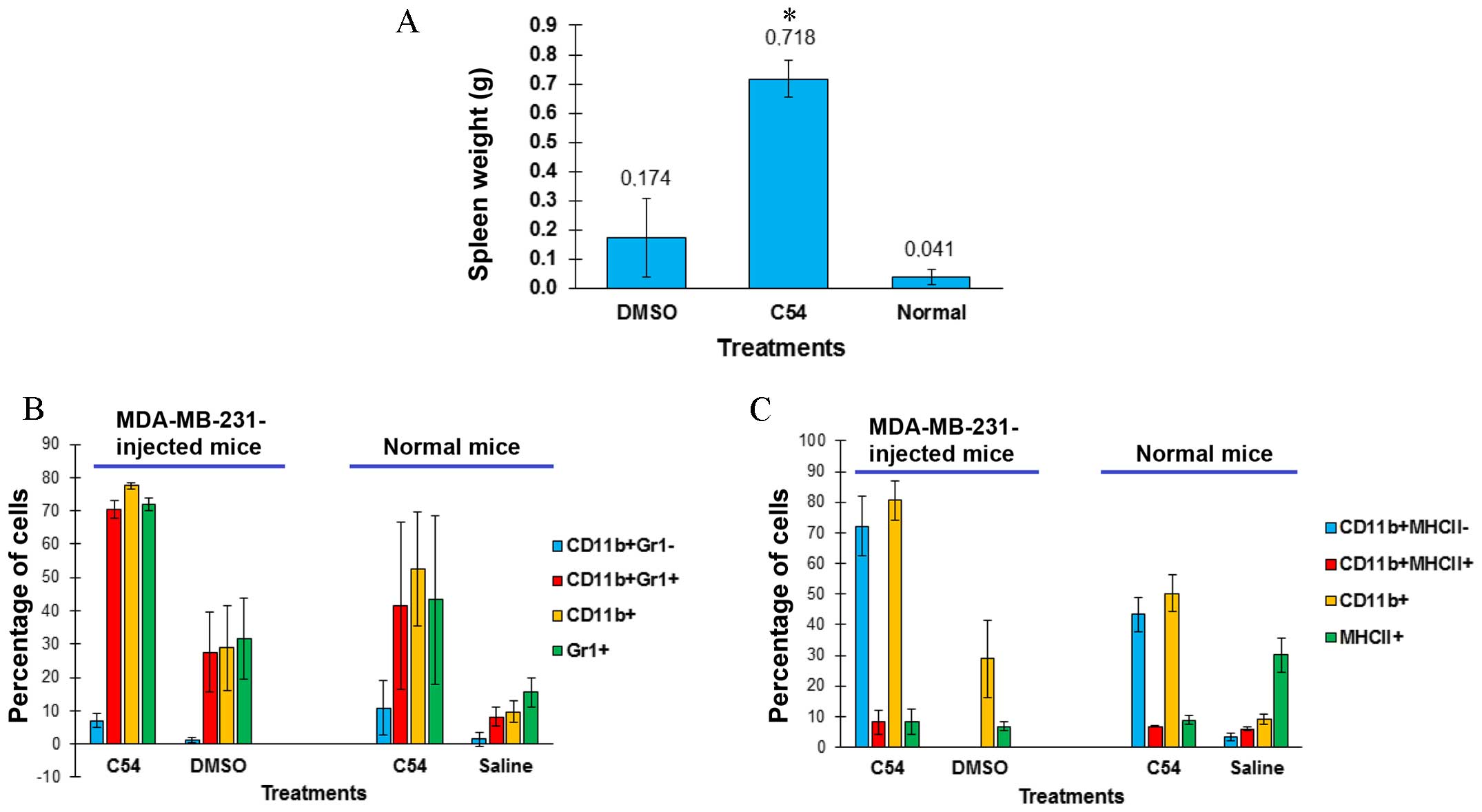
Subsequently, the effect of C54 on breast cancer
progression in an animal model of MDA-MB-231 cells orthotopically
transplanted into the mammary fat pad of SCID mice was examined. In
this experiment, C54 did not inhibit the growth of tumors in SCID
mice (Fig. 3D). It was observed
that, while the spleens of DMSO-treated leukemic mice were much
larger compared with those of control SCID mice, the C54-treated
leukemic group revealed an even more marked splenomegaly (Fig. 4A). Enlargement of the spleen was not
due to tumor infiltration, as culturing splenocytes did not result
in re-establishment of the MDA-MB-231 cell line (data not shown).
Splenomegaly induced in immunodeficient SCID mice following the
injection of MDA-MB-231 had been previously reported (23), although the underlying mechanism had
not been elucidated. Notably, our findings revealed that injection
of MDA-MB-231 increased the number of
CD11b+/Gr1+ monocytes in spleens of
DMSO-treated mice, and that spleen size and the
CD11b+/Gr1+ monocyte population further
increased in C54-treated mice (Fig.
4B). CD11b+/Gr1+ monocytes contribute to
inflammation, which, in turn, is capable of supporting cancer
progression through inflammatory factors (24). Normal BALB/c mice treated with C54
also developed splenomegaly, characterized by a higher infiltration
by CD11b+/Gr1+ monocytes compared with
controls (Fig. 4B). The
CD11b+-positive splenocytes isolated from C54-treated,
tumor-bearing or normal mice were predominantly negative for the
expression of the MHCII antigen, which is mostly found on the
surface of mature macrophages (Fig.
4C) (25).
CD11b+/Gr1+ and
CD11b+/MHCII− phenotypes are the
characteristic features of myeloid-derived suppressor cells
(MDSCs), which are known to inhibit the immune system and
accelerate tumor progression (25,26).
Taken together, these data have demonstrated that the induction of
inflammation by C54 may outweigh its potent cell toxicity, thereby
limiting its anti-cancer activity in vivo.
Discussion
In the past decade, increasing attention has been
given to TCMs to satisfy the public's growing demands for
alternative medicine. This has resulted in an acceleration in drug
development and production in China and Western countries. In our
laboratory, ~150 TCM formulas were screened, which are used for
various diseases in China in view of their anti-cancer activity: A
biological activity that was not the principal purpose of the
majority of these drugs. Among these, it has been revealed that the
drug, C54, exhibited potent toxicity in all cancer cell lines
tested in vitro. Despite this marked anti-cancer activity
in vitro, C54 only moderately inhibited leukemogenesis in
vivo, and did not exert any effect on the progression of
transplanted human breast cancer in mice. To understand the
underlying mechanism of this failed tumor inhibition, a number of
experiments were designed, and it has been demonstrated that C54
may induce prompt inflammation in mice, leading to an accumulation
of CD11b+/Gr1+ MSDCs in tumor masses and in
the spleen of tumor-bearing, as well as normal, mice. These results
indicate that the presence of both anti- and pro-tumorigenic
compounds within C54 may hamper the potential of this drug to be
used as an anti-cancer medicine for the treatment of various
malignancies.
The anti-cancer activity of C54 is likely to be
induced by its components, arsenic sulfide (Realgar) and Borneol,
which are known to induce apoptosis in several cancer cell lines
(13,14). In China, Realgar and Borneol have
been incorporated into many TCM formulas over a long period, and
are considered to be safe, albeit with a few side-effects. Realgar
appears to be the active ingredient of the Angong Niuhuang pill,
used for protection against lipopolysaccharide (LPS)-induced
neuroinflammation (27). Realgar
with Cinnabar is also found in Wan-Sheng-Hua-Feng-Dan formulas,
which have been demonstrated to protect against LPS-induced
neurotoxicity (28). A similar
anti-inflammatory capacity was recently reported for Borneol
(29). The anti-inflammatory effects
of Realgar and Borneol are primarily attributed to their ability to
inhibit inflammatory factors (28,29). It
is noteworthy that two other components of C54, Bos taurus
domestius Gmelin and cinobufagin venom toad, are also commercially
being sold on the basis of their anti-inflammatory action. Thus,
further research is required to identify the cause of inflammation
by C54.
Treatment of erythroleukemic cells with C54 resulted
in apoptosis, associated with a marked dowregulation of Fli-1. As
Fli-1 expression serves an important role in the survival of
erythroleukemic cells, and Fli-1 is known to be expressed in
various leukemic cells, C54 represents a powerful drug for the
treatment of hematological malignancies (16,30).
Thus, administration of C54 with a powerful anti-inflammatory drug
could be a promising approach for the treatment of
Fli-1-overexpressing leukemias. In support of this premise, our
group has previously demonstrated that F-MuLV-induced
erythroleukemogenesis was significantly inhibited when
cancer-bearing mice were treated with either an anti-Fli-1 compound
or an anti-inflammatory cyclo-oxygenase-2 inhibitor, Celebrex
(6,31).
Myeloid cells normally undergo differentiation to
become granulocytes (neutrophils, basophils, eosinophils),
macrophages and dendritic cells. However, under chronic
inflammatory conditions, myeloid differentiation is skewed towards
the expansion of MDSCs (24,25). The suppressor function of MDSCs lies
in their ability to inhibit adaptive and innate immune responses
(32,33). MDSCs are also known to secrete
factors that are able to stimulate tumor growth through increased
angiogenesis and metastasis (32,33). In
the mouse, MDSCs were phenotypically characterized as expressing
high levels of CD11b (a classical myeloid lineage marker) and Gr1
(a granulocytic marker). The presence of higher numbers of MDSCs in
tumor-bearing mice treated with C54 is likely to be the cause of
failed tumor inhibition by this drug. These MDSCs are able to exert
their tumor-promoting activity in normal and immunodeficient SCID
mice. Thus, targeting MDSCs should strengthen the tumor-inhibiting
ability of C54, leading to a slowing down of cancer.
Although C54 is not administered in the clinic for
cancer treatment, this drug is being sold in China for the
treatment of symptoms associated with having a cold and a sore
throat. In this situation, this drug may exert its anti-viral and
anti-bacterial activities through cell cytotoxicity and the
induction of inflammation, which is beneficial for the patient in
terms of curing the infectious disease. However, we propose that
patients diagnosed with cancer should not take C54 to treat
symptoms of cold, since the drug could worsen the cancer
status.
In conclusion, we have identified a TCM drug with
marked cytotoxicity in culture, although with limited or negligible
tumor inhibitory activity in mice. This drug was shown to inhibit
the expression and function of the oncogene Fli-1, involved in
leukemogenesis. In addition, C54 enriched inflammatory blood
monocytes in a cancer environment-a phenomenon that is known to
accelerate cancer progression. Therefore, the anti-cancer activity
of this formulation may potentially be improved when combined with
an anti-inflammatory drug.
Acknowledgements
The present study was supported by research grants
from the Science and Technology Department of Guizhou Province
innovation and project grant (2013-6012), Thousand Talent Program
of China (WQ20135200171) and The Natural Science Foundation of
China (no. 81472609) to Y.B.D.
References
|
1
|
Shaw D: Toxicological risks of Chinese
herbs. Planta Med. 76:2012–2018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu XY, Tang JL, Mao C, Yuan JQ, Qin Y and
Chung VC: Systematic reviews and meta-analyses of traditional
chinese medicine must search chinese databases to reduce language
bias. Evid Based Complement Alternat Med. 2013:8121792013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leung AY: Traditional toxicity
documentation of Chinese Materia Medica-an overview. Toxicol
Pathol. 34:319–326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang JX and Wang XM: Progress in studies
on anti-hepatoma effect of traditional Chinese medicine by
adjusting immune function. Zhongguo Zhong Yao Za Zhi. 32:281–284.
2007.(In Chinese). PubMed/NCBI
|
|
5
|
Yang D and Tian G: Review of experimental
study on treatment of lung cancer with traditional Chinese
medicine. Zhongguo Zhong Yao Za Zhi. 34:2405–2409. 2009.(In
Chinese). PubMed/NCBI
|
|
6
|
Li YJ, Zhao X, Vecchiarelli-Federico LM,
Li Y, Datti A, Cheng Y and Ben-David Y: Drug-mediated inhibition of
Fli-1 for the treatment of leukemia. Blood Cancer J. 2:e542012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li YJ, Liu G, Xia L, Xiao X, Liu JC,
Menezes ME, Das SK, Emdad L, Sarkar D, Fisher PB, et al:
Suppression of Her2/Neu mammary tumor development in mda-7/IL-24
transgenic mice. Oncotarget. 6:36943–36954. 2015.PubMed/NCBI
|
|
8
|
Usenko T, Li YJ, Haeri M, Li Y,
Vecchiarelli-Federico LM, Zhao X, Prchal JT and Ben-David Y:
Enrichment of Sca1+ hematopoietic progenitors in polycythemic mice
inhibits leukemogenesis. Blood. 114:1831–1841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
Relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. METHODS. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hermawan A, Wagner E and Roidl A:
Consecutive salinomycin treatment reduces doxorubicin resistance of
breast tumor cells by diminishing drug efflux pump expression and
activity. Oncol Rep. 35:1732–1740. 2016.PubMed/NCBI
|
|
11
|
Yu X, Yang L, Cairns MJ, Dass C, Saravolac
E, Li X and Sun LQ: Chemosensitization of solid tumors by
inhibition of Bcl-xL expression using DNAzyme. Oncotarget.
5:9039–9048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu SJ, Man S, Bani MR, Adachi D, Hawley
RG, Kerbel RS and Ben-David Y: Retroviral insertional mutagenesis
as a strategy for the identification of genes associated with
cis-diamminedichloroplatinum(II) resistance. Cancer Res.
55:1139–1145. 1995.PubMed/NCBI
|
|
13
|
Pastorek M, Gronesova P, Cholujova D,
Hunakova L, Bujnakova Z, Balaz P, Duraj J, Lee TC and Sedlak J:
Realgar (As4S4) nanoparticles and arsenic trioxide (As2O3) induced
autophagy and apoptosis in human melanoma cells in vitro.
Neoplasma. 61:700–709. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Li L, Su J, Li B, Chen T and Wong
YS: Synergistic apoptosis-inducing effects on A375 human melanoma
cells of natural borneol and curcumin. PLoS One. 9:e1012772014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu Y, Chen X, Du S, Wu Q, Yao Z and Zhai
Y: The in situ and in vivo study on enhancing effect of borneol in
nasal absorption of Geniposide in rats. Arch Pharm Res. 33:691–696.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Luo H, Liu T, Zacksenhaus E and
Ben-David Y: The ets transcription factor Fli-1 in development,
cancer and disease. Oncotarget. 34:2022–2031. 2015.
|
|
17
|
Ben-David Y, Giddens EB, Letwin K and
Bernstein A: Erythroleukemia induction by Friend murine leukemia
virus: Insertional activation of a new member of the ets gene
family, Fli-1, closely linked to c-ets-1. Genes Dev. 5:908–918.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee CR, Cervi D, Truong AH, Li YJ, Sarkar
A and Ben-David Y: Friend virus-induced erythroleukemias: A unique
and well-defined mouse model for the development of leukemia.
Anticancer Res. 23:2159–2166. 2003.PubMed/NCBI
|
|
19
|
Athanasiou M, Mavrothalassitis G,
Sun-Hoffman L and Blair DG: FLI-1 is a suppressor of erythroid
differentiation in human hematopoietic cells. Leukemia. 14:439–445.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zochodne B, Truong AH, Stetler K, Higgins
RR, Howard J, Dumont D, Berger SA and Ben-David Y: Epo regulates
erythroid proliferation and differentiation through distinct
signaling pathways: Implication for erythropoiesis and Friend
virus-induced erythroleukemia. Oncogene. 19:2296–2304. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lakhanpal GK, Vecchiarelli-Federico LM, Li
YJ, Cui JW, Bailey ML, Spaner DE, Dumont DJ, Barber DL and
Ben-David Y: The inositol phosphatase SHIP-1 is negatively
regulated by Fli-1 and its loss accelerates leukemogenesis. Blood.
116:428–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ben-David Y, Giddens EB and Bernstein A:
Identification and mapping of a common proviral integration site
Fli-1 in erythroleukemia cells induced by Friend murine leukemia
virus. Proc Natl Acad Sci USA. 87:1332–1336. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Emi M, Kim R, Tanabe K, Uchida Y and Toge
T: Targeted therapy against Bcl-2-related proteins in breast cancer
cells. Breast Cancer Res. 7:R940–952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nold MF, Mangan NE, Rudloff I, Cho SX,
Shariatian N, Samarasinghe TD, Skuza EM, Pedersen J, Veldman A,
Berger PJ and Nold-Petry CA: Interleukin-1 receptor antagonist
prevents murine bronchopulmonary dysplasia induced by perinatal
inflammation and hyperoxia. Proc Natl Acad Sci USA.
110:14384–14389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meyer C, Sevko A, Ramacher M, Bazhin AV,
Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M and
Umansky V: Chronic inflammation promotes myeloid-derived suppressor
cell activation blocking antitumor immunity in transgenic mouse
melanoma model. Proc Natl Acad Sci USA. 108:17111–17116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang F, Lu Y, Liu J and Shi J: Realgar is
active ingredient of Angong Niuhuang pill in protection against
LPS-induced neuroinflammation. Zhongguo Zhong Yao Za Zhi.
35:3333–3338. 2010.(In Chinese). PubMed/NCBI
|
|
28
|
Zhang F, Lu Y, Wu Q, Yan J, Shi J and Liu
J: Role of cinnabar and realgar of WSHFD in protecting against
LPS-induced neurotoxicity. J Ethnopharmacol. 139:822–828. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong W, Cui Y, Yu Q, Xie X, Liu Y, Wei M,
Ci X and Peng L: Modulation of LPS-stimulated pulmonary
inflammation by Borneol in murine acute lung injury model.
Inflammation. 37:1148–1157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cui JW, Vecchiarelli-Federico LM, Li YJ,
Wang GJ and Ben-David Y: Continuous Fli-1 expression plays an
essential role in the proliferation and survival of F-MuLV-induced
erythroleukemia and human erythroleukemia. Leukemia. 23:1311–1319.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cervi D, Klement G, Stempak D, Baruchel S,
Koki A and Ben-David Y: Targeting cyclooxygenase-2 reduces overt
toxicity toward low-dose vinblastine and extends survival of
juvenile mice with Friend disease. Clin Cancer Res. 11:712–719.
2005.PubMed/NCBI
|
|
32
|
Motallebnezhad M, Jadidi-Niaragh F,
Qamsari ES, Bagheri S, Gharibi T and Yousefi M: The immunobiology
of myeloid-derived suppressor cells in cancer. Tumour Biol.
37:1387–1406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parker KH, Beury DW and Ostrand-Rosenberg
S: Myeloid-derived suppressor cells: Critical cells driving immune
suppression in the tumor microenvironment. Adv Cancer Res.
128:95–139. 2015. View Article : Google Scholar : PubMed/NCBI
|