Introduction
Extranodal natural killer-T cell lymphoma (ENKL) is
a subtype of non-Hodgkin lymphoma (1), occurring more frequently in Asia
compared with Western countries (2).
According to a recent survey, ENKL is the second most common
extranodal lymphoma subtype in China after large B-cell lymphoma
(3). ENKL is very aggressive,
characteristically damaging vessels and surrounding tissues and
initiating cytotoxic responses. The mean incidence is in middle age
(43.1 years) (3). Over half of
patients present with local symptoms, and the nasal region is the
most common primary site (4). ENKL
may also involve the skin, gastrointestinal tract, lung and liver,
while soft tissue involvement is rare (5). The majority of the patients have B
symptoms, whereas bone marrow involvement is rare (4). Patients typically have evidence of
Epstein-Barr virus infection (6).
There is a range of reported treatments for ENKL,
with varied efficacy. A Chinese report of 58 patients with
early-stage disease (7) indicated
that primary radiotherapy may increase the progression-free
survival and 3-year overall survival. Chauchet et al
(8) analyzed the optimal therapeutic
options and factors affecting prognosis in 36 European patients
with ENKL and concluded that early radiotherapy, concomitantly or
sequentially with chemotherapy, may improve patient outcomes.
However, Ma et al (9), in
agreement with a recent meta-analysis (10), reported that the 5-year survival
rates were not different between the radiotherapy and
chemoradiotherapy groups in their study of 64 patients with
early-stage nasal-type ENKL (57.9 and 61.5% respectively, P=0.47).
The 2015 National Comprehensive Cancer Network (NCCN) guidelines
(11) recommend several treatment
regimens for ENKL for patients with different clinical
characteristics. Chemotherapy and radiotherapy are currently the
main options, but their efficacy remains controversial among
different studies and in different countries.
The present study was conducted to retrospectively
analyze the clinical characteristics and compare different
treatment regimens for ENKL by investigating the survival of 69
patients with this disease, with the aim to summarize the
experience of treating ENKL in clinical practice and provide a
reference for future clinical treatment.
Patients and methods
Inclusion criteria
The inclusion criteria of our study were as follows:
Pathologically confirmed ENKL with complete pathological data and
absence of comorbid conditions. Follow-up data were complete,
including physical examination, laboratory and imaging results,
treatments administered and dates of recurrence and death.
Objective
This retrospective study was conducted at The First
Affiliated Hospital of the Medical College of Xi'an Jiaotong
University (Xi'an, China), and included 69 ENKL patients who were
clinicopathologically diagnosed between 2004 and 2015. Pathological
diagnosis was made according to the World Health Organization
classification criteria (12).
Pathological data were collected from the Pathology Department of
our hospital. The certain risk factors of ENKL were evaluated
according to the 2015 NCCN guidelines, along with the International
Prognostic Index (IPI), including age, serum lactate dehydrogenase
(LDH), B symptoms, lymph node status, Ann Arbor stage, Eastern
Cooperative Oncology Group performance status (ECOG PS) and number
of extranodal sites.
Statistical analysis
Statistical analyses were performed using SPSS 21.0
statistical software (IBM Corp., Armonk, NY, USA) and GraphPad
Prism software (GraphPad Software, San Diego, CA, USA). Clinical
factors were estimated using Kaplan-Meier analysis and the log-rank
test was used to assess significance of survival. The Chi-square
test was used to compare the clinical factors between the
radiotherapy and non-radiotherapy groups. Cox regression models
were used for multivariate survival analysis. P-values <0.05
were considered to indicate statistically significant
differences.
Results
Clinical characteristics and treatment
regimens
The study included 50 men and 19 women, and 17
patients were aged ≥60 years at diagnosis. The nasal cavity was the
most common primary site (58.0%). A total of 15 patients (21.7%)
had ECOG PS scores ≥2, and 14 patients (20.3%) had IPI scores of
3–5. A total of 44 (63.8%) and 25 (36.2%) patients had Ann Arbor
stage I/II and III/IV disease, respectively. B symptoms were
present at diagnosis in 33 patients (47.8%), and 33 patients also
had lymph node involvement, while only 3 patients had bone marrow
involvement. The LDH levels were increased in 33 patients.
In our study, 5 patients underwent surgery, 7
underwent radiotherapy, 30 received chemotherapy alone and 29
received chemoradiotherapy. Chemotherapy combined with autologous
hematopoietic stem cell transplantation (HSCT) was administered to
2 patients. In addition, 3 patients only received palliative care
due to their poor health status. The initial chemotherapy regimens
included cyclophosphamide, adriamycin, vincristine and prednisone
(CHOP) in 36 cases, etoposide + CHOP (ECHOP) in 6 cases,
gemcitabine + platinum (GP) in 4 cases, dexamethasone,
methotrexate, ifosfamide, L-asparaginase and etoposide (SMILE) in 4
cases, and other regimens (e.g., L-asparaginase-based regimens) in
the remaining 9 cases. Locoregional radiotherapy with a dose of
45–62 Gy was administered to the patients who received
radiotherapy.
Univariate survival analysis
All the patients were followed until death or
through to April 20, 2015 (deadline date of our study), with a
median follow-up of 38 months (range, 1–132 months). The median OS
(mOS) was 96 months for all patients, with a 5-year survival rate
of 68.0%. However, these data were obviously below average for
patients with extranasal primary tumors (63 months, 43.4%), ECOG
score ≥2 (10 months, 0%), IPI score 3–5 (9 months, 21.4%), stage
III/IV disease (20 months, 43.6), presence of B symptoms (70
months, 55.7%), lymph node involvement (63 months, 57.4%), and
those not receiving radiotherapy (63 months, 55.9%) (Table I).
 | Table I.Univariate analysis of overall
survival by clinical factors (n = 69). |
Table I.
Univariate analysis of overall
survival by clinical factors (n = 69).
|
|
| Survival rate
(%) |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Clinical factors | No. | 1-year | 5-year | mOS (months) | P-value
(log-rank) | HR | 95% CI |
|---|
| Gender |
|
| Men | 50 | 81.9 | 71.1 | 96 | 0.5698 | 1.31 | 0.51–3.37 |
| Women | 19 | 73.3 | 58.8 | 118 |
|
|
|
| Age (years) |
|
| ≤60 | 52 | 80.7 | 65.4 | 96 | 0.8103 | 1.13 | 0.43–2.96 |
| >60 | 17 | 76.0 | 76.0 | 87 |
|
|
|
| Primary site |
|
| Nasal | 40 | 84.8 | 81.5 | 132 | 0.0079a | 0.33 | 0.14–0.75 |
| Extranasal | 29 | 72.4 | 43.4 | 63 |
|
|
|
| ECOG score |
|
| 0–1 | 54 | 92.6 | 83.8 | 132 | <
0.0001a | 0.01 | 0.003–0.03 |
| ≥ 2 | 15 | 33.3 | 0 | 10 |
|
|
|
| IPI |
|
| 0–2 | 55 | 90.8 | 80.2 | 132 | <
0.0001a | 0.02 | 0.006–0.08 |
| 3–5 | 14 | 35.7 | 21.4 | 9 |
|
|
|
| Ann Arbor stage |
|
| I/II | 44 | 95.5 | 82.5 | 132 | <
0.0001a | 0.16 | 0.06–0.39 |
| III/IV | 25 | 52.0 | 43.6 | 20 |
|
|
|
| B symptoms |
|
| Yes | 33 | 63.5 | 55.7 | 70 | 0.0183a | 2.72 | 1.19–6.24 |
| No | 36 | 94.3 | 79.1 | 96 |
|
|
|
| Serum LDH (U/L) |
|
| Normal | 36 | 83.2 | 69.9 | 132 | 0.1861 | 0.58 | 0.26–1.30 |
| Elevated | 33 | 75.6 | 65.7 | 70 |
|
|
|
| Bone marrow
involvement |
|
| Yes | 3 | 66.7 | 66.7 | 132 | 0.8552 | 1.22 | 0.14–10.98 |
| No | 66 | 80.1 | 68.1 | 96 |
|
|
|
| Lymph node
involvement |
|
| Yes | 33 | 66.7 | 57.4 | 63 | 0.0084a | 0.34 | 0.15–0.76 |
| No | 36 | 91.7 | 75.3 | 132 |
|
|
|
| Surgery |
|
| Yes | 5 | 100.0 | 100.0 | 95 | 0.1413 | 0.33 | 0.08–1.45 |
| No | 64 | 77.9 | 65.5 | 96 |
|
|
|
| Radiotherapy |
|
| Yes | 36 | 94.3 | 77.9 | 132 | 0.0041a | 0.30 | 0.13–0.68 |
| No | 33 | 64.7 | 55.9 | 63 |
|
|
|
| Chemotherapy |
|
| Yes | 59 | 81.3 | 69.8 | 96 | 0.5267 | 0.67 | 0.20–2.29 |
| No | 10 | 70.0 | 58.3 | 132 |
|
|
|
| HSCT |
|
| Yes | 2 | 100.00 | 100.00 | 34 | 0.4089 | 0.35 | 0.03–4.23 |
| No | 67 | 78.9 | 67.2 | 96 |
|
|
|
| All patients | 69 | 79.6 | 68.0 | 96 |
|
|
|
The following characteristics were found to indicate
poor survival: Primary tumors arising outside the nasal region
(HR=0.33, P=0.0079), ECOG score ≥2 (HR=0.01, P<0.0001), IPI
score 3–5 (HR=0.02, P<0.0001), stage III/IV disease (HR=0.16,
P<0.0001), presence of B symptoms (HR=2.72, P=0.0183), lymph
node involvement (HR=0.34, P=0.0084) and absence of radiotherapy
(HR=0.30, P=0.0041 (Table I).
Comparison between radiotherapy and
non-radiotherapy groups
A comparison between the radiotherapy and
non-radiotherapy groups by Chi-square tests indicated that patients
who received radiotherapy had higher rates of ECOG 0–1 (88.89 vs.
66.67%, P=0.0254), IPI 0–2 (91.67 vs. 66.67%, P=0.0099), Ann Arbor
stage I/II disease (75.00 vs. 51.51%, P=0.0426), absence of B
symptoms (63.89 vs. 39.39%, P=0.0419), normal serum LDH level
(63.89 vs. 39.39%, P=0.0419), and absence of lymph node involvement
(63.89 vs. 39.39%, P=0.0419) (Table
II). Therefore, the survival results of the two groups cannot
be compared directly.
 | Table II.Comparison of clinical factors
between different treatment groups with the Chi-square test. |
Table II.
Comparison of clinical factors
between different treatment groups with the Chi-square test.
| Clinical
factors | Radiotherapy n=36
(%) | Non-radiotherapy
n=33(%) | P-value |
|---|
| Gender |
|
| Men | 26 (72.22) | 24 (72.73) | 0.9626 |
| Women | 10 (27.78) | 9 (27.27) |
|
| Age (years) |
|
| ≤ 60 | 27 (75.00) | 25 (75.76) | 0.9418 |
| >60 | 9 (25.00) | 8 (24.24) |
|
| Primary site |
|
| Nasal | 24 (66.67) | 16 (48.48) | 0.1264 |
| Extranasal | 12 (33.33) | 17 (51.51) |
|
| ECOG score |
|
| 0–1 | 32 (88.89) | 22 (66.67) | 0.0254a |
| ≥ 2 | 4 (11.11) | 11 (33.33) |
|
| IPI |
|
| 0–2 | 33 (91.67) | 22 (66.67) | 0.0099a |
| 3–5 | 3 (8.33) | 11 (33.33) |
|
| Ann Arbor
stage |
|
| I/II | 27 (75.00) | 17 (51.51) | 0.0426a |
| III/IV | 9 (25.00) | 16 (48.48) |
|
| B symptoms |
|
| Yes | 13 (36.11) | 20 (60.60) | 0.0419a |
| No | 23 (63.89) | 13 (39.39) |
|
| Serum LDH |
|
| (U/l) |
|
| Normal | 23 (63.89) | 13 (39.39) | 0.0419a |
| Elevated | 13 (36.11) | 20 (60.60) |
|
| Bone marrow
involvement |
|
| Yes | 1 (2.78) | 2 (6.06) | 0.5042 |
| No | 35 (97.22) | 31 (93.94) |
|
| Lymph node
involvement |
|
| Yes | 13 (36.11) | 20 (60.60) | 0.0419a |
| No | 23 (63.89) | 13 (39.39) |
|
| Surgery |
|
| Yes | 4 (11.11) | 1 (3.03) | 0.1959 |
| No | 32 (88.89) | 32 (96.97) |
|
| Chemotherapy |
|
| Yes | 29 (72.22) | 30 (90.91) | 0.2223 |
| No | 7 (19.44) | 3 (9.09) |
|
| HSCT |
|
| Yes | 0 (0.0) | 2 (6.06) | 0.1339 |
| No | 36 (100.00) | 31 (93.94) |
|
Multivariate survival analysis
Cox regression multivariate analysis yielded the
following significant prognostic factors for OS in patients with
ENKL: ECOG score (HR=9.537, P<0.0001), disease stage (HR=5.500,
P=0.006), IPI (HR=3.650, P=0.022), presence of B symptoms
(HR=3.070, P=0.039) and radiotherapy (HR=2.970, P=0.046),
indicating that these factors were independently associated with
survival of ENKL (Table III).
 | Table III.Multivariate analysis of survival
with Cox proportional hazards model. |
Table III.
Multivariate analysis of survival
with Cox proportional hazards model.
|
| 95% CI for Exp
(B) |
|---|
|
|
|
|---|
| Clinical
factors | B | SE | Wald | df | Sig. | Exp (B) | Lower | Upper |
|---|
| Age | 0.025 | 0.015 |
2.800 | 1 | 0.094 | 1.026 | 0.996 |
1.057 |
| ECOG PS score | 2.255 | 0.598 | 14.220 | 1 | 0.000a | 9.537 | 2.954 | 30.796 |
| Stage | 1.705 | 0.614 |
7.702 | 1 | 0.006a | 5.500 | 1.650 | 18.334 |
| IPI | 1.295 | 0.564 |
5.276 | 1 | 0.022a | 3.650 | 1.209 | 11.016 |
| B symptoms | 1.122 | 0.545 |
4.240 | 1 | 0.039a | 3.070 | 1.056 |
8.928 |
| Radiotherapy | 1.089 | 0.546 |
3.969 | 1 | 0.046a | 2.970 | 1.018 |
8.667 |
Analysis of the effect of diverse
therapies on survival
To further assess the effect of different regimens,
the patients were divided into subgroups by different treatment
regimens, different stages and different primary sites. Considering
that the radiotherapy group included patients who had received
radiotherapy alone as well as chemoradiotherapy, survival was
analyzed among the chemoradiotherapy, chemotherapy and radiotherapy
groups. The mOS of patients was 118, 63 and 132 months,
respectively, and the corresponding 5-year survival rates were
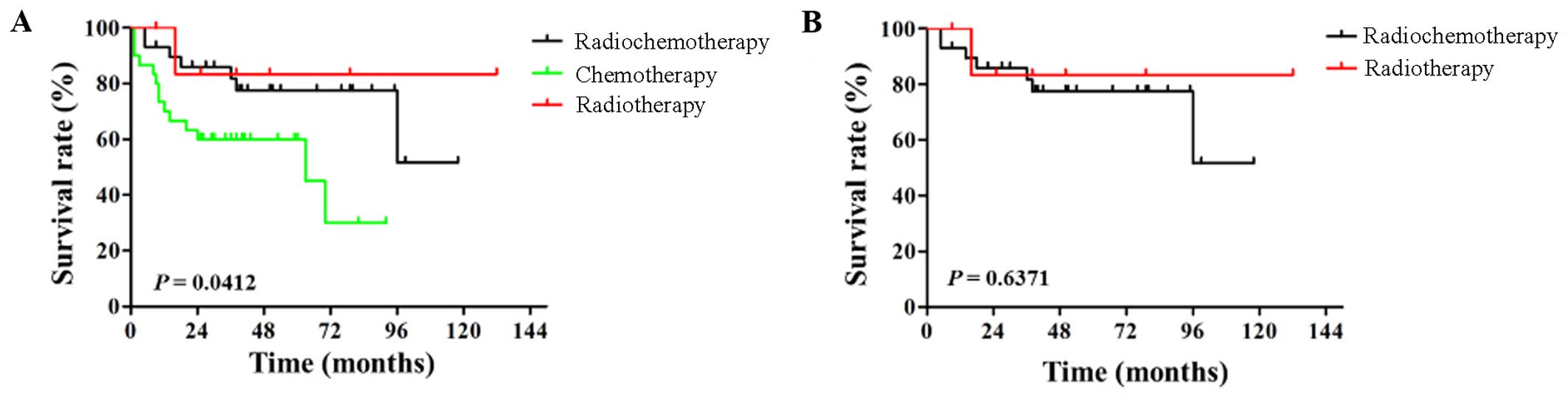
77.5, 45.0 and 83.3%, respectively (P=0.0412) (Fig. 1A); however, no statistically
significant difference was found between the chemoradiotherapy and
radiotherapy groups (HR=1.534, P=0.6371) (Fig. 1B). These results indicated that the
effects were different among the three treatment regimens, but
chemoradiotherapy did not provide a significant advantage over
radiotherapy. Since the chemotherapy regimens were mainly
anthracycline-based, the patients were divided into the
anthracycline-based and non-anthracycline based regimen groups;
however, no significant differences were found between these groups
in terms of mOS and 5-year survival rates (Table IV).
 | Table IV.Subgroup survival analysis. |
Table IV.
Subgroup survival analysis.
|
| Nasal | Extranasal | Total |
|---|
|
|
|
|
|
|---|
|
| No. | mOS (mo) | 5-year survival
(%) | P-value | No. | mOS (mo) | 5-year survival
(%) | P-value | No. | mOS (mo) | 5-year survival
(%) | P-value |
|---|
| Stage I/II |
|
| CT | 12 | 70 | 75.0 | 0.1003 | 5 | 90.5 | 100.0 | 0.5817 | 17 | 70 | 61.7 | 0.3243 |
|
RT/CT | 19 | 132 | 87.8 |
| 8 | 118 | 62.5 |
| 27 | 132 | 79.4 |
|
| A | 18 | 99 | 82.3 | 0.8836 | 9 | 118 | 75.2 | 0.2945 | 27 | 118 | 80.3 | 0.3821 |
|
Non-A | 7 | 67 | 85.7 |
| 3 | 95 | 100.0 |
| 10 | 95 | 90.0 |
|
| Stage III/IV |
|
| CT | 4 | 32 | 0 | 0.7472 | 9 | 12 | 0 | 0.0229a | 13 | 12 | 30.8 | 0.0656 |
|
RT/CT | 5 | 54 | 0 |
| 4 | 81.5 | 100 |
| 9 | 96 | 80.0 |
|
| A | 7 | 54 | 0 | 0.4692 | 8 | 96 | 51.9 | 0.7137 | 15 | 96 | 54.5 | 0.5927 |
|
Non-A | 2 | 27.5 | 0 |
| 5 | 24 | 0 |
| 7 | 24 | 0 |
|
| Total |
|
| CT | 16 | 70 | 68.8 | 0.1133 | 14 | 43.5 | 50.0 | 0.0543 | 30 | 63 | 45.0 | 0.0102a |
|
RT/CT | 24 | 132 | 81.9 |
| 12 | 96 | 72.9 |
| 36 | 132 | 78.6 |
|
| A | 25 | 99 | 77.9 | 0.3792 | 17 | 96 | 51.3 | 0.5319 | 42 | 96 | 69.9 | 0.6902 |
|
Non-A | 9 | 67 | 66.7 |
| 8 | 71 | 50.0 |
| 17 | 95 | 64.7 |
|
The survival of patients was then compared by
different stages, different primary sites and different treatment
regimens. The results revealed a difference between the
radiotherapy and non-radiotherapy groups for patients with
extranasal-type stage III/IV disease. The mOS for patients with
extranasal-type stage III/IV disease in the radiotherapy and
non-radiotherapy groups was 81.5 and 12 months, respectively, and
the 5-year survival rates differed significantly between the groups
(100 vs. 0%, respectively; P=0.0229). No significant differences
were found between other groups in terms of mOS and 5-year survival
rates. Our data demonstrated that patients with advanced disease
may also benefit from radiotherapy (Table IV).
Discussion
ENKL is a rare extranodal malignancy with poor
prognosis. This retrospective study analyzed the clinical
characteristics of patients with ENKL. In agreement with previous
studies (2,13), our data demonstrated that ECOG score,
stage, IPI, presence of B symptoms and treatment with radiotherapy
were independently associated with the survival of ENKL. However,
the LDH level did not exhibit an independent association with
survival, which differed from the results of other studies
(1,8), despite the fact that the mOS of
patients with elevated serum LDH was below average (70 vs. 96
months) (Table I). A larger sample
size is required to further investigate this point.
Radiotherapy is one of the most important treatments
for ENKL and has been confirmed in previous studies to improve
patient outcomes (7,8). Consistent with these prior reports, our
results demonstrated that radiotherapy was able to increase the mOS
and 5-year survival rate (132 months, 77.9% vs. 63 months, 55.9%,
P=0.0041) of ENKL (Table I) and was
one of the independent prognostic factors for survival (Table III). However, the majority of the
studies on ENKL evaluated nasal-type and early-stage disease
(14), while few focused on
extranasal and late-stage disease. In our study, we also observed
that, for patients with stage III/IV disease, the mOS and 5-year
survival rate of those receiving radiotherapy were 96 months and
80.0%, respectively, which were more favorable compared with those
of patients not receiving radiotherapy (12 months and 30.3%,
respectively), although this difference did not quite reach
statistical significance with the number of investigated patients
(P=0.0656). In the subgroup analysis stratified by stage and
primary site, a statistical difference was observed in the stage
III/IV extranasal subgroup between the radiotherapy and
non-radiotherapy groups (P=0.0229), but the samples were relatively
small and unbalanced (Table IV).
Taken together, our results indicated that patients with advanced
disease may also benefit from radiotherapy, although further
studies are required to support our findings.
Recently, a meta-analysis of 8 clinical studies
compared the efficacy of chemoradiotherapy and chemotherapy for
treating early-stage ENKL (10),
concluding that chemoradiotherapy did not provide a significant
advantage over radiotherapy for early-stage nasal-type ENKL.
Similarly, we observed that patients in the radiotherapy group
achieved a significantly longer mOS and higher 5-year survival
rates compared with those in the chemoradiotherapy and the
chemotherapy groups (132 months, 83.3% vs. 118 months, 77.5% vs. 63
months, 45.0%, respectively; P=0.0412) (Fig. 1A). In our study, however, the mOS and
5-year survival rates were not statistically different in the I/II
nasal-type subgroups, possibly because the number of patients with
stage I/II nasal-type disease was small (n=31). A recent study
(15) suggested that, unlike
patients with stage I nasal-type disease, who may achieve favorable
outcomes when treated with radiotherapy alone, patients with stage
II disease or with paranasal involvement should be treated with
systemic therapy. Thus, radiotherapy may still be the optimal
choice, but whether patients with unfavorable prognosis should be
treated with chemotherapy and the optimal chemotherapy regimens
require further investigation.
ENKL is often resistant to anthracycline-based
chemotherapy (1). Although the
prognosis of some patients was improved by non-anthracycline-based
chemotherapy, the prognosis of patients with advanced disease
remains poor (13). Au et al
(16) investigated 136 patients with
ENKL and did not find differences between the nasal and extranasal
groups in subgroups treated with anthracycline-based chemotherapy,
radiotherapy, or a combination of the two. Similarly, in our study,
42 of the 69 patients were administered an anthracycline-based
regimen as first-line therapy and the mOS and 5-year survival rates
did not differ between those treated with or without
radiotherapy.
Obama (17) reported
that, for radiotherapy-resistant disease, chemoradiotherapy,
including the topoisomerase inhibitors etoposide (VP-16) and
irinotecan (CPT-11), may be a therapeutic option. The present study
included only 6 cases treated with ECHOP, among which 5 received
chemoradiotherapy; thus, more data are required to evaluate this
type of treatment. SMILE has been reported as a potential
first-line chemotherapy regimen for ENKL patients (5,18,19). A
phase II study (20) also
demonstrated that SMILE chemotherapy may be a choice for patients
with stage IV ENKL with relapsed or refractory disease. The present
study included 4 patients treated with the SMILE chemotherapy
regimen, but only 1 received sequential radiotherapy. That patient
received only 2 cycles of chemotherapy, due to myelosuppression.
The SMILE regimen may achieve better outcomes for ENKL, but it is
associated with a high risk of myelosuppression during the long
treatment period. Furthermore, its clinical value and ability to be
used in combination with radiotherapy remain uncertain.
HSCT is a potential therapeutic option, allowing
some patients to achieve remission (21). The present study included only 2
patients who had been treated with HSCT, and they have achieved 30
and 34 months of remission. However, further investigation is
required to identify more effective therapeutic options for this
disease.
In conclusion, ECOG PS score, disease stage, IPI,
presence of B symptoms and treatment with radiotherapy were the
independent prognostic factors of OS in patients with ENKL.
Radiotherapy achieved better outcomes, but no difference was
observed between chemoradiotherapy and radiotherapy. Patients with
advanced disease may also benefit from radiotherapy.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 81172169).
References
|
1
|
William BM and Armitage JO: International
analysis of the frequency and outcomes of NK/T-cell lymphomas. Best
Pract Res Clin Haematol. 26:23–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Wang WD, Geng QR, Wang L, Chen
XQ, Liu CC and Lv Y: Serum levels of interleukin-9 correlate with
negative prognostic factors in extranodal NK/T-cell lymphoma. PloS
One. 9:e946372014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun J, Yang Q, Lu Z, He M, Gao L, Zhu M,
Sun L, Wei L, Li M, Liu C, et al: Distribution of lymphoid
neoplasms in China: Analysis of 4,638 cases according to the World
Health Organization classification. Am J Clin Pathol. 138:429–434.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee
JH, Lee DH, Huh J, Oh SY, Kwon HC, et al: Extranodal natural killer
T-cell lymphoma, nasal-type: A prognostic model from a
retrospective multicenter study. J Clin Oncol. 24:612–618. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jo JC, Yoon DH, Kim S, Lee BJ, Jang YJ,
Park CS, Huh J, Lee SW, Ryu JS and Suh C: Clinical features and
prognostic model for extranasal NK/T-cell lymphoma. Eur J Haematol.
89:103–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Liao WH, Zhou JH, Liu BA, Wen JF and
Hu ZL: NK/T cell lymphoma involving mediastinum: Report of a case
and review of literature. Int J Clin Exp Pathol. 7:6399–6402.
2014.PubMed/NCBI
|
|
7
|
Wang ZY, Liu QF, Wang H, Jin J, Wang WH,
Wang SL, Song YW, Liu YP, Fang H, Ren H, et al: Clinical
implications of plasma Epstein-Barr virus DNA in early-stage
extranodal nasal-type NK/T-cell lymphoma patients receiving primary
radiotherapy. Blood. 120:2003–2010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chauchet A, Michallet AS, Berger F,
Bedgedjian I, Deconinck E, Sebban C, Antal D, Orfeuvre H, Corront
B, Petrella T, et al: Complete remission after first-line
radio-chemotherapy as predictor of survival in extranodal NK/T cell
lymphoma. J Hematol Oncol. 5:272012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma HH, Qian LT, Pan HF, Yang L, Zhang HY,
Wang ZH, Ma J, Zhao YF, Gao J and Wu AD: Treatment outcome of
radiotherapy alone versus radiochemotherapy in early stage nasal
natural killer/T-cell lymphoma. Med Oncol. 27:798–806. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang B, Zhu F, Liu X, Zhao J, Li M, Li Y,
Huang J, Zou L and Chang Q: Radiotherapy combined with chemotherapy
versus radiotherapy alone for early stage nasal natural
killer/T-cell lymphoma: A meta-analysis. J Chemother. 28:65–71.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin's
Lymphomas, Version 2. 2015.https://www.nccn.org/about/nhl.pdfAccessed July
13, 2015.
|
|
12
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO classification of
tumours of haematopoietic and lymphoid tissues. 2. 4th. World
Health Organization; 2008
|
|
13
|
Kim SJ, Hong M, Do IG, Lee SH, Ryu KJ, Yoo
HY, Hong JY, Ko YH and Kim WS: Serum survivin and vascular
endothelial growth factor in extranodal NK/T-cell lymphoma, nasal
type: Implications for a potential new prognostic indicator.
Haematologica. 100:e106–e109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li YX, Wang H, Jin J, Wang WH, Liu QF,
Song YW, Wang ZY, Qi SN, Wang SL, Liu YP, et al: Radiotherapy alone
with curative intent in patients with stage I extranodal nasal-type
NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 82:1809–1815.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li YX, Liu QF, Wang WH, Jin J, Song YW,
Wang SL, Liu YP, Liu XF, Zhou LQ and Yu ZH: Failure patterns and
clinical implications in early stage nasal natural killer/T-cell
lymphoma treated with primary radiotherapy. Cancer. 117:5203–5211.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Au WY, Weisenburger DD, Intragumtornchai
T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO and Liang R:
International Peripheral T-Cell Lymphoma Project: Clinical
differences between nasal and extranasal natural killer/T-cell
lymphoma: A study of 136 cases from the International Peripheral
T-Cell Lymphoma Project. Blood. 113:3931–3937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Obama K: Concomitant use of radiotherapy
and two topoisomerase inhibitors to treat adult T-cell leukemia
with a radiotherapy-resistant bulky disease: A case series. Am J
Blood Res. 4:106–109. 2014.PubMed/NCBI
|
|
18
|
Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T,
Tse E, Leung AY and Chim CS: SMILE for natural killer/T-cell
lymphoma: Analysis of safety and efficacy from the Asia Lymphoma
Study Group. Blood. 120:2973–2980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim YL, Pack HS, Park JE, Oh JR and Kong
JH: Extranodal natural killer/T-cell lymphoma of the tenosynovium
of the hand. Korean J Intern Med. 30:122–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamaguchi M, Kwong YL, Kim WS, Maeda Y,
Hashimoto C, Suh C, Izutsu K, Ishida F, Isobe Y, Sueoka E, et al:
Phase II study of SMILE chemotherapy for newly diagnosed stage IV,
relapsed, or refractory extranodal natural killer (NK)/T-cell
lymphoma, nasal type: The NK-Cell Tumor Study Group study. J Clin
Oncol. 29:4410–4416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
LaPorte J, Morris L and Koepke J: Long
complete remission achieved with the combination therapy of
Cisplatin and gemcitabine in a patient with aggressive natural
killer cell leukemia. Case Rep Hematol. 2015:7156152015.PubMed/NCBI
|