Introduction
Biliary tract cancer (BTC) is a relatively uncommon
type of cancer. However, the incidence of BTC appears to be
increasing worldwide over the last few decades, particularly in
Latin America and East Asia (1). BTC
is currently the sixth leading cause of cancer-related mortality in
Japan, and patients with BTC have a poor prognosis (2). Therefore, effective treatment
strategies are urgently required. Surgical resection currently
represents the only potentially curative treatment for BTC.
However, the majority of patients are diagnosed at an advanced
stage, when curative resection is no longer feasible; in addition,
even in cases where surgery may be performed, there is a
significant likelihood of relapse (3). Patients with unresectable or recurrent
disease appear to be clinical candidates for systemic chemotherapy
(4,5). After the ABC-02 study reported that
gemcitabine/cisplatin (GC) combination therapy significantly
prolonged median survival time (MST) from 8.1 to 11.7 months over
gemcitabine monotherapy in patients with advanced BTC (6), this combination therapy has become the
standard treatment for BTC worldwide.
Recently, Kanai et al determined the optimal
dose of GC/S-1 (GCS) combination therapy for patients with advanced
BTC in a phase I study (7) and
reported a promising MST of >15 months in a phase II study
(8). Based on these results, a phase
III randomized trial is underway to demonstrate the superiority of
GCS therapy compared with GC in patients with unresectable BTC
(UMIN000014371/NCT02182778). We herein report the case of a patient
with unresectable intrahepatic cholangiocarcinoma (ICC) who
underwent conversion surgery and ultimately achieved a
pathologically complete response using GCS combination therapy.
Case report
A 68-year-old woman was referred to Toyonaka
Municipal Hospital with increased levels of biliary enzymes in
March, 2015. The patient suffered from hypertension and rheumatoid
arthritis, which were controlled with oral medication. A physical
examination revealed no abdominal abnormalities, but the laboratory
tests revealed abnormal liver function, including an elevated serum
alkaline phosphatase level of 839 U/l [upper limit of normal (ULN),
328 U/l), a γ-glutamyltranspeptidase level of 324 U/l (ULN, 64 U/l)
and an alanine aminotransferase level of 82 U/l (ULN, 40 U/l).
Additionally, the serum carcinoembryonic antigen (CEA) and
carbohydrate antigen (CA) 19-9 levels were 9.8 ng/ml (ULN, 5.0
ng/ml) and 32,806 U/ml (ULN, 37 U/ml), respectively. Computed
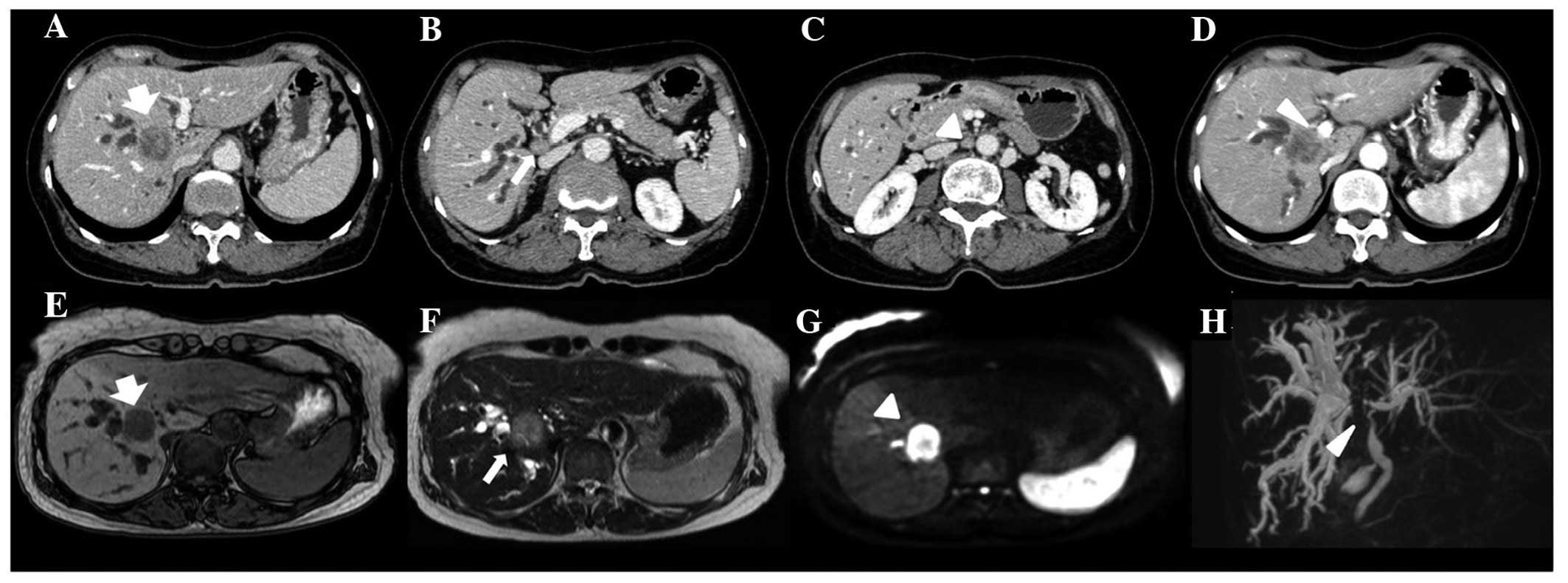
tomography (CT) revealed a 3-cm mass in the 1/8 segment of the
liver, resulting in dilated bile ducts in both hepatic lobes,
enlarged para-aortic and regional lymph nodes, and invasion of the
portal vein (PV) (Fig. 1A). Magnetic
resonance imaging (MRI) revealed an intrahepatic mass as a
hypointense lesion compared with normal liver tissue in T1-weighted
images. T2-weighted images revealed mild hyperintensity compared
with the liver parenchyma. Furthermore, diffusion-weighted MRI
revealed high signal intensity (Fig.
1B).
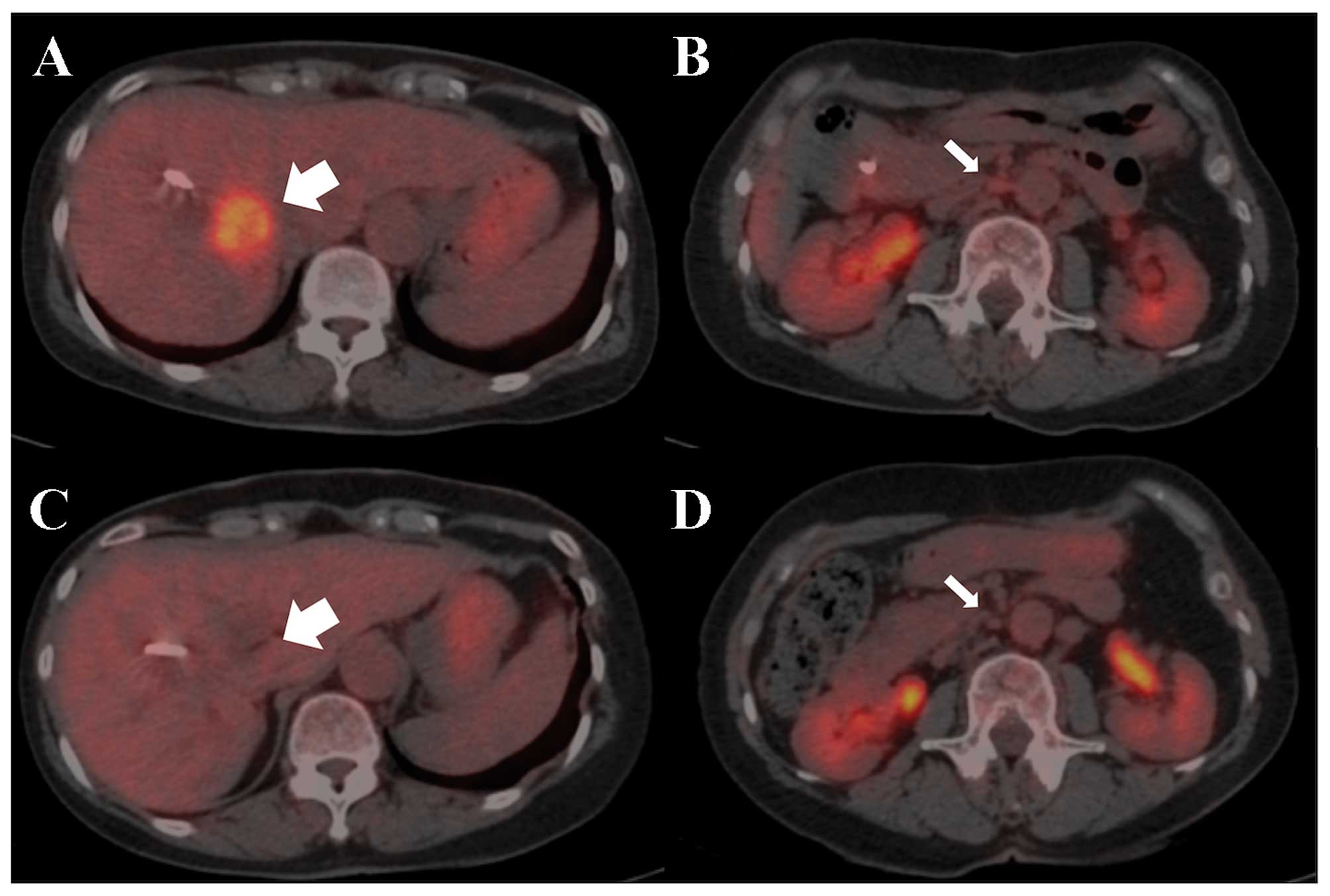
Positron emission tomography with
18F-fluoro-D-deoxyglucose (18F-FDG PET)/CT
revealed abnormal uptake of the primary tumor and para-aortic lymph
nodes, with an SUVmax of 5.8 and 2.5, respectively
(Fig. 3A). On imaging, the patient
was diagnosed with unresectable intrahepatic BTC with para-aortic
and hilar lymph node metastasis [cT3N1M1, cStage IVb according to
the Union for International Cancer Control classification system
(http://www.uicc.org/sites/main/files/private/TNM_Classification_of_Malignant_Tumours_Website_15%20MAy2011.pdf)].
Endoscopic retrograde cholangiopancreatography
(ERCP) revealed a 2-cm irregular stricture of the hilar bile duct
with a lobulated tumor (Fig. 2A).
The brush cytology specimens revealed atypical cells in a
three-dimensional cluster and an isolated pattern strongly
suggestive of adenocarcinoma (Fig.
2B). Based on these findings, the patient was diagnosed with
unresectable ICC with para-aortic lymph node metastasis and,
therefore, systemic chemotherapy was considered.
The patient underwent endoscopic biliary drainage
using plastic stent placement for the biliary stricture prior to
therapy. Endobiliary stents (7F 12- and 7-cm at a light angle) were
successfully placed through the narrowed lumen at initial ERCP,
resulting in successful biliary decompression.
The patient was subsequently enrolled in a phase III
randomized trial (UMIN000014371/NCT02182778) and randomly assigned
to receive GCS combination therapy.
Gemcitabine and cisplatin were administered
intravenously at doses of 1,000 or 25 mg/m2 on day 1,
and oral S-1 was administered daily at a dose of 80
mg/m2 on days 1–7 every 2 weeks. The patient received 12
cycles of the regimen for 6 months. On the first day of the 8th
cycle, the patient presented with grade 3 malaise after receiving
chemotherapy. This adverse effect was, however, manageable and
improved within 2 days by fluid replacement therapy. The scheduled
treatment was completed in accordance with the protocol without
delay. After the scheduled 12 cycles of the regimen, CT revealed a
marked reduction of the primary tumor and metastatic lymph nodes.
18F-FDG-PET/CT also revealed diminished abnormal uptake
of the primary lesion and para-aortic lymph nodes (Fig. 3B). Additionally, the serum CEA and
CA19-9 levels decreased to within the normal range (1.5 ng/ml and
11 U/ml, respectively).
Imaging examination showed no macroscopic evidence
of factors rendering the tumor unresectable. The patient achieved a
good partial response to GCS therapy and was allowed to undergo
conversion surgery. Intraoperative frozen section analysis of the
lymph nodes showed no malignant findings. Therefore, the patient
underwent extended right hepatic lobectomy, lymph node dissection
and left hepaticojejunostomy. Macroscopically, curative resection
was achieved.
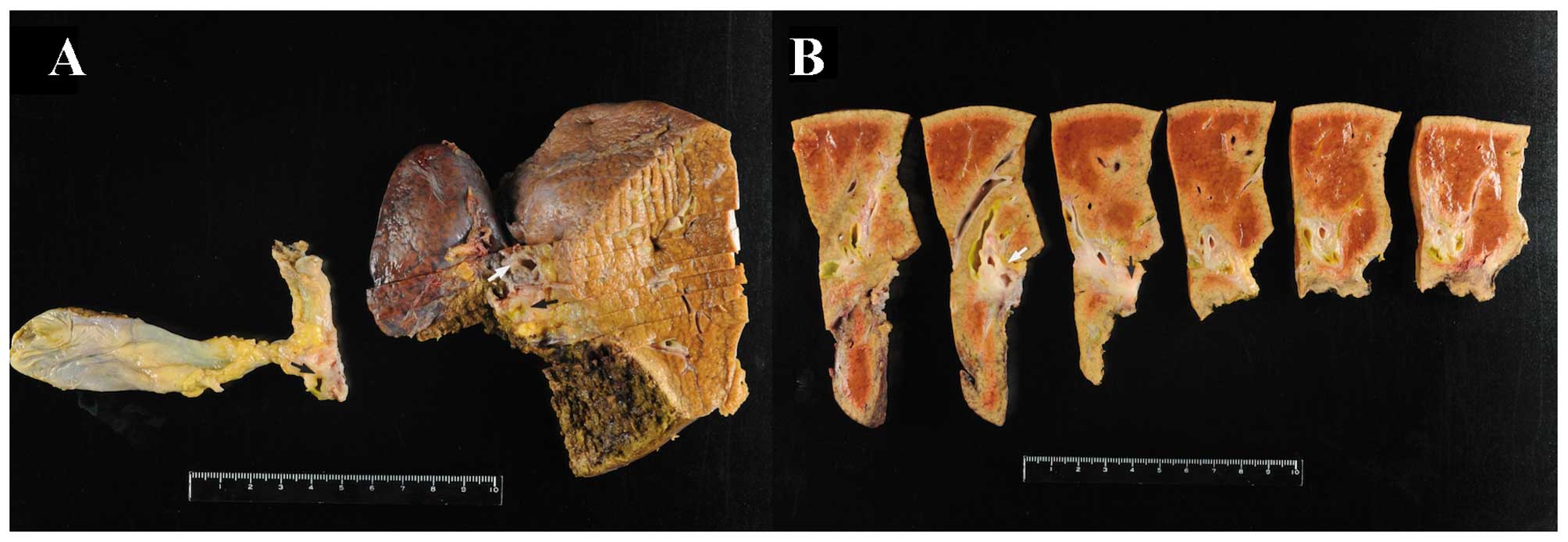
The resected specimen exhibited almost complete
occlusion of the right hepatic duct immediately before the junction
(Fig. 4). The hepatic parenchyma was
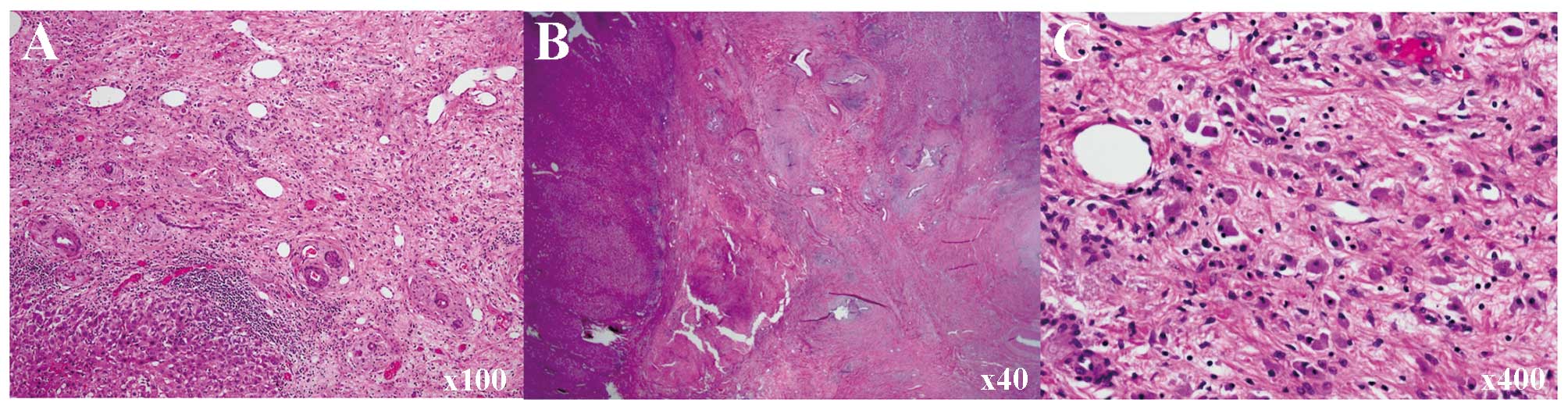
eroded and replaced by fibrotic tissue. Histological examination
revealed scattered pigmented macrophages in the fibrotic tissue,
suggesting that necrotic cells were scavenged from this location.
Although atypical epithelia in the bile duct were identified,
invasive carcinoma and intraepithelial carcinoma components were
not found, even following thorough examination. There were no
viable carcinoma cells in the dissected nodes, but some contained
fibrotic foci (Fig. 5).
In summary, a patient with unresectable ICC at
presentation achieved a pathologically complete response after
undergoing preoperative GCS combination chemotherapy. At the last
follow-up, 9 months after the operation (September, 2016), the
patient remained alive and recurrence-free, without adjuvant
therapy.
Discussion
BTC, which originates in the intrahepatic and
extrahepatic bile ducts, is a relatively uncommon type of cancer,
comprising ~3% of all gastrointestinal malignancies (9,10). The
majority of BTC patients are diagnosed at an advanced stage due to
the lack of abdominal symptoms, and the prognosis is generally poor
(1,2). Therefore, surgery is the optimal
therapeutic approach, although systemic chemotherapy is considered
for patients with unresectable BTC. In the ABC-02 study, Valle
et al reported that GC combination therapy was associated
with a significant survival advantage compared with gemcitabine
alone, with a overall MST of 11.7 months compared with 8.1 months,
respectively (hazard ratio = 0.64; P<0.001) (6). Based on that study, GC combination
therapy has been the standard palliative chemotherapy for patients
with advanced BTC worldwide. The BT-22 study used the same regimen
as the ABC-02 study and evaluated the efficacy and safety for
patients with advanced BTC in a Japanese population; the study
revealed that its outcome was similar to that of the ABC-02 study,
as the MST for GC combination therapy and G alone was 11.2 and 7.7
months, respectively, and the adverse events did not significantly
differ between the two groups, although the incidence of
hematotoxicity was higher with GC combination therapy compared with
G alone (GC vs. G: Leukopenia, 29.3 vs. 19.0%; neutropenia, 56.1
vs. 38.1%; thrombocytopenia, 39.0 vs. 7.2%; and decreased
hemoglobin level, 36.6 vs. 16.6%, respectively) (11). Therefore, this indicates that the new
regimen exhibited a higher efficacy and fewer adverse events.
S-1 is an oral fluoropyrimidine prodrug that is
widely used for various solid tumors (12–15), and
it is approved in Japan as a chemotherapeutic agent for BTC
(16). S-1 monotherapy has shown
promising outcomes associated with mild toxicity in BTC patients
(13). Additionally, in combination
with gemcitabine, S-1 has also achieved favorable response rates
(30–34%) and MST (11.6–12.7 months) (14,15).
On the basis of these reports, Kanai et al
expected that the addition of S-1 would exert an additive or
synergistic effect with GC combination therapy to improve treatment
results with respect to efficacy and safety. In a phase I study,
the regimen described below had the fewest grade 3–4 adverse events
(maculopapular rash, vasovagal reaction and anemia). Based on the
incidental rates of adverse events in the phase I study, Kanai
et al established a recommended dose of the GCS combination
therapy, which consisted of intravenous administration of
gemcitabine (1,000 mg/m2) and cisplatin (25
mg/m2) on day 1 and oral administration of S-1 (80
mg/m2) on days 1–7, every 2 weeks (7). Next, the authors evaluated the efficacy
of the GCS regimen. A phase II study demonstrated significantly
prolonged MST (16.2 months, 95% confidence interval 10.2–22.2
months) without uncontrollable adverse events compared with that of
the ABC-02 study (11.7 months, hazard ratio = 0.64; P<0.001).
Interestingly, two patients (4%) were able to achieve curative
secondary resection after tumor downstaging following chemotherapy
(8). Thus, GCS combination therapy
should not only be considered to be a standard first-line
chemotherapy, but also a ‘conversion surgery or neoadjuvant
chemotherapy’. On the basis of these findings, a randomized phase
III study has now been launched (UMIN000014371/NCT02182778).
Conversion surgery is radical resection performed
for previously unresectable cases that become resectable as a
result of regression following chemotherapy; it should be
distinguished from neoadjuvant chemotherapy, although a strict
distinction between these two strategies is occasionally clinically
difficult, as the definition of unresectable cancer varies among
physicians. Conversion surgery or neoadjuvant chemotherapy for
unresectable cancers, including gastric, colorectal and pancreatic
cancer, has been frequently reported (17–19). Kim
et al demonstrated that clinically curative conversion
therapy resulted in the highest survival rate and best prognosis in
gastric cancer patients with peritoneal seeding (cStage IV). The
MST of patients undergoing clinically curative conversion therapy
and non-curative resection was 37 and 18 months, respectively, and
the 3-year survival rates were 50 and 0%, respectively (17). For colorectal and pancreatic cancer,
several recent reports also demonstrated a clinical advantage with
neoadjuvant chemotherapy (18,19).
However, the feasibility and efficacy of neoadjuvant chemotherapy
for BTC has not been determined (20). Kato et al reported that
patients with initially unresectable locally advanced BTC who
underwent neoadjuvant chemotherapy (gemcitabine) had a
significantly longer survival time compared with those unable to
undergo surgery (2-year overall survival rate of 45 and 19%,
respectively) (21). Furthermore, a
case of curative resection after GCS chemotherapy for initially
unresectable biliary duct cancer and a case of a patient with
extrahepatic cholangiocarcinoma after undergoing preoperative
gemcitabine-based chemotherapy have been reported (22). Based on these reports, conversion
therapy has recently attracted attention, although there is
insufficient evidence regarding the safety and efficacy of
performing conversion surgery. To the best of our knowledge, the
present case is the first report of a patient diagnosed with
unresectable BTC who ultimately achieved a pathologically complete
response with GCS combination therapy. Further studies are required
to verify the efficacy of conversion surgery in patients with BTC
using a prospective study design.
Acknowledgements
We would like to thank the American Journal Expert
copy editing company for the linguistic revision of the
manuscript.
References
|
1
|
Randi G, Malvezzi M, Levi F, et al:
Epidemiology of biliary tract cancers: an update. Ann Oncol.
20:146–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsuda T and Marugame T: International
comparisons of cumulative risk of gallbladder cancer and other
biliary tract cancer, from Cancer Incidence in Five Continents Vol.
VIII. Jpn J Clin Oncol. 37:74–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leonard GD and O'Reilly EM: Biliary tract
cancers: Current concepts and controversies. Expert Opin
Pharmacother. 6:211–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glimelius B, Hoffman K, Sjoden PO, et al:
Chemotherapy improves survival and quality of life in advanced
pancreatic and biliary cancer. Ann Oncol. 7:593–600. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eckel F and Schmid RM: Chemotherapy in
advanced biliary tract carcinoma: A pooled analysis of clinical
trials. Br J Cancer. 96:896–902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: ABC-02 Trial Investigators: Cisplatin plus gemcitabine
versus gemcitabine for biliary tract cancer. N Engl J Med.
362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanai M, Hatano E, Kobayashi S, Fujiwara
Y, Sakai D, Kodama Y, Ajiki T, Nagano H and Ioka T: Phase I trial
of oral S-1 combined with gemcitabine and cisplatin for advanced
biliary tract cancer (KHBO1002). Cancer Chemother Pharmacol.
69:1181–1188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanai M, Hatano E, Kobayashi S, Fujiwara
Y, Marubashi S, Miyamoto A, Shiomi H, Kubo S, Ikuta S, Yanagimoto
H, et al: A multi-institution phase II study of
gemcitabine/cisplatin/S-1 (GCS) combination chemotherapy for
patients with advanced biliary tract cancer (KHBO 1002). Cancer
Chemother Pharmacol. 75:293–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sia D, Tovar V, Moeini A and Llovet JM:
Intrahepatic cholangiocarcinoma: Pathogenesis and rationale for
molecular therapies. Oncogene. 32:4861–4870. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vauthey JN and Blumgart LH: Recent
advances in the management of cholangiocarcinomas. Semin Liver Dis.
14:109–114. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okusaka T, Nakachi K, Fukutomi A, Mizuno
N, Ohkawa S, Funakoshi A, Nagino M, Kondo S, Nagaoka S, Funai J, et
al: Gemcitabine alone or in combination with cisplatin in patients
with biliary tract cancer: A comparative multicentre study in
Japan. Br J Cancer. 103:469–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ichinose Y, Yoshimori K, Sakai H, et al:
S-1 plus cisplatin combination chemotherapy in patients with
advanced non-small cell lung cancer: a multi-institutional phase II
trial. Clinical cancer research: an official journal of the
American Association for Cancer Research. 10:7860–7864. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furuse J, Okusaka T, Boku N, Ohkawa S,
Sawaki A, Masumoto T and Funakoshi A: S-1 monotherapy as first-line
treatment in patients with advanced biliary tract cancer: A
multicenter phase II study. Cancer Chemother Pharmacol. 62:849–855.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaki T, Isayama H, Nakai Y, Ito Y,
Kogure H, Togawa O, Toda N, Yasuda I, Hasebe O, Maetani I, et al:
Multicenter, phase II study of gemcitabine and S-1 combination
chemotherapy in patients with advanced biliary tract cancer. Cancer
Chemother Pharmacol. 65:1101–1107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanai M, Yoshimura K, Tsumura T, Asada M,
Suzuki C, Niimi M, Matsumoto S, Nishimura T, Nitta T, Yasuchika K,
et al: A multi-institution phase II study of gemcitabine/S-1
combination chemotherapy for patients with advanced biliary tract
cancer. Cancer Chemother Pharmacol. 67:1429–1434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasaki T, Isayama H, Nakai Y, Mizuno S,
Yamamoto K, Yagioka H, Yashima Y, Kawakubo K, Kogure H, Togawa O,
et al: Multicenter phase II study of S-1 monotherapy as second-line
chemotherapy for advanced biliary tract cancer refractory to
gemcitabine. Invest New Drugs. 30:708–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SW: The result of conversion surgery
in gastric cancer patients with peritoneal seeding. J Gastric
Cancer. 14:266–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adam R, Wicherts DA, de Haas RJ, Ciacio O,
Lévi F, Paule B, Ducreux M, Azoulay D, Bismuth H and Castaing D:
Patients with initially unresectable colorectal liver metastases:
Is there a possibility of cure? J Clin Oncol. 27:1829–1835. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morganti AG, Massaccesi M, La Torre G,
Caravatta L, Piscopo A, Tambaro R, Sofo L, Sallustio G, Ingrosso M,
Macchia G, et al: A systematic review of resectability and survival
after concurrent chemoradiation in primarily unresectable
pancreatic cancer. Ann Surg Oncol. 17:194–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grendar J, Grendarova P, Sinha R and Dixon
E: Neoadjuvant therapy for downstaging of locally advanced hilar
cholangiocarcinoma: A systematic review. HPB Oxf. 16:297–303. 2014.
View Article : Google Scholar
|
|
21
|
Kato A, Shimizu H, Ohtsuka M, Yoshidome H,
Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Kimura F and
Miyazaki M: Surgical resection after downsizing chemotherapy for
initially unresectable locally advanced biliary tract cancer: A
retrospective single-center study. Ann Surg Oncol. 20:318–324.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furukawa K, Uwagawa T, Sakamoto T, Shiba
H, Tsutsumi J and Yanaga K: Curative resection after gemcitabine,
cisplatin and S-1 chemotherapy for initially unresectable biliary
duct cancer: A case report. Anticancer Res. 35:4203–4206.
2015.PubMed/NCBI
|